Abstract
The unfolded protein response of Escherichia coli is triggered by the accumulation of unassembled outer membrane proteins (OMPs) in the cellular envelope. The PDZ-protease DegS recognizes these mislocalized OMPs and initiates a proteolytic cascade that ultimately leads to the σE-driven expression of a variety of factors dealing with folding stress in the periplasm and OMP assembly. The general features of how OMPs activate the protease function of DegS have not yet been systematically addressed. Furthermore, it is unknown how the PDZ domain keeps the protease inactive in the resting state, which is of crucial importance for the functioning of the entire σE stress response. Here we show in atomic detail how DegS is able to integrate the information of distinct stress signals that originate from different OMPs containing a ϕ-x-Phe C-terminal motif. A dedicated loop of the protease domain, loop L3, serves as a versatile sensor for allosteric ligands. L3 is capable of interacting differently with ligands but reorients in a conserved manner to activate DegS. Our data also indicate that the PDZ domain directly inhibits protease function in the absence of stress signals by wedging loop L3 in a conformation that ultimately disrupts the proteolytic site. Thus, the PDZ domain and loop L3 of DegS define a novel molecular switch allowing strict regulation of the σE stress response system.
Keywords: Unfolded protein response, regulatory proteolysis, protein quality control, HtrA
All living organisms face a wide variety of environmental stresses, which severely affect the stability of protein molecules and promote their unfolding. Damaged proteins might accumulate as large aggregates that often interfere with cellular function as is evident in several neurodegenerative diseases (for review, see Macario and Conway de Macario 2005). To efficiently cope with misfolded proteins, cells evolved a sophisticated protein quality-control system that precisely monitors the correct folding of individual proteins and coordinates repair and degradative functions (Gottesman et al. 1997; Wickner et al. 1999; Duguay and Silhavy 2004). Due to the vital importance of this process, all cells have multiple stress response pathways that adjust the levels of molecular chaperones and proteases (Raivio 2005). Within these pathways, compartment-specific signaling cascades sense the presence of misfolded protein and transmit this information to dedicated transcriptional regulators.
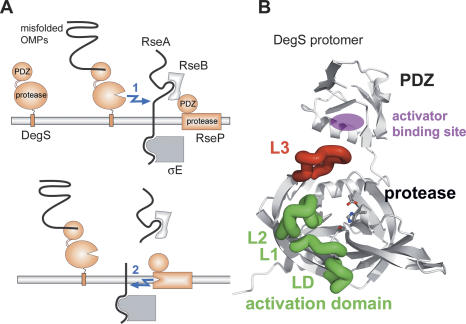
The envelope stress response of Gram-negative bacteria uses the mechanism of “regulated intramembrane proteolysis” (Brown et al. 2000) to transmit the stress signal across the inner membrane to the cytoplasmic transcriptional system (Fig. 1A; Alba et al. 2002). Ultimately, the response is carried out by the alternative σ factor E (σE), which orchestrates the expression of genes encoding periplasmic chaperones, folding catalysts, proteases, and enzymes involved in cell-wall homeostasis (Rouviere et al. 1995; Rhodius et al. 2006). Under nonstress conditions, σE is captured in an inactive state by the cytoplasmic domain of the transmembrane anti-σ factor RseA. Folding stress causes mislocalization of outer membrane proteins (OMPs) to the periplasm, thereby triggering a proteolytic cascade that leads to the complete digestion of RseA. Initially, the site-1 protease DegS, a membrane-tethered protease facing the periplasmic space, becomes activated by the C termini of unassembled OMPs and introduces the first cut in RseA (Walsh et al. 2003). This cut removes the bulk of the periplasmic domain of RseA, thereby abolishing inhibition of the site-2 protease RseP by the RseB regulator protein (De Las Penas et al. 1997; Cezairliyan and Sauer 2007; Kim et al. 2007). Subsequently, RseP cleaves RseA within its transmembrane segment, thereby releasing the N-terminal domain of RseA with the tightly bound σE into the cytoplasm (Ades et al. 1999; Kanehara et al. 2002; Akiyama et al. 2004). Further degradation events of RseA catalyzed by Clp proteolytic complexes liberate σE to interact with RNA polymerase and to up-regulate stress response promoters (Flynn et al. 2004; Levchenko et al. 2005; Chaba et al. 2007).
Figure 1.
Function of DegS in the σE stress response. (A) Schematic presentation of the σE stress response. (B) Ribbon presentation of one subunit of the DegS trimer highlighting loop L3 (red) that mediates communication between the PDZ and protease domains, and the activation domain (loops L1, L2, and LD, green) that allows formation of a functional catalytic triad comprising residues His96, Asp126, and Ser201, which are shown in ball-and-stick mode.
DegS plays a key role within the σE pathway as it directly couples the sensing of the stress signal with the triggering of the response pathway (Walsh et al. 2003; Young and Hartl 2003) and furthermore catalyzes the rate-limiting step in the proteolytic cascade (Chaba et al. 2007). DegS belongs to the widely conserved HtrA family of serine proteases involved in various aspects of protein quality control and cellular signaling (Clausen et al. 2002). Like other family members, DegS is composed of a trypsin-like protease domain and a C-terminal PDZ domain. PDZ domains are well-characterized protein–protein interaction motifs that bind to the C terminus of target proteins (Fanning and Anderson 1996). They contain specificity pockets for accommodating the C-terminal residues at the 0, −2, and −3 positions, where position 0 refers to the C-terminal residue (Fig. 2A). A distinctive feature of HtrA proteins is that their proteolytic activity can be reversibly switched on and off (Ehrmann and Clausen 2004; Hasenbein et al. 2007). For DegS, it was shown that the OmpC C terminus binds to the PDZ domain and turns on protease activity (Walsh et al. 2003). Structural elements involved in the activation process comprise several active site loops of the protease domain. Loop L3 (residues 177–189; for nomenclature, see Fig. 1B and Perona and Craik 1995) senses the presence of the PDZ-bound activator and transmits this information to the activation domain that is formed by loops L1 (residues 195–200), L2 (residues 218–230), and LD (residues 161–166). This domain is homologous to the classical activation domain of serine proteases, which generates the functional proteolytic site after propeptide cleavage (Huber and Bode 1978). The crystal structure of DegS complexed with an activating peptide mimicking the C terminus of OmpC (Wilken et al. 2004) showed that the PDZ-bound activator interacts specifically with the protease domain via the side chain of the glutamine in the −1 position, which forms a hydrogen bond with a main-chain oxygen of loop L3. This polar interaction triggers reorientation of loop L3, thereby inducing remodeling of the activation domain into its active conformation. However, both polar and hydrophobic residues are found in the −1 position of various OMPs (Struyve et al. 1991). Since several of the corresponding C termini are capable of activating DegS in vitro (Wilken et al. 2004), it remained puzzling how DegS interacts with these OMPs and integrates the information from different peptides serving as stress signals. Furthermore, it has been shown that a PDZ deletion mutant of DegS exhibits protease activity in vivo and in vitro, pointing to an inhibitory function of the PDZ domain (Walsh et al. 2003; Cezairliyan and Sauer 2007). The mechanism of how the PDZ domain keeps the protease inactive in the latent state and thus links DegS activity directly to the presence of the stress signal has not yet been determined. To study the molecular details of DegS regulation, which are of fundamental importance for the entire σE stress response, we performed a structure–function analysis of a PDZ-deletion mutant and of DegS complexes with activating peptides derived from various OMPs.
Figure 2.
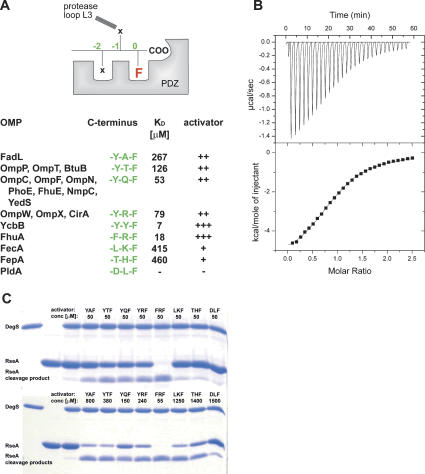
Binding and activation potential of different OMP-derived C termini. (A) Activating peptides used in this study. In the top panel, the general binding mode to the PDZ domain is indicated together with the nomenclature used here. For the ligand, the C-terminal phenylalanine present in all OMPs is highlighted. The table shown in the bottom panel summarizes the C termini of the analyzed peptides (green), the corresponding OMPs in E. coli, and the individual dissociation constants and activation capabilities. (B) ITC measurement of the binding of the FRF-peptide to the DegS wild-type protein. (Top panel) Ten-microliter aliquots of the FRF peptide (1 mM) were injected into the sample cell containing 85 μM DegS. (Bottom panel) The area under each peak was integrated and plotted against the molar ratio peptide/DegS inside the sample cell. The black line represents the fit to a binding isotherm, assuming one binding site per protomer. (C) SDS-PAGE of RseA cleavage by DegS. The periplasmic domain of RseA was present in 30 μM, DegS was present in 10 μM, and the activating peptides were present in the indicated concentrations. The reactions were stopped after 18 h. In the control reactions (first three lanes), the assay was conducted without activating peptide.
Results
OMP-derived peptides with a ϕ-x-Phe C-terminal motif function as stress stimuli activating DegS
It has been proposed that proteins with a Tyr-x-Phe C-terminal sequence should be capable of interacting with DegS (Walsh et al. 2003). Furthermore, it has been shown that most of the peptides carrying a Tyr-x-Phe motif are also able to activate protease function (Wilken et al. 2004). However, several OMPs of Escherichia coli do not contain a corresponding consensus motif and exhibit rather diverse C-terminal sequences. Therefore, we wished to explore the binding and activation potential of various E. coli OMPs. We performed isothermal titration calorimetry (ITC) binding studies and RseA cleavage assays using derivatives of the NH2-DNRLGLVXXX-COOH peptide, where the ultimate three residues matched the sequences of the individual OMPs indicated in Figure 2A. In the following, we refer to the OMP-derived peptides on the basis of their C-terminal residues; e.g., the “YQF peptide” refers to the activating peptide with a Tyr–Gln–Phe C terminus.
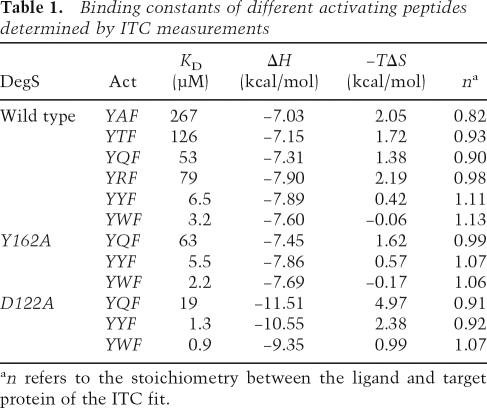
A typical ITC profile illustrating the binding of the FRF peptide to DegS is shown in Figure 2B. The ITC results demonstrated that all activating peptides, with the exception of the DLF peptide, can bind to DegS. Among the analyzed YxF peptides, DegS showed the highest affinity for YYF and YWF (KD of 3–6 μM) (Table 1); moderate affinity for YQF, YRF, and YTF (KD of 55–80 μM); and the weakest affinity for YTF and YAF (KD of 126 μM and 267 μM, respectively). These data suggest that YxF peptides are mainly bound to the PDZ domain by the phenylalanine and tyrosine in the 0 and −2 positions, whereas the residue in the −1 position appears to interact differently with the protease domain, causing the observed differences in affinity. Interestingly, peptides that deviate from the YxF consensus also interacted with DegS. While the LKF and THF peptides were bound weakly to DegS (KD of 300 and 450 μM, respectively), the FRF peptide (KD of 18 μM) was bound 4.5 times more strongly than the related YRF peptide. These data imply that the preferred residue in the −2 position might be a phenylalanine rather than a tyrosine and that small hydrophobic residues are also tolerated in the −2 position.
Table 1.
Binding constants of different activating peptides determined by ITC measurements
an refers to the stoichiometry between the ligand and target protein of the ITC fit.
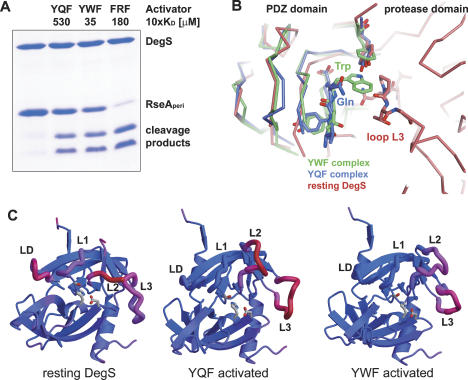
To explore the relevance of the ITC data for DegS activation, we studied the capacity of the OMP-derived peptides to induce RseA cleavage (Fig. 2C). First, we incubated DegS with distinct activating peptides present in 50 μM concentration. The cleavage assays clearly demonstrated that the FRF peptide most efficiently activates protease function, whereas YAF, YTF, YQF, and YRF activate DegS to a significantly lesser extent. For peptides LKF, THF, and DLF, no activation could be detected under these conditions. In order to account for the different KD values and to directly monitor the activation potential of the individual peptides, we repeated the RseA degradation assays with activating peptides present in concentrations corresponding to their threefold KD values. As shown in Figure 2C, all OMP-derived peptides, with the exception of the DLF peptide, were able to activate the DegS protease. Taken together, all OMPs with a ϕ-x-Phe C-terminal motif seem to be capable of activating DegS in vitro and thus could act as potential stress stimuli in vivo, however to various degrees.
Detachment of the PDZ domain from the protease domain allows activator binding but impairs protease activation
To analyze the requirement of a proper positioning of the protease and PDZ domain for peptide binding, we studied the binding characteristics of the D122A mutant. In the wild-type protein, Asp122 forms a short-distanced salt bridge with Arg256 that is part of the segment linking the protease and PDZ domain. Together with a few other polar interactions, it is this salt bridge that mainly determines the relative orientation of the two domains enabling their communication. Notably, the D122A mutant showed the highest affinity for all analyzed peptides as was evident from the KD values that are three- to fivefold lower than wild type. Interestingly, the binding constants determined for the D122A mutant resemble results of a previous ITC analysis, where the interaction of the YQF and YYF peptides to the isolated PDZ domain of DegS was studied (Walsh et al. 2003). The reported KD values of 15 and 0.63 μM, respectively, fit nicely to the corresponding KD values of 19 and 1.3 μM of the D122A mutant determined in this study. This analogy implies that the PDZ domain of the D122A mutant behaves like an “isolated” PDZ domain that is not fixed to the protein body, whereas in wild-type DegS, specific interactions between the PDZ and protease domain seem to impose constraints on ligand binding. Further lines of evidence support this hypothesis. First, the D122A mutant exhibited an entirely different thermodynamic signature (ΔH and −TΔS values) in the ITC experiments from the other DegS variants. Particularly the ΔH values were reduced by ∼2–4 kcal/mol. Although it is difficult to pinpoint the molecular basis for the different thermodynamic parameters, the ITC experiments indicated that the D122A mutation strongly affects the binding of activating peptides. Second, it should be noted that the D122A mutant resisted, in contrast to other DegS mutants, any attempt to crystallize and also showed a strikingly different behavior during NiNTA purification, most likely because the affinity tag was directly connected to the C terminus of the PDZ domain. Thus, our data suggest that the improved binding characteristics of the D122A mutant might be due to a differently oriented or more flexible PDZ domain. Since this mutant is able to bind the activator but does not cleave the RseA substrate, we conclude that a proper arrangement of protease and PDZ domain is essential to transfer the stress signal between these domains.
Destabilization of the activation domain does not influence activator binding
The ITC studies also showed that wild-type and Y162A DegS undergo similar contacts with OMP-derived peptides as the values for KD, ΔH, and −TΔS were almost identical (Table 1). Tyr162 is a central component of the activation domain of DegS and plays a key role in stabilizing its proteolytically active form upon binding the allosteric activator. Consistently, the Y162A mutant was inactive in the RseA cleavage assay (Wilken et al. 2004). The present results indicate that the conformation of the activation domain does not affect binding of the activator to the PDZ domain. However, the congruent KD values imply that loop L3 of the Y162A mutant retains its interactions with the activator and should thus be present in its “peptide-bound” conformation. Therefore, activator binding and protease activation appear to be two independent events coupled by the protease loop L3, which senses activating peptides bound to the PDZ domain and transmits this information to the protease domain.
Crystal structure of the complex between the YWF activator and DegS
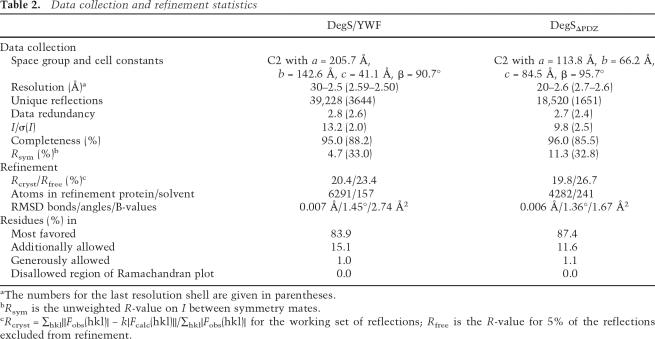
To explore the molecular mechanism of how DegS recognizes different stress signaling peptides, we set out to determine the crystal structures of complexes with the YWF, YYF, and FRF peptides, which represent the most potent DegS activators (Fig. 2C; Wilken et al. 2004). While crystallization trials with YYF and FRF were not successful, we managed to cocrystallize DegS with the YWF peptide. The structure of the complex was determined at 2.5 Å resolution (R-factor 20.4%, Rfree 23.4%) (Table 2) and revealed the binding mode of the activator (Fig. 3A).
Table 2.
Data collection and refinement statistics
aThe numbers for the last resolution shell are given in parentheses.
bRsym is the unweighted R-value on I between symmetry mates.
cRcryst = ∑hkl||Fobs(hkl)| − k|Fcalc(hkl)||/∑hkl|Fobs(hkl)| for the working set of reflections; Rfree is the R-value for 5% of the reflections excluded from refinement.
Figure 3.
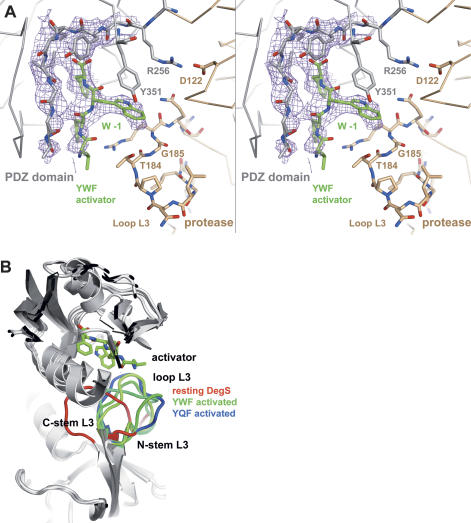
Structure of the complex between DegS and YWF. (A) Stereo presentation of the omit density of the bound YWF activator and of the main-chain-binding segment of the PDZ domain. The 3Fo − 2Fc electron-density map was calculated at 2.5 Å resolution (contoured at 1.0 σ.) after omitting the PDZ domain and the YWF activator from the refined model. Only the four C-terminal residues of the activator were defined by electron density. The YWF peptide (green)and the DegS residues that interact with the tryptophan in the −1 position are shown as a stick model. Furthermore, the Arg256:Asp122 salt bridge, which mainly defines the orientation of protease (brown) and the PDZ domain (gray), is indicated. (B) Ribbon plot of the superimposed structures of resting and activated DegS. The bound YWF peptide is shown as a stick model to indicate the position of the activator-binding site. Loop L3 is highlighted by color (resting DegS in red, YQF-activated in blue, and YWF-activated in green). Although the central segment of loop L3 differs in all activated forms, the stem regions show a mostly conserved conformation.
The stress peptide was bound as an additional β-strand to the small β-sheet of the PDZ domain with its carboxylate group being anchored by the carboxylate-binding loop Tyr258–Ile259–Gly260–Ile261 of DegS. The structure indicated that the 0 pocket, which is formed by residues Ile259, Thr318, Met319, and Val322, is the main anchoring site for the activator, since this pocket is clearly defined by electron density as is the bound phenylalanine side chain. In contrast, the −2 and −3 pockets and their bound residues are present in a rather flexible state. In analogy to the previously solved structure of the DegS/YQF complex, the residue in the −1 position, the tryptophan, interacts with the protease domain (Fig. 3A). Its indol side chain is wedged between the terminal β-strand of the PDZ domain and loop L3 of the protease domain. Particularly, residues Tyr351 and Gly185 are located in close proximity to the indol group (distances between 3.0 and 4.5 Å) and should support ligand binding by van der Waals interactions. Furthermore, the phenolic group of Tyr351 is oriented nearly parallel to the indol ring system of the −1 tryptophan of the activator and is thus in a good position for ring-stacking interactions.
Structural comparison with the DegS/YQF complex showed that binding of both activators triggers similar conformational changes in the protease domain leading ultimately to the formation of a functional proteolytic site. Consistently, the protease domains of both activated forms fit nicely to each other as indicated by the root-mean-square deviation (RMSD) of 0.61 Å for 208 Cα atoms, whereas superposition with the resting DegS yielded a RMSD of 0.90 Å for 185 Cα atoms. Structural differences between the two activated forms are mainly restricted to loop L3, which interacts differently with the −1 residue of the activator. While the glutamine of the YQF peptide forms a hydrogen bond to the main-chain carbonyl of Thr184, the tryptophan of YWF was bound by nondirected van der Waals interactions.
Protease loop L3 is able to integrate the information of different stress signaling peptides
Binding of the YWF peptide to DegS generated an asymmetric particle, in which the activator was bound differently in the three subunits (Fig. 3B). Although the activator generally underwent van der Waals interactions with Gly185, the backbone conformation of loop L3 differed significantly. In contrast, the active site loops L1, L2, and LD obtained almost identical conformations in the three protomers, yielding an entirely activated trimeric DegS particle. Superposition of the three YWF- and the YQF-complex structures highlights the structural flexibility of loop L3 in interacting with different activating peptides (Fig. 3B). Particularly, the central part of this loop comprising residues 180–186 was present in four different conformations. This inherent flexibility should form the basis to bind to different −1 residues of various activating peptides. Most interestingly, however, the stem regions of loop L3 comprising residues 177–179 and 187–189 adopt a similar fold in all activated DegS protomers. This conformation appears to be crucial to transmit the stress signal from the PDZ to the protease domain. In particular, the reoriented residues of the N-terminal stem segment are important for protease activation, as Ile179 induces formation of a hydrophobic cluster that forms the core of the functional activation domain, and Arg178 induces reorientation of the activation loop LD. Taken together, OMP-derived activating peptides can interact differently with DegS, but retain a common activation mechanism that depends on the repositioning of loop L3 in a conserved manner.
Activation potential of different activating peptides
It has been proposed that aromatic residues in the −1 position of allosteric ligands are most effective in activating the protease function of DegS (Wilken et al. 2004). Our ITC data indicate that the corresponding YYF and YWF activators have KD values that are one order of magnitude lower than other activators such as YQF and YRF. Thus the varying activation capability could simply reflect the affinity of peptide ligands to DegS. In order to test the activation potential of different ligands having a ϕ-x-Phe C terminus, we performed RseA degradation assays using peptide concentrations that were directly related to individual KD values. Surprisingly, the induced DegS activity varied gradually (Fig. 2C), suggesting that different allosteric ligands generate different allosterically activated complexes. To confirm these results, we repeated the degradation assays using activator peptides in saturating concentrations (i.e., 10-fold KD). As shown in Figure 4A, different activators, indeed, exhibited different capabilities to switch on DegS protease function. While the YWF activator was slightly more effective than the YQF activator, the FRF peptide clearly yielded the most active DegS protease. Unfortunately, it was not possible to obtain structural data of a DegS/FRF complex. However comparison of the YQF- and YWF-complex structures revealed structural features that might be mechanistically relevant and determine the activation potential of allosteric ligands. In contrast to the YQF peptide, binding of YWF to resting DegS would lead to a sterical clash between its tryptophan side chain and Pro183 of loop L3 (Fig. 4B). Hence the higher efficiency of YWF to stimulate DegS might be due to its ability to immediately displace loop L3 from its “inhibitory” position. Furthermore, the thermal motion factors of the activation domain of the YWF-activated DegS were considerably lower than of the YQF complex (Fig. 4C). Particularly, loop L2, which assembles the S1 specificity pocket and the main-chain-binding patch, was more flexible in the YQF complex. Thus it appears that binding of the YWF peptide generates a more rigid proteolytic site, which should have a direct impact on proteolytic activity. Similar to classical trypsin proteases, where the transition from zymogen to active protease is accompanied by a disorder–order transition (Huber and Bode 1978), such a well-defined proteolytic site should be crucial for cleaving the RseA substrate efficiently. In sum, these data indicate that additional factors other than the individual binding constants of stress peptides seem to affect the structural integrity of the activation domain and determine the degree of protease activation.
Figure 4.
Differences in the YQF- and YWF-activated DegS. (A) SDS-PAGE of the cleavage of the periplasmic domain of RseA (30 μM) by DegS (10 μM), applying saturating activator concentrations. For each activator, the 10-fold concentration of the individual KD value was applied. The reactions were carried out at 30°C and stopped after 3 h. The control reaction was carried out without activating peptide. (B) Comparison of YQF- and YWF-binding mode. Alignment of the PDZ domains with bound activator (YQF, blue; YWF, green) onto the resting DegS structure (red) illustrates the different binding positions of the −1 glutamine and tryptophan side chains, respectively. In contrast to the YQF peptide, binding of the indol ring of the YWF activator leads to a sterical clash with loop L3 of the resting protease. (C) Ribbon plot showing the protease domain of DegS with mapped thermal motion factors: (blue) rigid parts; (red) flexible parts. The relevant active site loops are labeled. For both activated complexes, the active site loops L1, L2, and LD are better defined as in resting DegS. However, loop L2 was more flexible in the DegS/YQF structure than in the DegS/YWF structure. The differences between the average thermal motion factors of loops LD/L1/L2 and the protease domain are as follows: 46.1, 29.7, and n.d. for resting DegS; −10.3, −9.9, and 49.7 for YQF-activated DegS; and −12.9, −9.7, and 36.3 Å2 for YWF-activated DegS.
Deletion of the PDZ domain yields a DegS protease with reduced activity that cannot be stimulated by activating peptides
Previous biochemical and structural data highlighted the regulatory function of the PDZ domain of DegS. The crystal structure of DegS in complex with the YQF peptide indicated that the PDZ domain offers a binding site for an allosteric activator that stimulates protease activity (Ehrmann and Clausen 2004; Wilken et al. 2004). In contrast to this “activation model,” the alternative “PDZ-inhibitory model” postulates that binding of OMP-like peptides to DegS relieves the inhibitory effect of the PDZ domain on protease function (Walsh et al. 2003). One important prediction of the latter model is that a PDZ deletion mutant should be fully active, whereas the activation model suggests that such a mutant should be proteolytically less active. Recent work could not clarify this point, as DegS PDZ deletion mutants showed different behaviors in vivo and in vitro (Walsh et al. 2003; Cezairliyan and Sauer 2007).
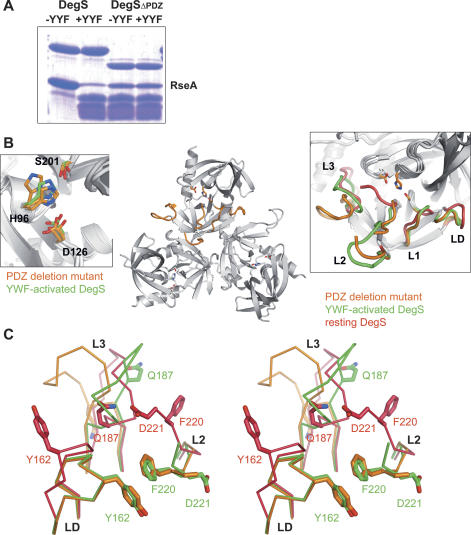
To distinguish between the two opposing models and to study the precise role of the PDZ domain in this process, we performed a structure–function analysis of a PDZ-deletion mutant DegSΔPDZ comprising residues 42–255. Like wild-type DegS, the purified DegSΔPDZ occurred as a trimer in solution but showed a lower solubility. It was, for example, not possible to concentrate the protein to >0.05 mM. However, the low solubility was not an indication of decreased stability, as the mutant did not aggregate and did not lose enzymatic activity over time. Protease assays revealed that DegSΔPDZ was able to cleave the RseA substrate at the same position as the parent construct. However, the proteolytic activity was significantly weaker and did not depend on the presence of an activating peptide (Fig. 5A). Next, we determined the crystal structure of DegSΔPDZ, which was refined at 2.6 Å resolution to an R-factor of 19.8% (Rfree = 26.7%) and exhibited good stereochemistry. The protease was present in a trimeric state, and each of the intersubunit interfaces was conserved with the wild-type protein. Alignment of DegSΔPDZ with the inactive and active forms of DegS yielded RMSD values of 0.99 Å (for 182 aligned Cαs) and 0.59 Å (for 191 aligned Cαs), respectively, indicating that the DegSΔPDZ structure was more similar to the activated form of DegS. Consistently, the activation domain of the deletion mutant was present in its functional state (Fig. 5B), promoting the formation of a properly formed oxyanion hole and S1-specifity pocket. However, the catalytic triad showed a higher conformational flexibility than activated DegS (Fig. 5B). Particularly the side chain of His96 was poorly defined by electron density and obtained different orientations in the distinct active sites of the DegS trimer. Furthermore Asp126 was located more distantly to His96 and thus should not be fully capable of activating the His96/Ser201 couple for nucleophilic attack on the RseA substrate, as was evident from RseA cleavage assays.
Figure 5.
Structure–function analysis of the PDZ deletion mutant DegSΔPDZ. (A) SDS-PAGE of RseA cleavage by DegS. The periplasmic domain of RseA was present in 30 μM, DegS and DegSΔPDZ were present in 10 μM, and the YYF peptide was present in 100 μM. The reaction was stopped after 5 h. (B) Overall fold of the DegSΔPDZ trimer. Mechanistic important loops L1, L2, L3, and LD of one monomer are colored orange. To indicate the position of the proteolytic site, the individual catalytic triads are shown as a stick model. (Left inset) Superposition of the catalytic triad of activated DegS (green) and DegSΔPDZ (orange). For the PDZ deletion mutant, the triad was observed in a different configuration in each subunit of the trimer, illustrating its flexibility. (Right inset) Superposition of active site loops of DegSΔPDZ (orange), resting DegS (red), and activated DegS (green). Notably, the loops of the activation domain (L1, L2, LD) of DegSΔPDZ and activated DegS fit nicely to each other. (C) Stereoview of the superposition of DegSΔPDZ (orange) with YWF-activated (green) and resting DegS (red), highlighting the different conformations of loop L3. The presence of the PDZ domain as well as the binding of activating peptides reorient loop L3 in a distinct but specific manner. Key residues for the interaction between loop L3 and loops L2 and LD of the activation domain are labeled and shown as a stick model. The superposition also illustrates the inherent flexibility of the central part of loop L3, which obtains a different conformation in each structure.
The PDZ domain of resting DegS captures loop L3 in a conformation that disrupts the activation domain of the protease
Next, we aimed to identify structural features that caused the observed differences in regulation and activity. Structural alignment of inactive and activated DegS with DegSΔPDZ indicated characteristic differences in the fold of the sensor loop L3. Although the three structures show significant variations in the central part of loop L3, the L3 stem segments of the PDZ deletion mutant and activated DegS aligned well to each other (Fig. 5C). As mentioned previously, these regions are important for propagating the stress signal from PDZ to protease domain. It is important to note that the sensor loop L3 of DegSΔPDZ is not oriented by the PDZ domain or activating peptide and should thus represent its “unrestrained” conformation. In full-length DegS, the PDZ domain captures loop L3 by wedging it in the interdomain space. Here, L3 undergoes numerous interactions with the PDZ domain including van der Waals interactions (L3 residues 179–183 with PDZ residues 319–323), hydrophobic interactions (Leu181 bound in hydrophobic pocket lined with residues 244, 254, and 256), and a short-distanced salt bridge between Arg178 and Asp320. Combined, these interactions bend loop L3 into a conformation that is strikingly different from its unrestrained conformation and that ultimately disrupts the activation domain (Fig. 5C). For example, Gln187 of loop L3 forms a hydrogen bond with Asp221, a residue defining the orientation of loop L2. By the Gln187:Asp221 interaction, loop L2 is pulled toward the PDZ domain and the activation domain is disrupted. The switch in activity is further advanced by Phe220 (L2), the residue adjacent to Asp221. In activated DegS and in DegSΔPDZ, Phe220 forms, together with Tyr162 (loop LD), the hydrophobic core of the functional activation domain, whereas in latent DegS, the side chain of Phe220 is oriented to the other side of the protein backbone, where it undergoes hydrophobic interactions with residues Gly185 and Phe189 of the PDZ-bound loop L3. Binding of activating peptides triggers a rearrangement of loop L3, which switches around by almost 180°, thereby releasing loop L2 to interact with loops L1 and LD and to set up the functional activation domain. Taken together, our results show that the PDZ domain directly prevents formation of a functional protease. It captures loop L3 in a conformation that destabilizes the activation domain. Binding of activating peptides triggers a conformational switch in loop L3 that relieves the destabilizing effect on the activation domain and ultimately enables DegS to cleave RseA.
Discussion
The envelope stress response of E. coli is mediated by a peptidic stress signal inducing a proteolytic cascade, in which DegS, RseP, and cytoplasmic ATP-dependent proteases sequentially degrade the anti-σ factor RseA, thereby activating σE, the transcriptional regulator of the σE regulon. Upon detection of mislocalized OMPs, DegS carries out the first proteolytic cut of RseA, which is also the rate-determining step in the entire signaling cascade. Furthermore, the amount of OMP-activated DegS is directly linked to the degree of σE activation (Chaba et al. 2007). Therefore, the tight regulation of the protease activity of DegS appears to be crucial for the robustness and performance of the σE stress response. The aim of this work was to elucidate the molecular details of the corresponding regulatory mechanisms.
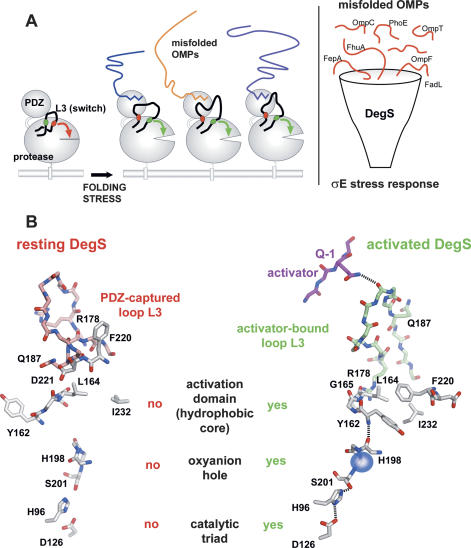
First, we were interested to better understand how the protease function of DegS is activated in general by OMPs. The biogenesis of OMPs requires ∼60% of proteins encoded in the σE regulon, many of which are chaperones and folding catalysts (Rhodius et al. 2006). Upon folding stress in the periplasm, misfolded proteins compete with unassembled OMPs for protein quality-control factors, which ultimately leads to increased amounts of free OMPs in the cellular envelope. Since the C termini of different OMPs are directly involved in the formation of the porin β-barrel and thus not accessible in the native state, the presence of free OMP C termini in the periplasm is an excellent indicator of folding stress. The last 10 residues of most OMPs have a similar amino acid composition and form an amphipathic β-strand with hydrophobic residues in the 0, −2, −4, −6, and −8 positions. Interestingly, such sequences are not present in periplasmic proteins, allowing the specific identification of mislocalized OMPs. Comparison of OMPs with different functions (e.g., receptors, enzymes, porins) from different bacteria indicates that the C-terminal 0-residue, a phenylalanine, is widely conserved, most likely due to its essential role in OMP assembly in the outer membrane (Struyve et al. 1991). Other residues are less conserved, and only position −2 has a slight preference for tyrosine. The present study indicates that the binding mode of potential activators to the PDZ domain of DegS matches this conserved pattern, since binding of the C-terminal phenylalanine appears to be the key interaction with the PDZ domain, and phenylalanine and tyrosine residues are preferred in the −2 position. However, some OMP C termini, which have a small hydrophobic residue in this position, are also capable of activating DegS, and thus the consensus sequence of an allosteric activator of DegS should be ϕ-x-Phe. As this motif is present in the majority of all OMPs, DegS seems to be capable of recognizing and getting activated by different mislocalized OMPs in a highly specific manner.
We also wished to study the binding mode of an activating peptide that does not contain the OmpC-like YQF C terminus and determined the crystal structure of DegS in complex with the YWF activating peptide. The structural data showed that the PDZ-bound YQF and YWF activators undergo different interactions with the protease domain but ultimately trigger activation of proteolytic function in the same manner. The integration of the stress signal is achieved by loop L3, which is composed of two functional parts. The structurally flexible central segment is able to variably interact with residues in the −1 position of different activating peptides, whereas the stem regions of loop L3 reorient in a largely conserved manner to trigger protease activation. Thus the specific composition of loop L3 enables DegS to become activated by different misfolded OMPs and to channel the corresponding information into the σE stress response (Fig. 6A). Our biochemical and structural data pinpoint several key residues that are crucial for the regulation of DegS and for propagating the stress signal through the molecule (Fig. 6B). In the resting state, the PDZ domain keeps DegS inactive by capturing loop L3 in a position that enables Gln187 to interact with Asp221 and that disrupts the hydrophobic core of the activation domain of the protease. Upon folding stress, OMP C termini are recognized by the PDZ domain and interact via their −1 residue with loop L3 of the protease domain. Rearrangement of L3 abolishes the interaction between Gln187 and Asp221 and allows formation of a functional activation domain, in which Tyr162 (LD), Leu164 (LD), Ile179 (L3), Phe220 (L2), and Ile232 (L2) form the hydrophobic core. The relocated Tyr162 is now capable of undergoing main-chain interactions with His198 and to flip the peptide bond between residues 198 and 199. Remodeling of the backbone of residues 197–201 establishes a properly formed oxyanion hole and catalytic triad.
Figure 6.
Model for the integration of different OMP-derived stress signals by DegS. (A) The left panel illustrates activation of DegS by different OMP C termini. Loop L3 is highlighted with its inhibitory (red) and activating (green) structural elements. Molecular details of both inhibitory and activating processes are given in B. In latent DegS, loop L3 directly inhibits protease function by disrupting the activation domain. Binding of the allosteric activator to the PDZ domain triggers a switch of this loop into its active position, where it now supports the setup of a functional proteolytic site. The right panel illustrates the cellular function of DegS acting as a mechanistic funnel to integrate the information from different mislocalized OMPs into the σE stress response. (B) Working model for how DegS switches from the resting to the activated state. Key residues that are important for regulation and for signal propagation are labeled. Loop L3 of the resting DegS is drawn in red, loop L3 of the active DegS is in green. Details of the signal transduction through the DegS molecule leading to the functional protease are described in the text.
Since DegS catalyzes the rate-limiting step in the σE cascade, its activity has to be tightly controlled to remain inactive under nonstress conditions and become activated only in the presence of exposed OMP C termini. The structure of the DegSΔPDZ mutant illustrates that the PDZ domain is absolutely required to inhibit proteolytic activity of DegS in the resting state by fixing loop L3 in a specific conformation that abolishes protease activity. In addition to its inhibitory function, the PDZ domain specifically recognizes the compartmental ϕ-x-Phe stress stimulus and thus enables DegS to regulate its protease function in strict dependence of the stress signal and to react immediately to varying amounts of mislocalized OMPs. The crystal structures of DegS in complex with YQF and YWF imply that OMP-like activating peptides use a common mechanism and abolish the inhibitory effect of the PDZ domain by releasing loop L3. However, clear differences could be observed in the degree of activation. For example, the FRF peptide was generating an activated DegS form with much higher proteolytic activity than the YxF peptides. Although the molecular details of this mechanism remain to be elucidated, our structural data reveal that different activating peptides induce different rearrangements of loop L3, which have a different effect on the active site geometry and rigidity. Similarly, loop L3 of the PDZ deletion mutant obtained a unique conformation that ultimately generated a less-ordered active site with reduced proteolytic activity.
Taken together, our data indicate that the PDZ domain of DegS exerts both inhibitory and activating functions. In the absence of allosteric ligands, it inhibits protease function by capturing loop L3, whereas upon folding stress, it offers a binding site for allosteric activators that release loop L3 and turn on protease function in a specific manner. Thus the PDZ domain and loop L3 of DegS set up a novel molecular switch that regulates the activity of DegS in strict dependence on the presence of mislocalized OMPs. We have shown previously that the switch in activity is reversible (Wilken et al. 2004). The reversible allosteric activation of a rate-limiting protease in a signal transduction pathway, such as DegS in the σE stress response, seems to represent a vital regulatory mechanism that might be compared, on the conceptual level, to phosphorylation/dephosphorylation switches of classical signaling cascades.
Materials and methods
Protein expression and purification
The ORF encoding the protease and PDZ domain (residues 42–354) of DegS and the D122A and Tyr162A mutants were expressed as C-terminal His tag proteins and purified as described previously (Wilken et al. 2004). Briefly, cells transformed with corresponding pET-15b plasmids were grown at 37°C in LB medium and overexpression was induced at OD600 = 0.6 with 1 mM IPTG. After 3 h of incubation, cells were harvested by centrifugation and lysed by sonication in 50 mM NaPO4 buffer (pH 7.5) and 200 mM NaCl. Proteins were purified using Ni-NTA resin (Qiagen) and a Superdex 200 column (GE Healthcare). Prior to crystallization, the protein buffer was exchanged by a NAP10 desalting column (GE Healthcare) to 10 mM NaPO4 (pH 7.5). Prior to ITC measurements and protease assays, the protein buffer was exchanged to 100 mM NaPO4 (pH 7.5), 150 mM NaCl, and 5 mM MgCl2.
The protease domain of DegS (residues 42–255) lacking the PDZ domain was amplified using the previously described degS construct as a template and cloned into pET21a (Novagen). The C-terminal His6-tagged degSΔPDZ was expressed in E. coli BL21(DE3) for 3 h at 37°C. The cultures were harvested by centrifugation, resuspended in 50 mM NaPO4 (pH 8.0) and 300 mM NaCl, and lysed by freeze–thawing and subsequent sonication on ice. To prevent proteolytic digestion, 0.1 mM PMSF, which does not inhibit DegS, was added during the initial purification steps. The PDZ deletion mutant was purified by Ni-NTA affinity chromatography. A Superdex-75 column was instrumental for further purification and to adjust the protein buffer to 20 mM Tris (pH 7.5) and 500 mM NaCl. Prior to crystallization, DegSΔPDZ was concentrated to 1.2 mg/mL.
Crystallization
For crystallization of the DegS–activator complex, the peptide NH2-DNRLGLVYWF-COOH (100 μM) was added to DegS and incubated for 30 min before setting up the cocrystallization trials. Crystals of the complex were grown in sitting drops at 19°C by mixing 4 μL of DegS/YWF with 2 μL of a crystallization solution containing 0.1 M HEPES (pH 7.5), 6% PEG 6000, 9% MPD, and 10 mM MgCl2. Crystal trials were set up in cryschem plates with a reservoir volume of 400 μL. For cryo measurements, crystals were transferred from the crystallization drop to the mother liquor supplemented with 18% MPD as cryo protectant and 100 μM YWF peptide.
Sitting-drop crystallization trials of DegSΔPDZ (1.2 mg/mL) were carried out at 19°C in 96-well plates with a reservoir volume of 100 μL. Crystals of DegSΔPDZ appeared after 2 d in 28% PEG400, 0.1 M HEPES (pH 7.5), and 0.2 M Li-sulfate after mixing 200 nL of protein with 100 nL of precipitant. For cryo measurement, crystals were directly transferred from the drop into the nitrogen gas stream.
Structure solution and quality of the structures
High-resolution data of the activator complex and the DegSΔPDZ mutant were collected in-house on a MarResearch image plate. Data were integrated using DENZO and scaled with SCALEPACK (Otwinowski and Minor 1997). Both crystal forms were of the monoclinic space group C2 with one DegS trimer in the asymmetric unit. The structure of the DegSΔPDZ mutant was determined by molecular replacement using the program MOLREP of the CCP4 package (Collaborative Computational Project in Macromolecular Crystallography 2002) and the protease domain (residues 43–251) of DegS as a search model (PDB ID 1soz). The structure of the YWF activator complex was solved by using the refined structure of the YQF-activated DegS, from which loops L1, L2, L3, and LD were omitted. For both structures, electron-density maps based on the coefficients 2Fo − Fc and 3Fo − 2Fc were calculated from the phases of the initial model. The resulting maps were used to build atomic models in O (Jones et al. 1991). Refinement, model rebuilding, and water incorporation proceeded smoothly via rigid body, positional, and later B-factor optimization in CNS (Brunger et al. 1998). Finally, the structures were checked using simulated annealing composite omit maps. Some protein segments including residues 221–229, 264–280, and 336–341 were hardly visible in these maps and were therefore omitted from the model. During refinement, clear electron density developed in the 3Fo − 2Fc omit density map for two of three activator molecules. The data collection and refinement parameters are summarized in Table 2. All graphical presentations were prepared using the program PYMOL (DeLano 2002).
In vitro cleavage assay
Proteolytic activity of wild-type DegS and DegSΔPDZ was measured by following cleavage of the periplasmatic domain of E. coli RseA (residues 121–216), which was expressed with an N-terminal His6 tag. After purification by NiNTA affinity chromatography, the RseA substrate was concentrated to 7.5 mg/mL. Cleavage assays were performed at 30°C in 100 mM NaPO4 (pH 7.5), 200 mM NaCl, 10% glycerol, 5 mM MgCl2, and 1 mM DTT. For the cleavage reaction, DegS and DegSΔPDZ were adjusted to 10 μM and RseA to 30 μM. The reactions were stopped by adding SDS-sample buffer and were analyzed by SDS-PAGE.
Isothermal titration calorimetry
The thermodynamic values of the interaction between DegS and different activating peptides were determined by isothermal titration calorimetry (MCS-ITC; Microcal). All experiments were conducted in overflow mode at 30°C. Solution (1.4-mL) of DegS (80–90 μM) was placed in the temperature-controlled sample cell and titrated with different OMP-derived peptides (1 mM), which were loaded in the 300-μL mixing syringe. For all experiments, 100 mM NaPO4 buffer (pH 7.5) supplemented with 150 mM NaCl and 5 mM MgCl2 was used as the buffer. Injections of 10 μL of peptide were dispensed into the sample cell using a 120-sec equilibration time between experiments and stirring at 300 rpm. Control experiments using the identical experimental setup were carried out in order to measure and correct the heat of dilution. Ultimately the data were analyzed using ORIGIN software following the instructions of the manufacturer.
Accession numbers
The Protein Data Bank accession numbers for the YWF-activated and the PDZ deletion forms of DegS are 2R3Y and 2R3U, respectively.
Acknowledgments
We thank Peggy Stolt-Bergner and the Clausen laboratory for critical reading of the manuscript and helpful discussions. The Research Institute of Molecular Pathology (IMP) is funded by Boehringer Ingelheim. M.E. was supported by the British Biology and Biotechnology Research Council, Deutsche Forschungsgemeinschaft, and the Fonds der Chemischen Industrie; T.C. was supported by the EMBO Young Investigator Program; and H.H, T.K., and J.S. were supported by the Austrian Science Fund (FWF P17881-B10).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.445307
References
- Ades S.E., Connolly L.E., Alba B.M., Gross C.A., Connolly L.E., Alba B.M., Gross C.A., Alba B.M., Gross C.A., Gross C.A. The Escherichia coli σ(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-σ factor. Genes & Dev. 1999;13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y., Kanehara K., Ito K., Kanehara K., Ito K., Ito K. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO J. 2004;23:4434–4442. doi: 10.1038/sj.emboj.7600449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba B.M., Leeds J.A., Onufryk C., Lu C.Z., Gross C.A., Leeds J.A., Onufryk C., Lu C.Z., Gross C.A., Onufryk C., Lu C.Z., Gross C.A., Lu C.Z., Gross C.A., Gross C.A. DegS and YaeL participate sequentially in the cleavage of RseA to activate the σ(E)-dependent extracytoplasmic stress response. Genes & Dev. 2002;16:2156–2168. doi: 10.1101/gad.1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.S., Ye J., Rawson R.B., Goldstein J.L., Ye J., Rawson R.B., Goldstein J.L., Rawson R.B., Goldstein J.L., Goldstein J.L. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Brunger A.T., Adams P.D., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Adams P.D., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Clore G.M., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., DeLano W.L., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Gros P., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Grosse-Kunstleve R.W., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Jiang J.S., Kuszewski J., Nilges M., Pannu N.S., Kuszewski J., Nilges M., Pannu N.S., Nilges M., Pannu N.S., Pannu N.S., et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Cezairliyan B.O., Sauer R.T., Sauer R.T. Inhibition of regulated proteolysis by RseB. Proc. Natl. Acad. Sci. 2007;104:3771–3776. doi: 10.1073/pnas.0611567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaba R., Grigorova I.L., Flynn J.M., Baker T.A., Gross C.A., Grigorova I.L., Flynn J.M., Baker T.A., Gross C.A., Flynn J.M., Baker T.A., Gross C.A., Baker T.A., Gross C.A., Gross C.A. Design principles of the proteolytic cascade governing the σE-mediated envelope stress response in Escherichia coli: Keys to graded, buffered, and rapid signal transduction. Genes & Dev. 2007;21:124–136. doi: 10.1101/gad.1496707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Southan C., Ehrmann M., Southan C., Ehrmann M., Ehrmann M. The HtrA family of proteases: Implications for protein composition and cell fate. Mol. Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project in Macromolecular Crystallography High-throughput structure determination. Proceedings of the 2002 CCP4 (Collaborative Computational Project in Macromolecular Crystallography) study weekend. January, 2002. York, United Kingdom. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1897–1970. [PubMed] [Google Scholar]
- DeLano W.L. 2002 The PyMol molecular graphics system. DeLano Scientific, Palo Alto, CA. http://www.pymol.org. [Google Scholar]
- De Las Penas A., Connolly L., Gross C.A., Connolly L., Gross C.A., Gross C.A. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol. Microbiol. 1997;24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- Duguay A.R., Silhavy T.J., Silhavy T.J. Quality control in the bacterial periplasm. Biochim. Biophys. Acta. 2004;1694:121–134. doi: 10.1016/j.bbamcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Ehrmann M., Clausen T., Clausen T. Proteolysis as a regulatory mechanism. Annu. Rev. Genet. 2004;38:709–724. doi: 10.1146/annurev.genet.38.072902.093416. [DOI] [PubMed] [Google Scholar]
- Fanning A.S., Anderson J.M., Anderson J.M. Protein–protein interactions: PDZ domain networks. Curr. Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Levchenko I., Sauer R.T., Baker T.A., Levchenko I., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. Modulating substrate choice: The SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes & Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Wickner S., Maurizi M.R., Wickner S., Maurizi M.R., Maurizi M.R. Protein quality control: Triage by chaperones and proteases. Genes & Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- Hasenbein S., Merdanovic M., Ehrmann M., Merdanovic M., Ehrmann M., Ehrmann M. Determinants of regulated proteolysis in signal transduction. Genes & Dev. 2007;21:6–10. doi: 10.1101/gad.1507807. [DOI] [PubMed] [Google Scholar]
- Huber R., Bode W., Bode W. Structural basis of activation and action of trypsin. Acc. Chem. Res. 1978;11:114–122. [Google Scholar]
- Jones T.A., Zou J.Y., Cowan S.W., Kjeldgaard M., Zou J.Y., Cowan S.W., Kjeldgaard M., Cowan S.W., Kjeldgaard M., Kjeldgaard M. Improved methods for building protein models in electron-density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kanehara K., Ito K., Akiyama Y., Ito K., Akiyama Y., Akiyama Y. YaeL (EcfE) activates the σ(E) pathway of stress response through a site-2 cleavage of anti-σ(E), RseA. Genes & Dev. 2002;16:2147–2155. doi: 10.1101/gad.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Jin K.S., Kwon E., Ree M., Kim K.K., Jin K.S., Kwon E., Ree M., Kim K.K., Kwon E., Ree M., Kim K.K., Ree M., Kim K.K., Kim K.K. Crystal structure of RseB and a model of its binding mode to RseA. Proc. Natl. Acad. Sci. 2007;104:8779–8784. doi: 10.1073/pnas.0703117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko I., Grant R.A., Flynn J.M., Sauer R.T., Baker T.A., Grant R.A., Flynn J.M., Sauer R.T., Baker T.A., Flynn J.M., Sauer R.T., Baker T.A., Sauer R.T., Baker T.A., Baker T.A. Versatile modes of peptide recognition by the AAA+ adaptor protein SspB. Nat. Struct. Mol. Biol. 2005;12:520–525. doi: 10.1038/nsmb934. [DOI] [PubMed] [Google Scholar]
- Macario A.J., de Conway Macario E., de Conway Macario E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Minor W., Minor W.1997. Processing of X-ray diffraction data collected in oscillation modeMeth. Enzymol. 276307–326. [DOI] [PubMed] [Google Scholar]
- Perona J.J., Craik C.S., Craik C.S. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivio T.L. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 2005;56:1119–1128. doi: 10.1111/j.1365-2958.2005.04625.x. [DOI] [PubMed] [Google Scholar]
- Rhodius V.A., Suh W.C., Nonaka G., West J., Gross C.A., Suh W.C., Nonaka G., West J., Gross C.A., Nonaka G., West J., Gross C.A., West J., Gross C.A., Gross C.A. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouviere P.E., Penas A.D.L., Mecsas J., Lu C.Z., Rudd K.E., Gross C.A., Penas A.D.L., Mecsas J., Lu C.Z., Rudd K.E., Gross C.A., Mecsas J., Lu C.Z., Rudd K.E., Gross C.A., Lu C.Z., Rudd K.E., Gross C.A., Rudd K.E., Gross C.A., Gross C.A. RpoE, the gene encoding the 2nd heat-shock σfactor, σ(E), in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyve M., Moons M., Tommassen J., Moons M., Tommassen J., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J. Mol. Biol. 1991;218:141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Alba B.M., Bose B., Gross C.A., Sauer R.T., Alba B.M., Bose B., Gross C.A., Sauer R.T., Bose B., Gross C.A., Sauer R.T., Gross C.A., Sauer R.T., Sauer R.T. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- Wickner S., Maurizi M.R., Gottesman S., Maurizi M.R., Gottesman S., Gottesman S. Posttranslational quality control: Folding, refolding, and degrading proteins. Science. 1999;286:1888–1893. doi: 10.1126/science.286.5446.1888. [DOI] [PubMed] [Google Scholar]
- Wilken C., Kitzing K., Kurzbauer R., Ehrmann M., Clausen T., Kitzing K., Kurzbauer R., Ehrmann M., Clausen T., Kurzbauer R., Ehrmann M., Clausen T., Ehrmann M., Clausen T., Clausen T. Crystal structure of the DegS stress sensor: How a PDZ domain recognizes misfolded protein and activates a protease. Cell. 2004;117:483–494. doi: 10.1016/s0092-8674(04)00454-4. [DOI] [PubMed] [Google Scholar]
- Young J.C., Hartl F.U., Hartl F.U. A stress sensor for the bacterial periplasm. Cell. 2003;113:1–2. doi: 10.1016/s0092-8674(03)00192-2. [DOI] [PubMed] [Google Scholar]