Abstract
The role of N-linked glycosylation and glycan trimming in the function of glycoproteins remains a central question in biology. Hepatitis B virus specifies three glycoproteins (L, M, and S) that are derived from alternate translation of the same ORF. All three glycoproteins contain a common N-glycosylation site in the S domain while M possesses an additional N-glycosylation site at its amino terminus. In the presence of N-butyl-deoxnojirimycin (an inhibitor of α-glucosidase) virions and the M protein are surprisingly retained. Preliminary evidence suggests that the retained M protein is hyperglucosylated and localized to lysosomal vesicles. In contrast, the S and L proteins are secreted, and their glycosylation state is unaffected by the presence of the inhibitor. Site-directed mutagenesis provides evidence that virion secretion requires the glycosylation sequon in the pre-S2 domain of M. This highlights the potential role of the M protein oligosaccharide as a therapeutic target.
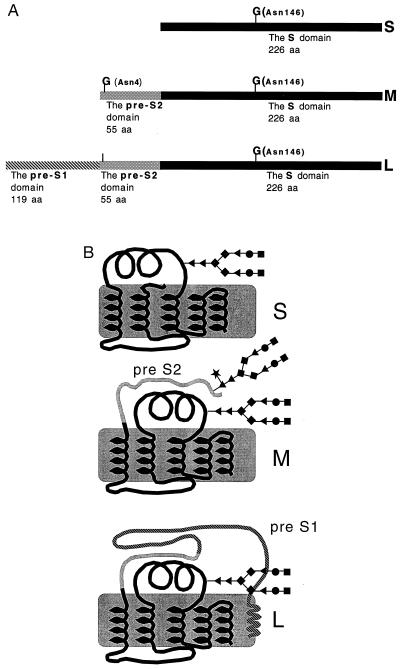
Hepatitis B virus (HBV) is the human member of the hepadnaviradae family of viruses which infects over 300 million people worldwide. The HBV genome encodes for three related envelope proteins termed L, M, and S (Fig. 1A). The three envelope proteins are produced from a single ORF through alternative translation start sites. All three proteins have a common N-linked glycosylation site at position 146 of the S domain (Fig. 1A), while the M protein alone contains an additional site at position 4 of the pre-S2 domain (1).
Figure 1.
(A) The HBV envelope proteins. All three proteins have a common N-linked glycosylation site at position 146 of the S domain (marked with a G). The M protein contains an additional glycan site at amino acid 4 of the pre-S2 domain (marked with a G). The L protein, while containing the pre-S2 glycosylation sequon, only utilizes the shared S glycan site (1). (B) Structural diagram of the three HBV envelope proteins with attached N-linked glycans. The pre-S1 and pre-S2 domains are indicated. B is based upon ref. 1. The glycan structures are as follows: ♦, mannose; ▴, N-acetylglucosamine; •, galactose; ▪, sialic acid; ★, fucose.
A peculiar feature of these envelope glycoproteins is that they are secreted in the form of small, noninfectious, non-DNA-containing, lipoprotein particles. These subviral particles are secreted in vast numbers, often outnumbering the infectious HBV virion by 100,000:1 (1). Subviral particles are found in two forms: spheres, which consists mainly of the S and M proteins; and filaments, which contain a greater amount of the L protein (2).
All three envelope glycoproteins are important in the viral life cycle. While it has been shown that the L and S proteins are necessary for virion secretion (3), the role of M is in doubt (3, 4). The current model of virion formation involves DNA containing nucleocapsids budding into the lumen of the endoplasmic reticulum (ER) and secretion through the trans-Golgi network (5).
The N-linked glycosylation pathway is well defined, consisting of over 13 enzymes that are involved in processing within the ER and the Golgi apparatus (6). Specific inhibitors of this pathway can be used to probe the importance of N-linked glycosylation. The role of N-linked glycosylation in HBV has yet to be determined. Several reports have provided evidence that the N-linked glycans are not necessary for the secretion of the subviral particle (7, 8). In contrast, the secretion of virus requires both N-linked glycosylation (9) and N-linked glycan processing (10). However, it was unclear whether this resulted from a general effect of glycosylation or from a specific effect on a particular viral glycoprotein. To this end, we have now analyzed the glycosylation state and the secretion levels of the three envelope glycoproteins (S, M, and L) in the presence of the α-glucosidase inhibitor N-butyl-deoxnojirimycin (NB-DNJ). The α-glucosidases are the first enzymes involved in the glycan processing pathway and remove the terminal three glucose residues from the Glc3Man9GlcNAc2 glycoform after it has been transferred from the dolichol diphosphate to the growing polypeptide backbone (reviewed in ref. 6). Site-directed mutagenesis on the individual glycan sites of the three glycoproteins has allowed more detailed conclusions as to which glycosylation site is important in viral secretion. The data presented in this paper, together with our previous work with tunicamycin (9), lead to the conclusion that the Asn-4 glycan site in the pre-S2 domain of M plays an important role in the secretion of HBV.
METHODS
Cells and Transfections.
HepG2 cells were grown in RPMI 1640 medium (GIBCO/BRL) containing 10% fetal bovine serum (GIBCO/BRL). HepG2.2.15 cells were kindly provided by George Acs (Mt. Sinai Medical College, New York) and maintained as HepG2 cells but with the addition of 200 μg/ml of G418 (GIBCO/BRL). DNA transfection of HepG2 cells were performed as in ref. 3.
Detection of Intracellular Viral DNA.
HepG2.2.15 cells were either left untreated or treated with 1000 μg/ml of the α-glucosidase inhibitor NB-DNJ (provided by Monsanto/Searle) for the indicated times and the total DNA extracted as described (11). DNA (25 μg) was digested with HindIII, resolved through a 1.2% agrose gel, and transferred to nylon membranes (Micron Separations, Westboro, MA). Membranes were then hybridized with a 32P-labeled probe containing the total HBV genome and developed as described (11). The relaxed circular, linear, and closed circular DNA were confirmed by enzymatic digestion (data not shown).
Detection of HBV Proteins by ELISA.
After 6 days, culture medium from HepG2.2.15 cells left uninhibited or inhibited with the 1000 μg/ml NB-DNJ were resolved through a discontinuous sucrose gradient as described (10). Fractions were collected and subjected to antigen detection by the ELISA method as described (10).
Release of Glycans and Labeling of Sugars.
Subviral particles from untreated or NB-DNJ-treated cells were purified by double CsCl ultracentrifugation (2) and concentrated using an Amicon Centriplus 100,000-Da cut-off protein concentrator). Particles were further purified by P4 column chromatography (Bio-Rad) before sugar release. Glycans were removed by hydrazinolysis using a Glycoprep 1000 machine (Oxford Glycosciences, Abingdon, Oxfordshire, U.K.) and fluorescently labeled at their reducing end with 2-aminobenzamide using the Signal Labeling Kit (Oxford Glycosciences). Glycans were subsequently analyzed by weak anion exchange (WAX) chromatography (12) and normal phase HPLC (13). Glycan structures were identified using sequential exoglycosidase digestion as described (13).
Detection of HBV Proteins by Antigen Capture.
For detection of subviral particle secretion, cells transfected with the vectors PRV-HBV 1.5, Sg−, and Mg− were incubated with 200 μCi/ml [35S]methionine (ICN; 1 Ci = 37 GBq) for 1 h in the presence of methionine-free RPMI (Sigma). After 1 h the radioactive medium was removed, and the cells were washed with 1× PBS (150 mM NaCl/10 mM Na2HPO4/1.5 mM K2HPO4, pH 7.12) and replaced with complete RPMI (10% fetal bovine serum; GIBCO/BRL) for 24 h. The medium was collected and layered onto a discontinuous sucrose density gradient as described (10). The collected fractions were then analyzed for the presence of S antigen by an antigen capture method as described (14).
Mutagenesis.
A 3.5-kb EcoRV to EcoRV fragment from PRV-HBV 1.5 (kindly provided by Volker Bruss, University of Göttingen, Göttingen, Germany) containing the entire HBV genome was cloned into the EcoRV site of sk-Bluescript (Stratagene). This plasmid, referred to as sk-HBV EcoRV–EcoRV, was used for the mutagenesis experiments. Mutagenesis was performed using the Chameleon site-directed mutagenesis kit (Stratagene) as per instructions. Site-directed mutagenesis was performed only on the second nucleotide of the S ORF, which changed the third nucleotide of the underlining polymerase ORF. Hence, the only amino acid change occurred in the S ORF. After mutagenesis, the 3.5-kb EcoRV fragment from sk-HBV EcoRV–EcoRV was cloned back into PRV-HBV 1.5. The Sg− vector contained the mutation Asn-146 to Ser-146. The Mg− vector contained the change of Thr-6 to Ile-6, hence disrupting the Asn-4-XXX-Thr-6 glycosylation sequon. The Sg− Mg− vector contained both these mutations. No alterations within the underlining polymerase gene were created, and the mutants contained only the desired mutation as confirmed by DNA sequence analysis (data not shown).
Detection of Secreted Viral DNA.
Five days after transfection with the appropriate HBV expression vectors, the supernatant was collected and briefly centrifuged at 3000 rpm for 10 min followed by pelleting through 20% sucrose for 16 h at 35,0000 rpm. As the parent vector PRV-HBV 1.5 has been shown to only secrete enveloped viral particles (3), PCR was then performed on the pellets using primers and PCR conditions as described (11). PCR products were subsequently run on 1.5% agarose gels and stained with ethidium bromide.
RESULTS
Inhibition of α-Glucosidase Causes the Accumulation of the Replicative Forms of HBV DNA.
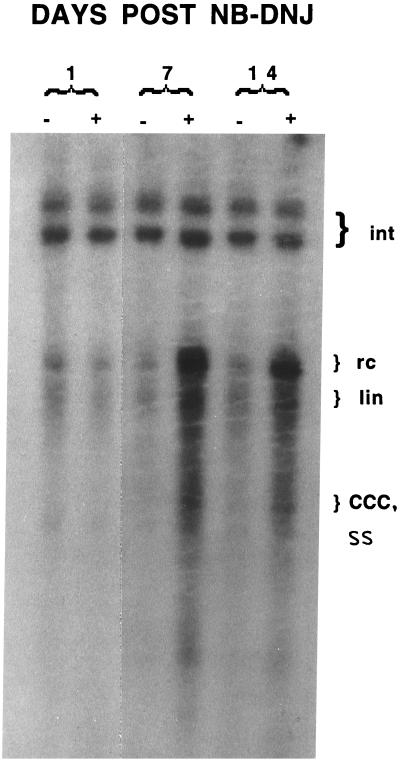
Inhibition of early glycan processing events leads to the retention and misfolding of some glycoproteins within the ER (15). This may be the result of triglucosylated glycoproteins being unable to interact with ER chaperones such as calnexin, which only recognize monoglucosylated glycoproteins (16, 17). Inhibitors of α-glucosidase, an ER early glycan processing enzyme, have also been shown to have antiviral activities against many viruses, including HBV (10). For example, the inhibition of α-glucosidase in HepG2.2.15 cells, a cell line that chronically secretes HBV (18), prevents the secretion of enveloped HBV DNA and causes the intracellular accumulation of viral DNA (10). Fig. 2 shows that in the presence of the α-glucosidase inhibitor, NB-DNJ, HepG2.2.15 cells accumulate large amounts of the replicative forms of HBV viral DNA. The upper bands represent the integrated forms of the HBV DNA, and their abundance remains constant in all samples. In contrast, the replicative forms of HBV DNA increase dramatically over time. At day 7 for example, there is over a 30-fold increase in the amount of relaxed circular and linear HBV DNA as compared with the untreated control. These data provide information as to which stages of the HBV life cycle have been interrupted by the α-glucosidase blockade. For example, accumulation of the replicative forms of HBV DNA suggest that while encapsidation of HBV viral nucleic acid has occurred, the envelopment process may be prevented. This is consistent with the α-glucosidase inhibitor NB-DNJ affecting the viral glycoproteins and implies that they are functionally impaired.
Figure 2.
Southern blot of accumulated intracellular HBV DNA from untreated and α-glucosidase-inhibited HepG2.215 cells. HepG2.2.15 cells were either left untreated or treated with 1000 μg/ml of the α-glucosidase inhibitor NB-DNJ for the indicated times and the intracellular HBV DNA detected as described. The location of the HBV integrated (Int), relaxed circular (rc), linear (lin), covalently closed circular (CCC), and single-stranded (SS) DNA are indicated. Increases in the rc and lin form of HBV DNA are clearly evident. As the HBV SS DNA comigrates with the CCC DNA it is difficult to determine which of these increases.
Secreted Subviral Particles from Cells in Which α-Glucosidase Has Been Inhibited Lack the M Protein.
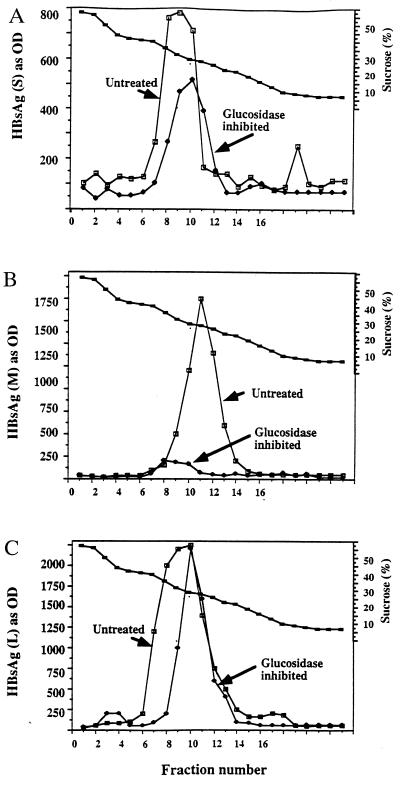
Our previous study showed that subviral particles were still secreted in the presence of NB-DNJ (10). However, a more detailed analysis revealed that the individual HBV envelope antigens had varying degrees of sensitivity to α-glucosidase function (Fig. 3). As shown in Fig. 3, while the secretion of the L and S proteins within subviral particles was reduced by only 20–30%, the secreted subviral particles were virtually devoid of the M protein. That is, while subviral particles containing mostly the L and S glycoproteins are secreted near normally, as are many host glycoproteins from α-glucosidase-inhibited cells (15, 19, 20), there was a 16-fold reduction the secretion of M-containing subviral particles. Preliminary evidence suggest that the subviral particles containing the M protein carry hyperglucosylated glycan structures and are targeted to lysosomal compartments (14). The lack of secretion of M-containing subviral particles is thought to be the result of the effect of the α-glucosidase inhibitor on the M protein. The extreme sensitivity of M, compared with L and S, is interesting because all three proteins are derived from alternate translation of the same ORF and hence have great amino acid similarity (1).
Figure 3.
Detection of the HBV surface antigens in subviral particles secreted from untreated or α-glucosidase-inhibited cells by sucrose density gradient. Medium from untreated cells and cells treated with 1000 μg/ml of the α-glucosidase inhibitor NB-DNJ for 10 days was collected and resolved through sucrose density gradients as described. HBV-specific envelope proteins were detected by ELISA using antibodies and methods described previously (10). (A–C) Detection of individual envelope antigens using antibodies for the HBV S, M, and L proteins, respectively. Markers are untreated (▪) or 1000 μg/ml (•) of NB-DNJ.
Subviral Particles Secreted from α-Glucosidase-Inhibited Cells Contain Fully Processed Oligosaccharides.
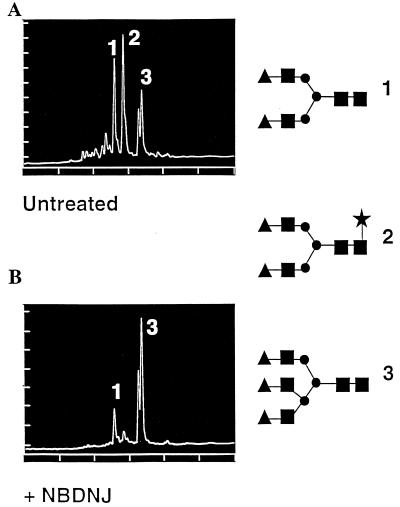
If the failure to secrete the M protein, relative to L and S, is due to the accumulation of triglucosylated glycan structures on the M protein, then HBV glycoproteins secreted from α-glucosidase-inhibited cells should not contain any unprocessed glycan (their N-glycans should be complex). Therefore the glycan structures from subviral particles secreted from untreated and α-glucosidase-inhibited cells were determined and are shown in Fig. 4 A and B, respectively. As shown in Fig. 4, the S and L proteins secreted from α-glucosidase-inhibited cells contain complex glycan structures. The lack of the fucosylated structure from α-glucosidase-inhibited cells is assumed to be the result of the lack of M protein (and its corresponding glycans) in subviral particles obtained from cells treated with NB-DNJ. Consistent with this is a previous report localizing the fucosylated biantennary structure, lacking in the α-glucosidase-treated sample, to the M protein (21). The complete processing of the L and S glycans in the presence of NB-DNJ arises from their ability to overcome the α-glucosidase blockade via the endomannosidase pathway (19). This pathway allows for the formation of complex glycan structures in the presence of inhibitors of the α-glucosidases.
Figure 4.
Analysis of the major glycan structures on subviral particles purified from untreated (A) and α-glucosidase-inhibited (B) cells. Glycans were removed from subviral particles by hydrazinolysis and fluorescently labeled at their reducing end as described. The determined glycan structures are shown along the right. The glycan structures are as follows: ▴, galactose; ▪, N-acetylglucosamine; •, mannose; ★, fucose.
HBV Glycosylation Knockout Vectors All Secrete HBV Subviral Particles.
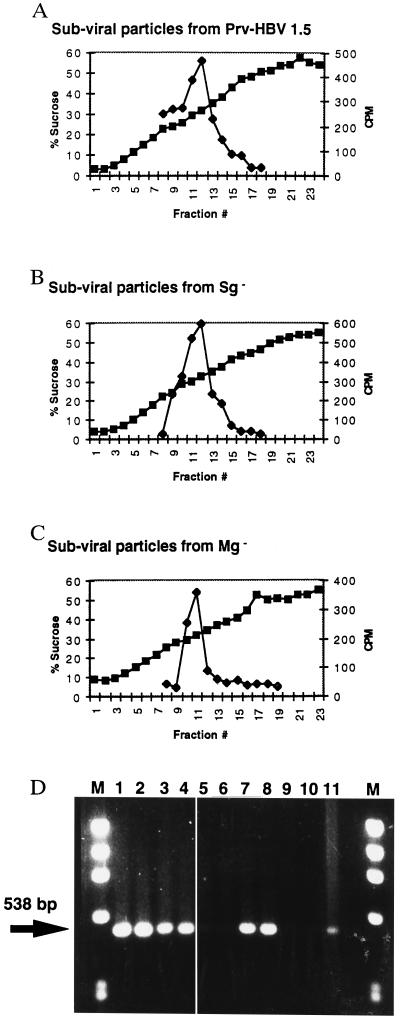
To determine which, if any, glycan was necessary to mediate secretion of virus, HBV glycosylation knockout vectors were created in the background of the HBV expression vector PRV-HBV 1.5 (3). Three mutant vectors were created that either (i) lacked the shared glycan at Asn-146 of the S domain (Sg−), (ii) lacked both the shared glycan at Asn-146 and the additional pre-S2 glycan at Asn-4 of the M protein (Sg− Mg−), or (iii) lacked only the Asn-4 glycan in the pre-S2 domain of the M protein (Mg−). All vectors contained amino acid changes only within the env ORF, with no alterations of the overlapping HBV DNA polymerase as determined by DNA sequence analysis (data not shown). These vectors, along with the parent vector, were transfected into HepG2 cells and assayed for the ability to induce the production of subviral and viral particles. Fig. 5 A–C shows that all vectors produced subviral particles with sedimentation profiles similar to wild type as determined by a sucrose density gradient. The Sg− Mg− vector also induced the secretion of subviral particles as determined by UV absorbance at the correct sucrose density (data not shown). The expression vectors also maintained the ability to secrete all three envelope antigens, implying that no extreme misfolding had occurred as the result of the mutagenesis or lack of glycosylation (data not shown). This is consistent with the results obtained with tunicamycin (7–9), and further shows that the secretion of subviral particles is independent of N-linked glycosylation.
Figure 5.
Analysis of the subviral and viral particles released from the glycosylation-incompetent expression vectors. Indicated expression vectors were transfected into HepG2 cells and analyzed for the ability to support the secretion of subviral and viral particles as described in materials and methods. Secretion of subviral particles from the (A) PRV-HBV 1.5 vector, (B) Sg− vector, and (C) Mg− vector, as resolved through a sucrose density gradient. (D) Secretion of virus as detected by PCR. The arrow indicates the location of the 538-bp PCR product. First and last lanes marked “M” are DNA molecular weight standards. Lanes: 1 and 2, HepG2.2.15 cells; 3 and 4, from the control vector PRV-HBV 1.5; 5 and 6, from the Sg− Mg− vector which secretes no virus; 7 and 8, from the Sg− vector; 9 and 10, the Mg− vector which also secretes no virus; 11, a control for the PCR.
Expression Vectors That Lack the pre-S2 Glycosylation Site Do Not Secrete Viral DNA.
The ability of the expression vectors to support the secretion of virion particles is shown in Fig. 5D. Fig. 5D shows that HBV DNA can be recovered from culture media of HepG2.2.15 cells (lanes 1 and 2) and cells transfected with the parent vector PRV-HBV 1.5 (lanes 3 and 4). No detectable HBV DNA was recovered from the culture media of cells transfected with the Sg− Mg− expression vector (lanes 5 and 6). This also confirms the results obtained with tunicamycin and demonstrates that the N-glycans of HBV are required for virion secretion. Surprisingly, the results obtained following the transfection of the Sg− glycan vector (lanes 7 and 8) indicated that the common glycan in L, M, and S does not play a role in virion secretion. However the removal of the pre-S2 glycan (lanes 9 and 10) prevents the secretion of virus. Southern analysis of pelleted media from all HBV expression vectors confirmed that the amplified DNA originated from HBV viral particles (data not shown).
DISCUSSION
Inhibitors of α-glucosidase are known to have different effects on different glycoproteins (15, 20). However, the differential effect of the α-glucosidase inhibitor on the three HBV envelope proteins (L, M, and S) proved quite surprising. All three proteins are closely related, since they are derived from the same ORF. These proteins are also thought to have similar three dimensional structures (Fig. 1B; ref. 1). The major difference between the S and M protein is the additional 55 amino acids of the pre-S2 domain on M and importantly the additional glycan site within this region at Asn-4 (Fig. 1A). Although the L protein contains the pre-S2 domain as well, this glycosylation site is not utilized. The importance of glycosylation in the pre-S2 domain of M is consistent with the full utilization of this site as opposed to the variable utilization of the glycan sequon in the S domain (1).
The data obtained with tunicamycin and the glycan mutants demonstrate the importance of glycosylation for the secretion of viral particle (Table 1). Previous experiments with deoxymannojirimycin (DMJ), an inhibitor of Golgi mannosidases, have shown that glycan processing outside of the ER is not important for the secretion of either viral or subviral particles. It is therefore the glycosylation events in the ER that must be important for the secretion of the virus. It is in this cellular compartment that protein folding, oligomerization, and the viral budding process occur (5). For glycoproteins, the processing of the initial oligosaccharide precursor from the Glc3Man9GlcNAc2 glycoform to the Glc1Man9GlcNAc2 glycoform in the ER can lead to an interaction with chaperones such as calnexin (16). Calnexin, which binds only to glycoproteins containing Glc1Man9GlcNAc2 structures, is thought to assist the folding of some glycoproteins (17). NB-DNJ, by inhibiting the formation of the Glc1Man9GlcNAc2 glycoform, prevents the interaction with calnexin. As the M protein is not secreted and is targeted toward lysosomes in the presence of NB-DNJ (14), we hypothesize that this type of interaction may be required for M, but not for the other envelope proteins. Support for this comes from the analysis that only the glycosylation state of M is altered by the presence of NB-DNJ. Presumably, subviral particles containing predominately S and L proteins (Fig. 3) can utilize the Golgi-endomannosidase pathway, which can remove the triglucosylated cap and allows further processing to proceed normally. The same glycosylation profile of these proteins in the presence or absence of NB-DNJ is also indicative of their correct folding (22).
Table 1.
Viral and subviral particle secretion from cells treated with inhibitors of the N-linked glycosylation pathway or transfected with the glycan mutants
| Treatment/glycan mutant* | Virions† | Subviral particles‡ |
|---|---|---|
| Tunicamycin§ | No | Yes |
| NB-DNJ¶ | No | Yes; but no M |
| DMJ§ | Yes | Yes |
| Glycan mutant‖ | ||
| Sg−, Mg− | No | Yes |
| Sg− | Yes | Yes |
| Mg− | No | Yes |
HepG2.2.15 cells were treated with indicated inhibitor (8, 9), or HepG2 cells were transfected with indicated HBV expression vectors and analyzed for the ability to secrete viral and subviral particles as described.
The secretion of viral DNA either in the presence of the indicated inhibitor or as induced by the transfected HBV expression vectors.
The secretion of subviral particles detected as described. Unless otherwise noted, all envelope proteins secreted.
As in ref. 9.
As in ref. 10.
The glycan mutants are as indicated in Fig. 5D.
The site-directed mutagenesis experiments highlight the important role of the M glycan in HBV. HBV expression vectors that lacked either all the glycan sites, or the additional pre-S2 glycan site, were unable to secrete enveloped virus. However, alterations of the common S glycan site had no effect on virion secretion. Because the L protein also contains the pre-S2 region, alterations in the M glycan site could affect the properties of the L protein. However the detection of the L protein as part of a subviral particle from the Mg− vector suggest that the L protein is unaffected by the amino acid change at Asn-4 (data not shown).
The results of the site-directed mutagenesis experiments suggest that the lack of viral particle secretion from tunicamycin-treated cells arises from the deficient glycosylation on the pre-S2 glycan on the M protein. They also imply that the effect with NB-DNJ arises from the improper glycosylation of M. Thus the M glycan plays a crucial role in the formation of the HBV viral particle (Table 1).
In searching for a mechanism, it is interesting to note the antiviral effects of NB-DNJ on HIV (23). Inhibition of α-glucosidase prevents the proper folding of the V1/V2 loop of HIV gp120 (24). However, in contrast to HBV, enveloped HIV virus is formed and secreted (although it is noninfectious). Another example in which the inhibition of glycan processing with NB-DNJ resulted in regional misfolding is that of tyrosinase, an enzyme involved in the production of melanin (25). In this case, the hyperglucosylated enzyme is correctly transported to melanosomes but is inactive as a result of being unable to bind the copper required for activity (25). In the case of the HBV M protein, the most likely effect of NB-DNJ would be that the M protein is severely misfolded. This is supported by the finding of aggregated, hyperglucosylated M in the lysosomes (14). That the M protein is secreted in the presence of tunicamycin and from the Mg− vector suggest that the degree of misfolding is less in these cases. However, unlike the secretion of subviral particles, viral envelopment may require the precise three-dimensional geometry of its assembled envelope glycoproteins. Even the slight misfolding of the individual M glycoprotein within the viral pre-envelope may be enough to prevent virion formation.
A crucial role for the M glycan is surprising as there is evidence that HBV can be secreted in the absence of the M protein (3, 26). Perhaps the M protein acts in a dominant negative manner. That is, it is hypothesized that when the M protein is present and misfolded, it has the ability to destabilize the viral envelope in such a way as to hinder viral but not subviral particle secretion.
Overall the results presented here demonstrate that alterations within the pre-S2 glycosylation site can prevent the secretion of HBV. The realization that the interference of a single glycan site so profoundly influences HBV secretion suggests both an opportunity for antiviral intervention and offers a simplified system to study the importance of N-linked glycosylation in the life cycle of HBV and possibly other animal viruses.
Acknowledgments
We thank Dr. Volker Bruss for the expression vector PRV-HBV 1.5. Drs. P. Rudd, S. Petrescu, F. Platt, and T. Mattu are thanked for their careful reading of the manuscript and providing useful comments. This work was supported by the Hepatitis B Foundation of America, Monsanto Searle, Inc., and the North Atlantic Treaty Organization. B.S.B. is supported by U.S. Public Health Service Grant CA-06927 from the National Institutes of Health and by an appropriation from the Commonwealth of Pennsylvania.
ABBREVIATIONS
- HBV
hepatitis B virus
- ER
endoplasmic reticulum
- NB-DNJ
N-butyl-deoxnojirimycin
References
- 1.Heermann K H, Gerlich W H. In: Molecular Biology of HBV. Maclachlan A, editor. Boca Raton, FL: CRC; 1992. pp. 109–143. [Google Scholar]
- 2.Heermann K H, Goldman I, Schwartz W, Seyffarth T, Baumgarten H, Gerlich W H. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss V, Ganem D. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda K, Tsurimoto T, Matsubara K. J Virol. 1991;65:3521–3529. doi: 10.1128/jvi.65.7.3521-3529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganem D. Curr Top Microbiol Immunol. 1991;168:61–84. doi: 10.1007/978-3-642-76015-0_4. [DOI] [PubMed] [Google Scholar]
- 6.Datema S, Olofsson P, Romero P A. Pharmacol Ther. 1987;33:221–286. doi: 10.1016/0163-7258(87)90066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheu S Y, Lo S J. J Gen Virol. 1994;75:3031–3039. doi: 10.1099/0022-1317-75-11-3031. [DOI] [PubMed] [Google Scholar]
- 8.Patzer E, Nakamura G, Yaffe A. J Virol. 1984;51:346–353. doi: 10.1128/jvi.51.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Mehta A, Butters T, Dwek R A, Block T M. Virology. 1995;213:660–665. doi: 10.1006/viro.1995.0038. [DOI] [PubMed] [Google Scholar]
- 10.Block T, Platt F, Lu X, Gerlich W, Foster G, Blumberg B, Dwek R A. Proc Natl Acad Sci USA. 1994;91:2235–2239. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X, Block T, Gerlich W H. J Virol. 1996;70:2277–2285. doi: 10.1128/jvi.70.4.2277-2285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guile G R, Wong S Y C, Dwek R A. Anal Biochem. 1994;222:231–235. doi: 10.1006/abio.1994.1478. [DOI] [PubMed] [Google Scholar]
- 13.Guile G R, Rudd P, Wing D, Prime S B, Dwek R A. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- 14.Lu, X., Mehta, A., Dadmarz, M., Dwek, R. A., Blumberg, B. S. & Block, T. M. (1997) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 15.Lodish H, Kong A. J Cell Biol. 1984;98:1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou W J, Cameron P H, Thomas D Y, Bergeron J M. Nature (London) 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- 17.Helenius A. Mol Biol Cell. 1994;5:253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sells M A, Zelent A, Shvartsman M, Acs G. J Virol. 1988;62:2836–2844. doi: 10.1128/jvi.62.8.2836-2844.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore S E, Spiro R G. J Biol Chem. 1990;265:1304–1312. [PubMed] [Google Scholar]
- 20.Karlsson G B, Butters T D, Dwek R A, Platt F M. J Biol Chem. 1993;268:570–576. [PubMed] [Google Scholar]
- 21.Poulter L, Burlingame A L. Methods Enzymol. 1990;193:661–689. doi: 10.1016/0076-6879(90)93444-p. [DOI] [PubMed] [Google Scholar]
- 22.Dwek R A. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 23.Fisher P B, Karlsson G B, Dwek R A, Platt F M. J Virol. 1996;70:7153–7160. doi: 10.1128/jvi.70.10.7153-7160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher P B, Karlsson G B, Butters T D, Dwek R A, Platt F M. J Virol. 1996;70:7143–7152. doi: 10.1128/jvi.70.10.7143-7152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrescu, S. M., Petrescu, A. J., Dwek, R. A., Platt, F. M. (1996) J. Biochem. (Tokyo), in press.
- 26.Fernholz D, Galle P R, Stemler M, Brunetto M, Bonino F, Will H. Virology. 1993;194:137–148. doi: 10.1006/viro.1993.1243. [DOI] [PubMed] [Google Scholar]