Short abstract
New diagnostic criteria for coeliac disease are warranted
Coeliac disease is an autoimmune‐mediated enteropathy triggered in genetically susceptible persons by the ingestion of a single dietary factor – wheat, rye and barley‐derived gluten. The permanency of gluten intolerance was suggested in early studies, which showed that symptoms and intestinal lesions recurred usually within 2 years when gluten was reintroduced to the diet.1,2 Later it was recognised that the process of gluten‐induced mucosal deterioration may take years or even decades in some individual cases.3,4
The paper by Matysiak‐Budnik et al5 in the present issue of Gut brings new aspects into the natural history of coeliac disease (see page 1379). They studied 61 adult patients who had had the diagnosis of coeliac disase in childhood, and who had regarded themselves as asymptomatic despite remaining on a gluten‐containing diet for 11 years (median, range 3–21 years). In the follow‐up examination, 48 out of these 61 patients had, as expected, developed small bowel mucosal villous atrophy with crypt hyperplasia; 70% of them were also suffering from osteopenia or osteoporosis, which may well have been due to untreated coeliac disease. However, the remaining 13 patients showed normal small bowel mucosal morphology. Surprisingly, two out of the 13 patients evinced mucosal atrophy shortly after the beginning of the gluten challenge, but their mucosa eventually normalised when the gluten ingestion continued. The authors concluded that there might be some patients with coeliac disease who may develop true latency and tolerance against dietary gluten.
The current diagnostic criteria of coeliac disease require the presence of small intestinal mucosal villous atrophy and crypt hyperplasia.2 However, villous atrophy comprises only the end stage in the clinical course of the disease: evidence shows that coeliac disease develops gradually from small bowel mucosal inflammation to crypt hyperplasia and finally to overt villous atrophy.6 The term latent coeliac disease is well recognised: a patient having a normal small bowel mucosal structure while on a gluten‐containing diet develops later typical villous atrophy.7,8,9,10 The concept that coeliac disease may exist in patients having a normal mucosa while eating a gluten‐containing diet, is supported by the existence of very late relapse after reintroduction of gluten.3,4 Matysiak‐Budnik et al elucidated the clinical course in the less common form of latent coeliac disease, where patients with coeliac disease did not show clinical or histological relapse on a long‐term gluten challenge.5 The late concordancy in the appearance of coeliac disease in monozygotic twins also suggests that the disorder has remained in a latent stage for long periods.11,12
The diagnosis of latent coeliac disease is usually retrospective, and it is difficult to interpret whether minor small bowel mucosal changes are due to early developing coeliac disease, or whether the infiltrativity is an unspecific finding. The value of γδ+ intraepithelial lymphocytes in discovering patients with early developing coeliac disease has been shown to be superior to the detection of mucosal Marsh‐1‐type lymphocytosis,10,13 which may also be seen in other conditions.14 In patients having normal small bowel mucosal villous morphology, positive serum endomysial or tissue transglutaminase (TG2) antibodies have predicted forthcoming coeliac disease.8,9,10,15 These autoantibodies are produced in the small bowel mucosa16 and they can deposit on extracellular TG2 in the intestinal mucosa even when not yet measurable in serum.17,18,19 Recently, the detection of such TG2‐targeted intestinal autoantibody deposits proved to be a powerful tool in diagnosing mild enteropathy coeliac disease without villous atrophy, showing a sensitivity and specificity of 93%.10
Coeliac disease is clearly not restricted to small bowel mucosal enteropathy, and the next inevitable question is whether patients having evidence of early developing coeliac disease should be treated with a gluten‐free diet even in the absence of intestinal mucosal villous atrophy. In dermatitis herpetiformis the spectrum of enteropathy varies, and 20% of patients show apparently normal small bowel mucosal architecture, but there are virtually always inflammatory changes consistent with latent coeliac disease.20,21 Thus, dermatitis herpetiformis constitutes a model for early developing coeliac disease, where treatment of gluten intolerance is indicated irrespective of small intestinal mucosal damage. Interestingly, it has been found that many adult patients with dermatitis herpetiformis show coeliac‐type dental enamel defects, which suggests that they have suffered from a gluten‐induced condition or malabsorption even in early childhood.22
Many patients having latent coeliac disease have in fact suffered from gluten‐dependent symptoms already before the development of small bowel mucosal villous atrophy;9,18,23 some had had osteopenia or osteoporosis, which definitely warrants the early treatment.23,24 It is not known whether untreated patients having early developing coeliac disease carry an increased risk of malignancies; there is only one case report indicating that intestinal lymphoma may appear in the latent stage of coeliac disease.25 Recently, it has been suggested that in coeliac disease gluten may also affect both the peripheral and central nervous system.26 In gluten ataxia patients developed severe neurological symptoms although only some of the patients evinced gluten‐dependent small bowel mucosal atrophy with crypt hyperplasia. All patients were found to have TG2‐specific autoantibody deposits in their intestinal mucosa – interestingly one of the patients also had similar TG2‐targeted IgA deposits in the small vessels in the brain.27 Nervous tissue, in general, is known to have poor regenerative capacity. Therefore, it has been suggested that only early treatment with a gluten‐free diet might be beneficial in patients with gluten ataxia, irrespective of intestinal mucosal villous morphology.28
Back to the study of Matysiak‐Budnik et al.5 It appears that the original diagnosis was correct in the 13 cases. Each had coeliac‐type HLA, meaning HLA DQ2 or DQ8;29 at the follow‐up examination five had positive serum autoantibodies and the densities of small bowel mucosal γδ+ and CD3+ intraepithelial lymphocytes were higher than in non‐coeliac controls. Two additional patients had developed symptomatic relapse of coeliac disease during the follow‐up. Provided that the 13 patients had childhood coeliac disease, did they develop tolerance to gluten after long‐term gluten challenge, or did they simply have coeliac disease without manifest villous atrophy, which should perhaps be treated?
Even though seemingly healthy, 40% of patients with latent coeliac disease were suffering from mild clinical symptoms, 62% had anaemia, 67% had low serum ferritin and one had low bone mineral density. Again, they also had mucosal inflammation compatible with coeliac disease and dermatitis herpetiformis. The demonstration of TG2‐specific autoantibody deposits in the intestinal mucosa would have further supported the diagnosis of coeliac disease.17 Altogether the findings suggest that the patients had not developed tolerance against gluten. We feel that the patients described here might benefit from a gluten‐free diet; with a dietary treatment mild anaemia and other nutritional deficiencies and minor clinical symptoms may improve and in the long run subsequent intestinal or extraintestinal complications may be avoided. Our goal in coeliac disease is not to treat small intestinal mucosa but to treat patients.
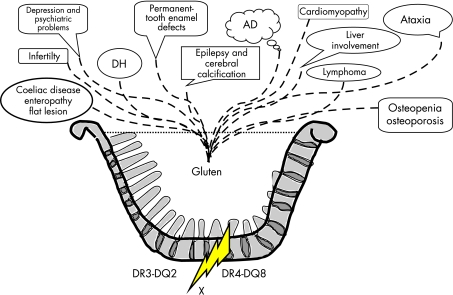
The current study again shows nicely that the “2 years rule” in gluten challenge is no longer valid. It further supports the view that coeliac disease clearly exists beyond villous atrophy. The current diagnostic criteria (the European Society for Pediatric Gastroenterology and Nutrition diagnostic criteria2) based on mucosal damage, and forgetting early developing coeliac disease and dermatitis herpetiformis, are no longer valid. Small intestinal villous atrophy is only one manifestation of genetic gluten intolerance (see fig 1).
Figure 1 Genetic gluten intolerance. The cooking pot shows that gluten‐induced disease entities in individuals with coeliac‐type HLA genetics splash out of the pot irrespectively of the grade of the small intestinal mucosal enteropathy. Coeliac disease with the classic flat mucosal lesion is only one of the disease entities. Dermatitis herpetiformis (DH) is the well‐known example of a gluten‐dependent disorder where the small intestinal villous morphology may be even normal. The fire of the cooking pot is the genetics and X indicates hitherto unknown susceptibility genes. AD, autoimmune diseases.
Footnotes
Competing interests: None declared.
References
- 1.Shmerling D H, Franckx J. Childhood celiac disease: a long‐term analysis of relapses in 91 patients. J Pediatr Gastroenterol Nutr 19865565–569. [PubMed] [Google Scholar]
- 2.Walker‐Smith J A, Guandalini S, Schmitz J.et al Revised criteria for diagnosis of coeliac disease. Arch Dis Child 199065909–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuitunen P, Savilahti E, Verkasalo M. Late mucosal relapse in a boy with coeliac disease and cow's milk allergy. Acta Paediatr Scand 198675340–342. [DOI] [PubMed] [Google Scholar]
- 4.Hogberg L, Stenhammar L, Falth‐Magnusson K.et al Anti‐endomysium and anti‐gliadin antibodies as serological markers for a very late mucosal relapse in a coeliac girl. Acta Paediatr 199786335–336. [DOI] [PubMed] [Google Scholar]
- 5.Matysiak‐Budnik T, Malamut G, Patey‐Mariaud de Serre N.et al Long‐term follow‐up of 61 coeliac patients diagnosed in childhood: evolution towards latency is possible on a normal diet. Gut 2007561379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh M N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue'). Gastroenterology 1992102330–354. [PubMed] [Google Scholar]
- 7.Mäki M, Holm K, Collin P.et al Increase in gamma/delta T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut 1991321412–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collin P, Helin H, Mäki M.et al Follow‐up of patients positive in reticulin and gliadin antibody tests with normal small bowel biopsy findings. Scand J Gastroenterol 199328595–598. [DOI] [PubMed] [Google Scholar]
- 9.Troncone R, the SIGEP working group on latent coeliac disease Latent coeliac disease in Italy. Acta Paediatr 1995841252–1257. [DOI] [PubMed] [Google Scholar]
- 10.Salmi T T, Collin P, Järvinen O.et al Immunoglobin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment Pharmacol Ther 200624541–552. [DOI] [PubMed] [Google Scholar]
- 11.Salazar de Sousa J, Ramos de Almeida J M, Monteiro M V.et al Late onset coeliac disease in the monozygotic twin of a coeliac child. Acta Paediatr Scand 198776172–174. [DOI] [PubMed] [Google Scholar]
- 12.Hervonen K, Karell K, Holopainen P.et al Concordance of dermatitis herpetiformis in monozygous twins. J Invest Dermatol 2000115990–993. [DOI] [PubMed] [Google Scholar]
- 13.Paparo F, Petrone E, Tosco A.et al Clinical, HLA, and small bowel immunohistochemical features of children with positive serum antiendomysium antibodies and architecturally normal small intestinal mucosa. Am J Gastroenterol 20051002294–2298. [DOI] [PubMed] [Google Scholar]
- 14.Kakar S, Nehra V, Murray J A.et al Significance of intraepithelial lymphocytosis in small bowel biopsy samples with normal mucosal architecture. Am J Gastroenterol 2003982027–2033. [DOI] [PubMed] [Google Scholar]
- 15.Iltanen S, Holm K, Partanen J.et al Increased density of jejunal gamma/delta + T cells in patients having normal mucosa — marker of operative autoimmune mechanisms. Autoimmunity 199929179–187. [DOI] [PubMed] [Google Scholar]
- 16.Marzari R, Sblattero D, Florian F.et al Molecular dissection of tissue transglutaminase autoantibody response in celiac disease. J Immunol 20011664170–4176. [DOI] [PubMed] [Google Scholar]
- 17.Korponay‐Szabo I R, Halttunen T, Szalai Z.et al In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 200453641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaukinen K, Peräaho M, Collin P.et al Small bowel mucosal transglutaminase 2‐specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized study. Scand J Gastroenterol 200540564–572. [DOI] [PubMed] [Google Scholar]
- 19.Salmi T T, Collin P, Korponay‐Szabo I.et al Endomysial antibody‐negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 2006551746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry L, Seah P P, McMinn R M H.et al Lymphocytic infiltration of epithelium in diagnosis of gluten‐sensitive enteropathy. BMJ 19723371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savilahti E, Reunala T, Mäki M. Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut 199233206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aine L, Mäki M, Reunala T. Coeliac‐type dental enamel defects in patients with dermatitis herpetiformis. Acta Derm Venereol 19927225–27. [PubMed] [Google Scholar]
- 23.Kaukinen K, Mäki M, Partanen J.et al Celiac disease without villous atrophy. Revision of criteria called for. Dig Dis Sci 200146879–887. [DOI] [PubMed] [Google Scholar]
- 24.Esteve M, Rosinach M, Fernandez‐Banares F.et al Spectrum of gluten‐sensitive enteropathy in first‐degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut 2006551739–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman H J, Chiu B K. Multifocal small bowel lymphoma and latent celiac sprue. Gastroenterology 1986901992–1997. [DOI] [PubMed] [Google Scholar]
- 26.Hadjivassiliou M, Gibson A, Davies‐Jones G A B.et al Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996347369–371. [DOI] [PubMed] [Google Scholar]
- 27.Hadjivassiliou M, Mäki M, Sanders D.et al Autoantibody targetting of brain and intestinal transglutaminase in gluten ataxia. Neurology 200666373–377. [DOI] [PubMed] [Google Scholar]
- 28.Hadjivassiliou M, Davies‐Jones G A B, Sanders D.et al Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry 2003741221–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karell K, Louka A S, Moodie S J.et al HLA types in celiac disease patients not carrying the DQA1*05‐DQB1*02 (DQ2) heterodimer: results from the European genetics cluster on celiac disease. Hum Immunol 200364469–477. [DOI] [PubMed] [Google Scholar]