Necrotising enterocolitis (NEC) is a severe gastrointestinal disease with a mortality of 20–40%, affecting predominantly premature neonates.1 In the early phase of the disease NEC continues to present a diagnostic challenge for the attending clinician. The signs and symptoms are often non‐specific, including gastrointestinal problems such as abdominal distension and feeding intolerance, which are also among the most prevalent presenting features of neonatal sepsis.2 The diagnosis is further hampered by the limited diagnostic accuracy of the laboratory and radiological tests currently in use.3,4
Histopathologically, NEC is characterised by intestinal coagulative or ischaemic necrosis, starting at the mucosa and extending into the submucosa and muscularis externa.1 We sought a non‐invasive test to find evidence of enterocyte cell death in infants with gastrointestinal symptoms suspicious of NEC, in order to differentiate NEC from other neonatal diseases that present with abdominal signs.
Intestinal fatty acid binding protein (I‐FABP) has been reported to be a useful plasma marker for early enterocyte cell death.5,6 The small (14–15 kDa) cytosolic I‐FABP is specifically present in mature enterocytes of small and large intestine and is released as soon as cell membrane integrity is compromised. I‐FABP is present in very small amounts in the plasma of healthy individuals, probably representing the normal turnover of enterocytes, but levels rise rapidly after episodes of acute intestinal ischaemia and inflammation, including NEC.5,6,7,8
Because of its low molecular weight, I‐FABP present in the systemic circulation passes through the glomerular filter (fractional renal excretion 28%; half life time 11 minutes) and can readily be detected in the urine.9 I‐FABP is not expressed in the urinary tract mucosa. Thus urinary values of I‐FABP provide specific information about the number of dying intestinal epithelial cells. Given the age and birth weight of our population of interest, there are important advantages to sampling urine instead of plasma.10,11
Over an 18 month period, 17 consecutive neonates were identified in the neonatal intensive care unit at the University Hospital Maastricht in whom NEC was suspected. Inclusion criteria were the presence of at least one clinical gastrointestinal sign (abdominal distension or discolouration, increased gastric residues, bloody stools), causing sufficient clinical concern to require an abdominal x ray or to stop enteral feeding or both.
Written consent was obtained from both parents before inclusion, and the study was conducted with approval from the local medical ethics committee.
Urine was collected daily using a bag catch method for at least one week once appropriate consent was obtained. All neonates produced urine at a minimal rate of 1.9 ml/kg/h. Samples were centrifuged at 4000×g for 15 minutes, aliquoted, and stored at −20°C until analysis. Urinary I‐FABP was measured using a commercially available I‐FABP enzyme linked immunosorbent assay (ELISA) that selectively detects human I‐FABP (standard, 20–5000 pg/ml), kindly provided by HBT (Uden, Netherlands). Values were expressed as a ratio (units pg/nmol) of I‐FABP (pg/ml) to creatinine (Cr, μmol/l), in order to compensate for variations in the concentration of the urine. Urinary I‐FABP:Cr levels were compared between neonates with NEC and those with other final diagnoses using the Mann–Whitney test.
Five of the 17 infants with suspected NEC subsequently developed NEC stage IIa (pneumatosis intestinalis on abdominal x ray, confirmed by a paediatric radiologist) or higher. Of the remaining 12 patients suspected of NEC, there were seven cases of sepsis, one of ileal atresia with intestinal necrosis, two patients for whom a diagnosis of constipation was ultimately made, one patient with Hirschsprung's disease, and finally one patient born with gastroschisis who later developed signs and symptoms of NEC after gastroschisis repair. In the neonates who developed NEC, the average gestational age was 32 weeks and 3 days, the average age at diagnosis was 14 days, and the average birth weight was 1500 g. For the neonates who subsequently received different diagnoses, the average gestational age was 30 weeks and 4 days and the average birth weight was 1487 g.
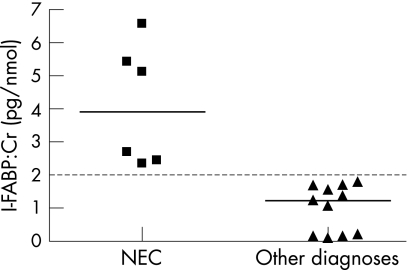
In the first urine sample, the median urinary I‐FABP:Cr ratio was significantly higher in neonates who ultimately developed NEC or intestinal necrosis (3.9 pg/nmol, range 2.3 to 6.6) than in those without NEC (1.2 pg/nmol, range 0.1 to 1.8) (p = 0.001) (fig 1). A value of 2 pg/nmol discriminated between neonates with NEC or intestinal necrosis and the remainder. None of the patients without either NEC or intestinal necrosis was found to have an I‐FABP:Cr ratio in excess of 2 pg/nmol over the first week.
Figure 1 In the first urine sample of neonates with suspected necrotising enterocolitis (NEC), the mean urinary I‐FABP:Cr ratio was significantly higher in neonates who ultimately developed NEC or intestinal necrosis (3.9 pg/nmol, range 2.3 to 6.6) than in those without NEC (1.2 pg/nmol, range 0.1 to 1.8) (p = 0.001). Cr, creatine; I‐FABP, intestinal fatty acid binding protein.
In this study urinary I‐FABP, a specific marker for intestinal mucosal damage, was measured using ELISA to identify patients with NEC. This study is the first to show that measurement of urinary I‐FABP offers valuable additional information in the early diagnosis of NEC. Furthermore, while previous studies have assessed the feasibility of using plasma I‐FABP or L‐FABP to identify patients with NEC compared with healthy controls,7,8 this is the first study which prospectively identified neonates with NEC among a population of preterm infants who presented with gastrointestinal symptoms.
In our small series, a value of 2 pg/nmol for the urinary I‐FABP:Cr ratio was capable of distinguishing the group with NEC or intestinal necrosis from the other diagnoses.
NEC is a complex and probably multifactorial disease for which the aetiology remains incompletely understood. However, intestinal epithelial cell death is an important early hallmark1 which is in line with the observed increase in urinary I‐FABP. Moreover, normalisation of the urinary I‐FABP:Cr ratio correlated well with clinical improvement, at least in the short term.
Given the ease and frequency with which urine can be collected without adverse consequences for the baby, the feasibility of urinary I‐FABPs as a screening tool for NEC needs to be further evaluated in a prospective trial. Finally, as the cumulative release of I‐FABP can be used to estimate the extent of intestinal damage, it may also help to guide treatment strategies, such as the timing of surgery (if required), the duration of antibiotic treatment, and selection of the ideal time for reintroducing enteral feeding.
Acknowledgements
This research was supported by AGIKO‐stipendium 920‐03‐438 (to JPMD) from the Netherlands Organisation for Health Research and Development.
Footnotes
Conflict of interest: None declared.
References
- 1.Lin P W, Stoll B J. Necrotising enterocolitis. Lancet 20063681271–1283. [DOI] [PubMed] [Google Scholar]
- 2.Fanaroff A A, Korones S B, Wright L L.et al Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. Paediatr Infect Dis J 199817593–598. [DOI] [PubMed] [Google Scholar]
- 3.Hällströma M, Koivistob A, Janasa M.et al Laboratory parameters predictive of developing necrotizing enterocolitis in infants born before 33 weeks of gestation. J Pediatr Surg. 2006;41: 792– 8, [DOI] [PubMed]
- 4.Tam Alda L, Camberos A, Applebaum H. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: predictive value of radiologic findings. J Pediatr Surg 2002371688–1691. [DOI] [PubMed] [Google Scholar]
- 5.Kanda T, Fujii H, Tani T.et al Intestinal fatty acid‐binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996110339–343. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman J M, Sacchettini J, Marks C.et al Human intestinal fatty acid binding protein: report of an assay with studies in normal volunteers and intestinal ischemia. Surgery 1997121335–342. [DOI] [PubMed] [Google Scholar]
- 7.Edelson M B, Sonnino R E, Bagwell C E.et al Plasma intestinal fatty acid binding protein in neonates with necrotizing entercolitis: a pilot study. J Pediatr Surg 1999341452–1457. [DOI] [PubMed] [Google Scholar]
- 8.Guthmann F, Börchers T, Wolfrum C.et al Plasma concentration of intestinal‐ and liver‐FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol Cell Biochem 2002239227–234. [PubMed] [Google Scholar]
- 9.van de Poll MCG, Derikx J PM, Buurman W A.et al Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. (in press). [DOI] [PMC free article] [PubMed]
- 10.Smoller B R, Kruskall M S. Phlebotomy for diagnostic laboratory tests in adults. Pattern of use and effect on transfusion requirements. N Engl J Med 19863141233–1235. [DOI] [PubMed] [Google Scholar]
- 11.Lin J C, Strauss R G, Kulhavy J C.et al Phlebotomy overdraw in the neonatal intensive care nursery. Pediatrics 2000106e19–e25. [DOI] [PubMed] [Google Scholar]