Because of improved survival of HIV infected patients following the development of effective antiviral drugs and the proven efficacy of interferon/ribavirin in the treatment of hepatitis C in this group of patients,1 gastroenterologists and hepatologists will more and more be involved in the clinical management of such patients. We report an adverse reaction associated with atazanivir, a recently introduced HIV protease inhibitor, which may be relevant for the management of such patients.
Atazanivir is a protease inhibitor with minimal effects on lipid and glucose homeostasis, which is given once daily in combination with other antiretroviral agents.2 This drug is known to cause reversible unconjugated hyperbilirubinaemia by competitive inhibition of the uridine diphosphate‐glucuronosyltransferase 1A1 (UGT1A1) in up to 50% of patients treated. In nine per cent of the patients, hyperbilirubinaemia is severe (grade 4), with serum bilirubin concentrations ⩾105 μmol/l.3 We investigated the effect of efavirenz, a non‐nucleoside reverse transcriptase inhibitor, on grade 4 hyperbilirubinaemia in an atazanivir/ritonavir treated patient with HIV infection and Gilbert's syndrome.
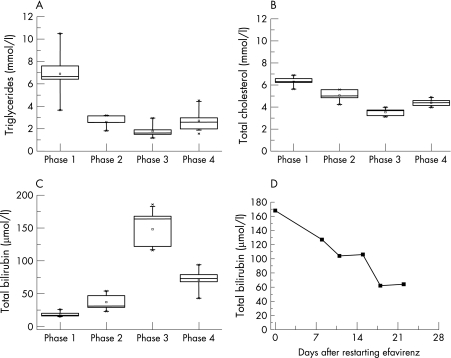
A 48 year old, treatment experienced HIV infected patient with genetically proven Gilbert's syndrome was treated with a combination of 150 mg lamivudine and 300 mg zidovudine (combivir, twice daily), efavirenz (600 mg, once daily) and ritonavir boosted lopinavir (400 mg lopinavir and 100 mg ritonavir, twice daily). Because of severe hyperlipidaemia, with a median fasting triglyceride concentration of 6.6 mmol/l (range 3.7 to 10.5, phase 1 (fig 1A)) and a total cholesterol concentration of 6.3 mmol/l (5.63 to 6.89, phase 1 (fig 1B)), lopinavir/ritonavir was replaced by atazanivir/ritonavir (ritonavir boosted atazanivir containing 300 mg atazanivir and 100 mg ritonavir, once daily). With this treatment, the patient developed moderate hyperbilirubinaemia with a median bilirubin concentration of 31 μmol/l (range 23 to 54) (phase 2 (fig 1C)). After stopping efavirenz, the patient presented with jaundice and grade 4 hyperbilirubinaemia, with a median bilirubin concentration of 152 μmol/l (range 116 to 186) (phase 3 (fig 1C)). In order to test whether discontinuation of efavirenz was responsible for the rise in bilirubin concentration, we re‐exposed the patient to efavirenz (600 mg once daily). The serum bilirubin concentration fell rapidly within three weeks (fig 1D). Over a time period of five months after restarting efavirenz, median serum bilirubin concentrations decreased to 73 μmol/l (range 43 to 94) (phase 4 (fig 1D)). Fasting triglyceride plasma concentrations increased, however, from 1.6 mmol/l (range 1.2 to 2.9) without efavirenz to 2.5 mmol/l (range 1.6 to 4.5) after restarting efavirenz (phases 3 and 4, respectively (fig 1A)). The plasma concentrations of atazanivir were monitored and were found to be within the therapeutic range.
Figure 1 Serum concentrations of fasting triglycerides (A), total cholesterol (B), and total bilirubin (C) during the four phases of different antiretroviral drug combinations, and total bilirubin serum concentration after restarting efavirenz in phase 4 (D). Drug combinations: phase 1: combivir (lamivudine/zidovudine) + lopinavir/ritonavir (ritonavir boosted lopinavir) + efavirenz; phase 2: combivir + atazanivir/ritonavir + efavirenz; phase 3: combivir + atazanivir/ritonavir; phase 4: combivir + atazanivir/ritonavir + efavirenz.
As our case demonstrates, the addition of efavirenz significantly decreased the raised serum bilirubin concentrations associated with atazanivir treatment in this patient with Gilbert's disease. Three weeks after restarting efavirenz, the serum bilirubin concentrations had fallen to less than half the starting value (fig 1D). Efavirenz is an activator of the human constitutive androstane receptor (hCAR).4 Activation of hCAR leads to an induction of the bilirubin conjugating capacity of UGT1A15 as well as to increased biliary excretion of conjugated bilirubin by induction of the efflux transporter multidrug resistance associated protein 2 (MRP2).6 Similar observations have been made in patients with unconjugated hyperbilirubinaemia caused by Crigler–Najjar syndrome type II (reduction of UGT1A1 activity to <10% of normal), when treated with the enzyme inducer phenobarbital.7,8
Combinations of atazanivir and efavirenz may be of benefit in HIV infected patients with atazanivir induced hyperbilirubinaemia.
Footnotes
Conflict of interest: None declared.
References
- 1.Payan C, Pivert A, Morand P.et al Rapid and early virological response to chronic hepatitis C treatment with IFN alpha‐2b or PEG‐ IFN alpha‐2b plus ribavirin in HIV/HCV co‐infected patients. Gut. 2007, March 22 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 2.Murphy R L, Sanne I, Cahn P.et al Dose‐ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral‐naive subjects: 48‐week results. Aids 2003172603–2614. [DOI] [PubMed] [Google Scholar]
- 3.Johnson M, Grinsztejn B, Rodriguez C.et al Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. Aids 200519685–694. [DOI] [PubMed] [Google Scholar]
- 4.Faucette S R, Zhang T C, Moore R.et al Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 200732072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie W, Yeuh M F, Radominska‐Pandya A.et al Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA 20031004150–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kast H R, Goodwin B, Tarr P T.et al Regulation of multidrug resistance‐associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X‐activated receptor, and constitutive androstane receptor. J Biol Chem 20022772908–2915. [DOI] [PubMed] [Google Scholar]
- 7.Crigler J F, Gold N I. Effect of sodium phenobarbital on bilirubin metabolism in an infant with congenital, nonhemolytic, unconjugated hyperbilirubinemia, and kernicterus. J Clin Invest 19694842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias I M, Gartner L M, Cohen M.et al Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyl transferase deficiency. Clinical, biochemical, pharmacologic and genetic evidence for heterogeneity. Am J Med 196947395–409. [DOI] [PubMed] [Google Scholar]