We read the recent article by Houghton et al (Gut 2007, Apr 19 [Epub ahead of print]), reporting that pregabalin, a new generation of α2δ ligand, increased sensory thresholds to normal levels in 26 patients with irritable bowel syndrome (IBS) and baseline rectal hypersensitivity, in a randomised double blind, placebo controlled, parallel group study. The authors concluded that α2δ ligands are worthy of further physiological and clinical investigations for diseases affecting gut sensory function. Experimental studies to date indicate that pregabalin prevents colorectal allodynia and hyperalgesia in rats exposed to intracolonic trinitrobenzene‐sulphonic acid1 or septic shock.2 Visceral hyperalgesia and symptoms in IBS are, however, characterised by the absence of overt colonic damage or mucosal abnormality. In the study we describe here, pregabalin given orally in a rat non‐inflammatory model of repeated tonic colorectal‐distension‐induced hypersensitivity3 prevented visceral hyperalgesia and blunted lumbosacral spinal neurone activation.
Adult male Sprague–Dawley rats with or without electrodes implanted in the abdominal muscles were given orally (po) either vehicle (water, 1 ml/rat) or pregabalin (10 or 30 mg/kg; Parke Davis, Fresnes, France) and placed individually in Bollman cages. After a 60 minute stabilisation period, basal abdominal contractions were monitored for 10 minutes, then isobaric colorectal distension (60 mm Hg for 10 minutes twice, with a 10 minute rest interval) was carried out as before.3 The visceromotor response was quantified either visually (contractions/10 min) or as the area under the curve of contraction (AUC) of the abdominal muscle electromyogram, as previously described.3 For the spinal neuronal activation study, naive rats received water (1 ml/rat, po) or pregabalin (30 mg/kg, po), or no treatment/no distension (sham control), and were placed in a Bollman cage. Sixty minutes later, colorectal distension was applied as above. At 60 minutes after the end of the second colorectal distension, or at the corresponding time for the sham control, the rats were killed and the lumbosacral spinal cord processed for Fos immunohistochemistry, as previously reported.4
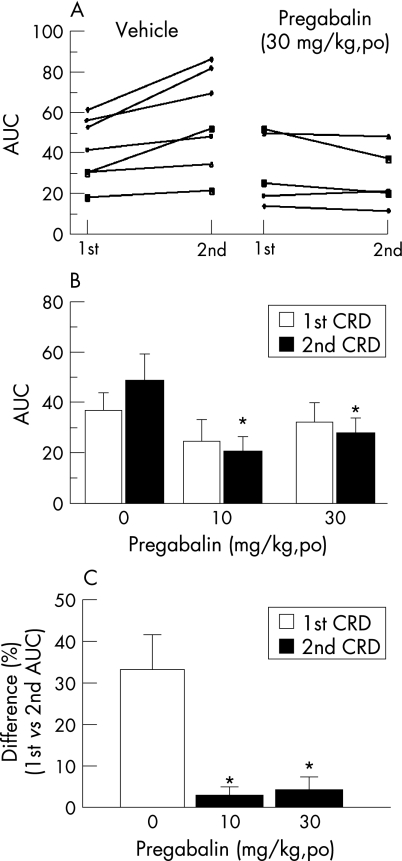
The data show that the first colorectal distension caused 23.6 (2.5) (mean (SEM)) abdominal contractions/10 min in the vehicle group (n = 17), while none or few were observed under basal conditions. Pregabalin (10 and 30 mg/kg) lowered colorectal‐distension‐induced contractions/10 min to 18.3 (1.3) (p>0.05, n = 7) and 12.4 (2.6) (p<0.05, n = 17), respectively. During the second distension, the abdominal contractions/10 min increased to 29.5 (2.4) in the vehicle group but not in the pregabalin group at both 10 mg/kg (19.1 (1.5)) and 30 mg/kg (11.3 (2.2)). The response to the second distension in the vehicle group was 25.1 (2.3)% greater than the first, suggesting occurrence of sensitisation. Pregabalin at both doses abolished the second distension induced sensitisation. Likewise, in rats fitted with abdominal electrodes and treated with vehicle, the AUC response to the second colorectal distension was increased by 33.2 (8.3)% compared with the first, while in rats treated with pregabalin (10 and 30 mg/kg), this hypersensitivity was blocked (29.5 (3.8) and 30.6 (6.5), respectively, vs vehicle 48.6 (10.8), p<0.05) (fig 1, panels A and D).
Figure 1 Oral pregabalin decreased the area under the curve of contraction (AUC) of the abdominal electromyogram to two successive tonic colorectal distensions in rats. (A) Individual rat's response to the first and second colorectal distension (60 mm Hg, 10 minutes each and a 10 minute interval) one hour after administration of vehicle (water) or pregabalin (30 mg/kg orally). (B) Group mean of the AUC in rats given vehicle or pregabalin 10 or 30 mg/kg. (C) Per cent difference in AUC between the second and first colorectal distensions in vehicle and pregabalin (10 and 30 mg/kg) treated rats. Values are mean, error bars = SEM. Differences within and between groups were analysed using one way analysis of variance (ANOVA) or a two way repeated measures ANOVA (one factor repetition). *p<0.05 vs the corresponding vehicle treated rats. CRD, colorectal distension; po, oral administration.
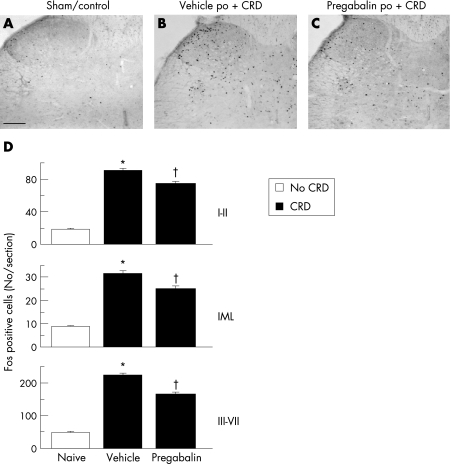
Sham control rats had no or little spinal neuronal activation (fig 2, panels A and C). Colorectal distension induced Fos activation in laminae I–II and the intermediolateral column (IML) (90.4 (2.8) and 31.6 (1.2) cells/section, respectively) in the vehicle group (fig 2, panels B and D). Pregabalin (30 mg/kg, po) significantly reduced the number of Fos positive cells in laminae I, II, and IML to 74.6 (2.6) and 25.1 (1.1), respectively (fig 2, panels C and D). Similarly, in laminae III–VII, the number of Fos positive cells/section induced by colorectal distension was reduced from 223.5 (5.6) in the vehicle group to 164.9 (7.7) with pregabalin (p<0.05) (fig 2, panels B to D).
Figure 2 Oral pregabalin blunted spinal activation of dorsal horn neurones induced by two colorectal distensions (60 mm Hg, 10 minutes each with a 10 minute interval) in rats. Photomicrographs of rat L6–S1 spinal cord section showing Fos expression 60 minutes after the last colorectal distension. Scale bar = 100 μm. (A) Sham/control rats. (B) Vehicle + colorectal distension. (C) Pregabalin (30 mg/kg, orally) + colorectal distension. (D) Number of Fos expressing cells in laminae I–II, the intermediolateral column (IML), and laminae III–VII. Fos positive cells were counted bilaterally in the superficial laminae I and II, in the deep layers III–VII, and in the IML in 50 sections of the L6–S1 spinal cord. Values are mean, error bars = SEM. *p<0.05 vs sham and pregabalin; †p<0.05 vs sham and vehicle groups; one way analysis of variance followed by Dunn's multiple comparison test.
These data show that a single oral dose of pregabalin prevents visceral sensitisation and dampens spinal neuronal activation induced by repeated episodes of colorectal distension in a conscious rat model of visceral hyperalgesia with no underlying colonic pathology.3 Colorectal distension in non‐inflamed rat colon activates neurones in the lumbosacral spinal cord where the nociceptive visceral inputs mainly reach the superficial lamina I, outer II, and deeper V and X, as shown by Fos expression and ERK 1/2 phosphorylation.3 The inhibitory effect of pregabalin on neurotransmitter release5 may contribute to the decreased spinal neuronal activation observed in the present study.
Acknowledgements
This work was supported by the NIH grants P50 AR 049550 and R01 DK 33061, CURE Center grant DK 41301 (Animal Model Core), VA Career Scientist Award, R21 DK 068155, and Parke Davis (Fresnes, France).
Footnotes
Conflict of interest: None declared.
References
- 1.Diop L, Raymond F, Fargeau H.et al Pregabalin (CI‐1008) inhibits the trinitrobenzene sulfonic acid‐induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther 20023021013–1022. [DOI] [PubMed] [Google Scholar]
- 2.Eutamene H, Coelho A M, Theodorou V.et al Antinociceptive effect of pregabalin in septic shock‐induced rectal hypersensitivity in rats. J Pharmacol Exp Ther 2000295162–167. [PubMed] [Google Scholar]
- 3.Million M, Wang L, Wang Y.et al CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 200655172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez V, Wang L, Mayer E.et al Proximal colon distention increases Fos expression in the lumbosacral spinal cord and activates sacral parasympathetic NADPHd‐positive neurons in rats. J Comp Neurol 1998390311–321. [PubMed] [Google Scholar]
- 5.Field M J, Cox P J, Stott E.et al Identification of the alpha2‐delta‐1 subunit of voltage‐dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci USA 200610317537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]