Studies from a variety of scientific fields point at the importance of biofilms in the gut. For example, Jeffrey Gordon and colleagues,1 evaluating data from immunologists, environmental engineers and glycobiologists, proposed that “symbionts inhabiting the polysaccharide‐rich mucus gel layer overlying the gut epithelium constitute a biofilm‐like community and that retention in such a matrix benefits the host by promoting functions served by the microbiota, including digestion of luminal contents and fortification of host defenses.” Evaluating our own immunological data, data from microbiologists and the medical literature, we independently came to the same conclusions.2
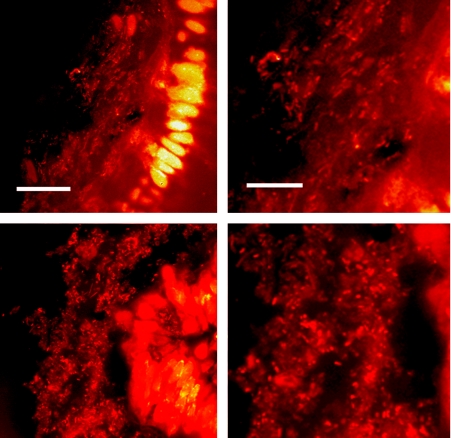
Direct observations of biofilms in the normal gut were lacking as recently as five years ago, probably because preservation of the epithelial glycocalyx, like the preservation of other glycocalyx structures, is technically challenging, as has long been known.3,4,5 In fact, we have found that manipulations as seemingly innocuous as washing with saline can disrupt biofilms from normal bowel tissue.6 With this in mind, we devised an approach that reproducibly preserves biofilms in the normal, unprepped (without flushing of the luminal contents) bowel,6 and examined fresh, unprepped human appendixes that had been removed from recipients during kidney–pancreas transplant procedures. Our laboratory has subsequently recapitulated our previously published observations using a fresh, normal, unprepped human appendix from a deceased organ donor (fig 1). To date, biofilms have been observed in the normal proximal (not distal) large bowel of mice,7 rats,6 baboons6 and humans.6 Thus, although enteric biofilms are likely in a steady state of shedding and regrowth, and although the percentage of epithelium covered with biofilms is unknown, there is no doubt that biofilms are indeed present in the normal bowel.
Figure 1 Biofilms adjacent to epithelium in a normal human appendix obtained from a deceased organ donor were observed using a confocal laser microscope following flash freezing, cryosectioning and rapid staining of the tissue with acridine orange as previously described.6 Two representative sections are shown, and images on the right show an enlarged section of the images on the left. Photos were taken of the areas at the border between the epithelium and the lumen. The smaller fluorescent spots are bacteria within the mucus layer stained with acridine orange, and the larger brightly stained areas are the nuclei of the epithelial cells that also stain with acridine orange. The bars = 30 μm (panels on left) and 15 μm (panels on right).
In a recent article (Gut 2007;56:343–50) Alexander Swidsinski and colleagues flatly dispute our findings of biofilms in the human appendix, referring to our work as “fiction”. The investigators, following a 6‐hour fixation procedure with a non‐aqueous solvent, find no biofilms in the human appendix and conclude that biofilms do not exist in the normal human bowel. We do not dispute or doubt the observations made by Swidsinski et al., since their results confirm what we have already demonstrated: biofilms in the normal colon are not stable using common preservation techniques.6
Of interest is the fact that Swidsinki's laboratory has been successful at preserving biofilms in biopsies of the diseased bowel of humans,8,9 in the normal bowel of laboratory mice,7 but not in the normal human appendix (Gut 2007;56:343–50). A likely explanation for these observations is that, for technical reasons, some tissues were more effectively preserved than others. The idea that the typically thick‐walled appendix is particularly difficult to preserve is supported by the fact that Swidsinski and colleagues used a 6‐hour fixation procedure for the appendix, but only a 2‐hour procedure for the biopsy samples. Another potential explanation for the observations made by Swidsinski's laboratory is that biofilms in the diseased human bowel may be more resilient than biofilms in the normal human gut. Indeed, biofilm formation in the normal bowel is probably supported by the immune system,10,11,12 and since inflammatory bowel disease is associated with an enhanced immune response, it is not surprising that biofilm formation in the diseased state would be profoundly increased.
Finally, we would point out that it is not overly surprising to observe biofilms in the normal colons of humans since similar observations have been made in the normal colons of laboratory animals as diverse as mice7 (observations from Swidsinski's own laboratory) and baboons6 (observations from our laboratory).
Footnotes
Competing interests: None.
References
- 1.Sonnenburg J L, Angenent L T, Gordon J I. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol 20045569–573. [DOI] [PubMed] [Google Scholar]
- 2.Everett M L, Palestrant D, Miller S E.et al Immune exclusion and immune inclusion: a new model of host‐bacterial interactions in the gut. Clinical and Applied Immunology Reviews 20045321–332. [Google Scholar]
- 3.Luft J H. The structure and properties of the cell surface coat. Int Rev Cytol 197645291–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen A, Pearson J P. The gastrointestinal adherent mucous gel barrier. Methods Mol Biol 200012557–64. [DOI] [PubMed] [Google Scholar]
- 5.Fassel T A, Edmiston C E., Jr Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy. Methods Enzymol 1999310194–203. [DOI] [PubMed] [Google Scholar]
- 6.Palestrant D, Holzknecht Z E, Collins B H.et al Microbial biofilms in the gut: visualization by electron microscopy and by acridine orange staining. Ultrastruct Pathol 20042823–27. [PubMed] [Google Scholar]
- 7.Swidsinski A, Loening‐Baucke V, Lochs H.et al Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol 2005111131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swidsinski A, Ladhoff A, Pernthaler A.et al Mucosal flora in inflammatory bowel disease. Gastroenterology 200212244–54. [DOI] [PubMed] [Google Scholar]
- 9.Swidsinski A, Weber J, Loening‐Baucke V.et al Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 2005433380–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollinger R R, Everett M L, Palestrant D.et al Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology 2003109580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orndorff P E, Devapali A, Palestrant S.et al Immunoglobulin‐mediated agglutination and biofilm formation by Escherichia coli K‐12 requires the type 1 pilus fiber. Infect Immun 2004721929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollinger R B, Everett M L, Wahl S.et al Secretory IgA and mucin‐mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol Immunol 200543378–387. [DOI] [PubMed] [Google Scholar]