Abstract
Advances in the delivery of targeted drug systems have evolved to enable highly regulated site specific localization to subcellular organelles. Targeting therapeutics to individual intracellular compartments has resulted in benefits to therapies associated with these unique organelles. Endocytosis, a mechanism common to all cells in the body, internalizes macromolecules and retains them in transport vesicles which traffic along the endolysosomal scaffold. An array of vesicular internalization mechanisms exist, therefore understanding the key players specific to each pathway has allowed researchers to bioengineer macromolecular complexes for highly specialized delivery. Membrane specific receptors most frequently enter the cell through endocytosis following the binding of a high affinity ligand. High affinity ligands interact with membrane receptors, internalize in membrane bound vesicles, and traffic through cells in different manners to allow for accumulation in early endosomal fractions or lysosomally associated fractions. Although most drug delivery complexes aim to avoid lysosomal degradation, more recent studies have shown the clinical utility in directed protein delivery to this environment for the enzymatic release of therapeutics. Targeting nanomedicine complexes to the endolysosomal pathway have serious potential for improving drug delivery for the treatment of lysosomal storage diseases, cancer, and Alzheimer’s disease. Although several issues remain for receptor specific targeting, current work is investigating a synthetic receptor approach for high affinity binding of targeted macromolecules.
Keywords: lysosomes, endosomes, receptor mediated endocytosis, clathrin, HPMA, riboflavin
1. Introduction
Macromolecular therapeutics are rapidly gaining interest in the area of nanomedicine for their ability to serve as alternatives to traditional drug regimens. Macromolecular conjugation has offered improvements in the delivery of low molecular weight drugs by preventing their passive diffusion into highly circulated tissue systems throughout the body resulting in lowered toxicity and volume of distribution. Drugs that would potentially benefit from such strategies are those exhibiting low bioavailability and limited therapeutic utility. A variety of macromolecules, such as oligonucleotides, peptides, proteins, and polymers, have been investigated for their biocompatibility and stability in vivo in serving as vehicles for drug delivery; however, even these systems still posses drawbacks, especially with regards to bioavailability [1].
The primary advantage in the use of macromolecules as a drug delivery vehicle is their mechanism(s) of cellular internalization. The cell membrane is naturally impermeable to complexes larger than 1 kDa; however, cells posses a variety of active internalization mechanisms to accommodate cellular entry of large molecular complexes. Here, the cell membrane will invaginate to engulf molecules and extracellular fluid in an intracellular membrane-bound vesicle, or endosome, that will subsequently traffic through the cell, a process known as endocytosis. Molecules may reside near the membrane or directly interact with membrane proteins to enable their retention in these vesicles. Analogous to the attachment of drug moieties to high molecular weight carriers, agents such as antibodies and high affinity ligands can be incorporated in order to exploit direct membrane interactions and target these complexes to specific cell populations in organ systems. Once inside the cell, the intracellular fate of the endosomal contents is an important determinant of successful drug delivery. Depending on the membrane interaction and components involved in vesicle formation, endosomes will mature into acidic vesicles which may or may not fuse with lysosomes, which can completely metabolize macromolecules using hydrolytic and enzymatic reactions. Targeting macromolecular complexes with high affinity ligands specific to membrane proteins, namely receptors, can aid in regulating not only the cellular recognition of these carriers but also the trafficking pathway and subcellular localization within the cell.
This review will discuss macromolecular drug delivery systems targeted to the endolysosomal systems of cells. Targeting to this pathway offers several advantages, including the ability to exploit upregulated membrane receptors in certain diseased organs and tissues, to control the intracellular fate for localization to acidic endosomes, and to allow for regulated release of therapeutics from bioresponsive linkers on the vehicle. Next, targeting the macromolecules to an intracellular endolysosomal pathway will enable therapeutic delivery to the unique organelles connected with these trafficking pathways, namely the endosomes and lysosomes. Pathological conditions associated with the endosomes and lysosomes would greatly benefit from therapies directed along these pathway. Finally, current delivery systems allowing for endolysosomal targeting will be discussed, with particular attention to the use of nanoscale carriers.
1.1 Clinical Significance of Targeted Drug Delivery to Endolysosomal Systems
The major objectives for targeted drug delivery are reducing the nondiscriminate uptake of toxic agents as well as enhancing drug accumulation at the target site. In order to target drugs to specific tissue systems within the body, drug molecules can be directly attached to a targeting agent or complexed with a vehicle, or macromolecule, that contains targeting moieties. Macromolecules can be bioengineered to incorporate a variety of synthetic and natural compounds including drugs, ligands, and radionuclides.
Receptor mediated endocytosis (RME) allows for a more rapid means of ligand targeted internalization compared to that of untargeted complexes. Depending on the receptor-dependent or independent endocytic path, the intracellular trafficking path can also be controlled (Table 1). For example, those macromolecules taken up by clathrin-dependent RME are typically destined for lysosomal degradation; whereas, clathrin-independent RME internalization leads to endosomal accumulation and sorting to a nondegradative path. Appropriate selection of targeting agents could therefore allow for controlled delivery to the lysosomes or endosomes to alleviate conditions associated with these individual organelles, including cancer [2], Alzheimer’s disease [3], and most importantly lysosomal storage diseases (LSDs).
Table 1.
Major well characterized endocytic targeting ligands for macromolecular drug delivery.
| Targeting Agent | Internalization Mechanism | Delivery Vehicle | Therapeutic Cargo |
|---|---|---|---|
| Folic Acid | Caveolin-Assist1ed | Ligand Alone | |
| Low-Density Lipoprotein | Caveolin-Assisted | Nanoparticles | Photodynamic Therapy Agent[64] |
| Cholera Toxin B | Lipid Raft Associated | Liposomes | Saliva-Binding Region[65] |
| Mannose-6- Phasphate | Clathrin-Dependent RME | Human Serum Albumin | Antifibrotic Drug[66] |
| Transferrin | Clathrin-Dependent RME | Doxorubicin | |
| Riboflavin | Clathrin-Dependent RME | Bovine Serum Albumin[69] | N/A |
| Arg-Gly-Asp (RGD) | Cell Adhesion Molecule (CAM) Directed | Biocompatible Polymer | Radionuclides [70] |
| ICAM-1 Antibody | CAM Directed | Nanocarriers | Lysosomal Enzyme[20] |
| Nicotinic Acid | Clathrin-Dependent RME | Biotinylated Polylysine | DNA[71] |
Previous modalities of treatment for LSDs have resulted in hepatic buildup of replacement enzymes causing rapid clearance and low bioavailability. The use of macromolecular delivery systems that are targeted to endocytic machinery enable both cell specific as well as organelle specific, in this case the lysosomes, interactions resulting in a more efficacious treatment for these disease states. Work with chemically modified lysosomal enzymes has acted as a proof-of-principle for this theory of directed delivery. For example, glucocerebrosidase, the enzyme deficient in Gaucher’s disease, was modified to expose mannose residues allowing for recognition by mannose receptors and subsequent receptor-mediated internalization of the enzyme and trafficking to the lysosomes [4].
The majority of targeted deliveries aim to avoid lysosomal trafficking in an effort to protect the drug molecule or biomolecules from enzymatic degradation. For our focus, this issue need only be addressed for those complexes which are expected to release drug molecules prior to endosomal fusion with lysosomal contents. This matter can easily be resolved by selecting a targeting agent that is known to be internalized via a clathrin-independent mechanism where contents are exposed to the acidic environment of endosomes but are typically not destined to the lysosomally degradative pathway. Alternatively, a growing number of polymer-based therapies are targeting therapeutics through a clathrin-dependent endocytic mechanism for drug localization and release in lysosomes. The endolysosomal pathway is of direct relevance to targeted intracellular drug delivery because not only does endocytosis allow for macromolecular internalization but it enables receptor- and lysosome-specific localization. Using the current knowledge of endocytic mechanisms and the key players involved, drug delivery systems can be bioengineered to exploit these pathways for more specialized intracellular delivery.
Another area with recent successes in macromolecular therapeutics is directed delivery of anticancer drugs. Chemotherapeutics are low molecular weight drugs that quickly diffuse into all tissues in the body thereby frequently causing toxicity leading to dose-related side effects. Modifying the delivery of these compounds, which remains the best form of cancer treatment, through macromolecular conjugation has offered promising results and has currently reached Phase 3 clinical trials. Success in this area can be attributed largely to the structural and physical nature of cancer tissue. To begin, solid tumors recruit new vasculature to support the rapidly dividing cells; however, the process of angiogenesis occurs insufficiently resulting in semipermeable blood vessels and allowing for the entry of otherwise impermeable molecules which are retained in the solid tumor tissue. This effect allows for a higher accumulation of drug conjugated complexes, which are taken up by normal cells through an indirect endocytic mechanism at a much slower rate. Furthermore, cancer cells typically contain upregulated amounts of membrane receptors. For example, the folate receptor is upregulated in over one-third of human cancers. With this in mind, drug delivery vehicles have been conjugated to high affinity targeting agents directed at specific cell surface receptors in cancer tissues including liver, breast, and brain [5]. Another advantage of macromolecular therapy targeting RME systems is the containment of therapeutic macromolecules within endocytic vesicles, thereby bypassing potential effects of multi-drug resistance associated efflux transporters (e.g. P-glycoprotein, MRP, BCRP), which are frequently upregulated in tumor tissues [6].
2. Understanding Endocytic Mechanisms for Directed Targeting
All eukaryotic cells absorb macromolecules through endocytosis, where large or polar substances are engulfed within the cell membrane and contained intracellularly in membrane bound vesicles. Materials, such as proteins and carbohydrates, come in close proximity to the cellular membrane where they may directly interact with membrane-embedded receptors or, indirectly by associating with the bilayer. The selective incorporation of materials is governed by cellular requirements and, depending on the mode of internalization, contents are transported to early endosomes where they can be recycled and exocytosed [7] or trafficked to organelles including the lysosomes, golgi, and mitochondria. The endosomal and lysosomal trafficking pathways following receptor-dependent and independent endocytosis are the focus of this review.
Receptor-mediated internalization occurs in pitted membrane regions lined either by the assistor proteins caveolin-1 or clathrin. Although vesicle formation for each endocytic mechanism is similar and requires the action of the pluripotent scission GTPase dynamin-2, the intracellular fate of endosomes and their contents are distinctly regulated. Clathrin-dependent internalization follows a well defined path where mature endosomes fuse with lysosomal vesicles resulting in enzymatic destruction of a variety of molecules including lipids, carbohydrates, nucleic acids, and viruses. In the case of clathrin-independent uptake, subcellular movement proceeds through a series of endosomal compartments of increasing acidity allowing for the hydrolytic breakdown of molecules. Characterization of proteins involved in the distinct paths for a number of RME receptors has been studied using biochemical modifiers along with fluorescence imaging and confocal microscopy [8].
2.1. Mechanisms for Lysosomal Delivery
In an effort to expand the therapeutic potential of macromolecular drug delivery systems, RME targeted approaches have been investigated for their use in chemotherapy and enzyme replacement therapy. The endolysosomal path serves as a scaffold for intracellular movement leading to fusion with lysosomes to allow for the breakdown of macromolecules and vesicular content. Two different mechanisms are utilized for internalization and trafficking to the lysosomes, namely fluid phase endocytosis (FPE) and clathrin-dependent endocytosis occurring in a receptor-dependent and independent manner, respectively. Unlike fluid phase endocytosis, RME involves complex intracellular signaling following binding of a high affinity ligand. Targeting macromolecular therapeutics to FPE and clathrin-dependent RME is accomplished with the use of hydrophobic or charged macromolecules and receptor-directed ligands, respectively.
2.1.1 Clathrin-Dependent Endocytosis
Clathrin-mediated endocytosis serves as the main mechanism of internalization for macromolecules and plasma membrane constituents for most cell types. Clathrin-dependent mechanisms that have been identified thus far include the well characterized RME pathway, including cell adhesion molecule (CAM) assisted RME. In these processes, intracellular vesicles form invaginations in the membrane that are coated by the triskelion protein clathrin populating the cytoplasmic face of the membrane. Coated pits cover 1–2% of the plasma membrane surface area and allow for rapid intracellular vesicle budding, occurring as quickly as 1 minute. Clathrin aids in vesicular formation from multiple sites, including endosomes and TGN. Assembly proteins, such as adaptins, dynamin, and numerous rab proteins, facilitate the binding, internalization, and post-internalization trafficking of vesicles. Adaptin acts to bind cell surface receptors on the extracellular face as well as binding clathrin heavy chains on the cytosolic side to allow for polymerization of clathrin subunits forming a polyhedral lattice scaffold. Without a specific cell receptor ligand to bind, macromolecules can only enter the cell through FPE, which is significantly slower but has analogous vesicular propagation. Proteins including amphiphysin and endophilin bring the surrounding membrane into close proximity. Following this, dynamin, a cystolic small GTPase, assembles around the neck of the budding vesicle and causes scission and intracellular release. The clathrin coat is quickly shed after intracellular invagination by an uncoating protein, namely heat shock protein (hsc70). Next, the endosomes traffic along the cytoskeleton, comprising microtubules, microfilaments, and molecular motor proteins, to fuse with early or sorting endosomes. At this stage the intravesicular contents will either recycle back to their original membrane domain from sorting endosomes, in an effort to regulate homeostasis, or continue along the endolysosomal pathway for degradation in the lysosomes [9]. It should be noted that ligands which return to the plasma membrane have limited utility in macromolecular therapy which would require intracellular accumulation for eventual therapeutic action. Alternatively, trafficking to early endosomes occurs in as quickly as 5 to 10 minutes and enables intracellular localization. Movement along this path can be monitored with early endosomal markers, such as early endosome antigen 1 (EEA1). Vacuolar ATPase pumps are recruited to the endosomes to pump in H+ ions causing an increase in acidity to approximately 5.5–6.0. This slightly acidic pH results in a conformational change for numerous receptors often resulting in the release of bound ligands from receptors [10]. From this point, ligands may dissociate from receptors and be transported in separate endosomes to the apical (retroendocytosis) and basolateral (transcytosis) membrane domains, or remain attached to receptors to continue along the endolysosomal pathway. Ultimately, endosomal carrier vesicles detach from the early endosome, forming multivesicular bodies, which will mature into late endosomes. During this vesicular maturation process, there is a gradual decrease in pH reaching as low as pH 5, creating an environment that is conducive to enzyme function. Lysosomal enzymes are marked for endosomal delivery by the incorporation of a mannose-6-phosphate (M6P) tag received in the endoplasmic reticulum followed by their translocation into the golgi apparatus. These acid hydrolases are retained in prelysosomal vesicles that will fuse with late endosomes, creating lysosomes, where macromolecular degradation occurs. Mechanisms including clathrin-assisted endocytosis, cell adhesion molecule assisted endocytosis, and FPE, all enable lysosomal fusion. Although targeted drug delivery pathways ideally aim to avoid lysosomal degradation, disease states that are directly associated with lysosomes would benefit greatly from direct intracellular delivery to this subcellular organelle. For example, targeted drug delivery strategies have exploited the clathrin-dependent receptor mediated endocytic events of glycoreceptors, including mannose-6-phosphate receptor and mannose receptor [11]. This delivery mechanism provokes a highly directed method for the delivery of deficient lysosomal enzymes as well as drug precursors.
2.1.1.1 Receptor-Mediated Endocytosis
Clathrin-dependent RME is the most investigated vesicular pathway for targeted drug delivery. In addition to a well characterized internalization scheme (Section 2.1.1), numerous receptors, including transferrin, asialoglycoprotein [12], epidermal growth factor (EGF) [13], and chemokine [14], serve as high affinity binding sites and have been investigated for their use in targeting to different cell types. Ligands bind specific cell surface receptors, signaling their directional movement towards clathrin underlined pits in the membrane. Receptor-ligand complexes accumulate in pits through the aid of multisubunit complexes, or adaptins, which bind to a four amino acid signaling sequence in the carboxy terminus of the receptor. Here, receptors are concentrated in the coated pits prior to invagination, minimizing the amount of extracellular fluid which can be retained in the vesicle. After binding and internalization, ligands and/or receptors may be recycled or ultimately enzymatically degraded in the lysosomal compartment depending on cellular requirements. Ligands such as transferrin (TF) and riboflavin (RF) [15] will transverse the cytosol along a sorting path allowing for receptor recycling as well as the uncoupling of the ligands and receptors.
The TF pathway has been studied extensively for its potential to serve as a high affinity target for the delivery of a variety of drug vehicles. For example, transferrin was recently recognized for its ability to efficiently target polyethylene glycol (PEG) coated nanoparticles containing azidothymidine (AZT) to brain tissue [16]. The brain sequesters iron from TF after internalization via the TF receptor (TFR) across the blood brain barrier (BBB). This advocates the use of TF as a targeting agent for drugs directed at brain disease [17]. Furthermore, internalization through this endocytic process will allow for partial avoidance of recognition by efflux transporters which are highly concentrated in the BBB. Analogous to transferrin, RF follows a traditional clathrin-assisted RME mechanism colocalizing with markers characteristic to this pathway [9]. Preliminary data in our lab suggests that the RF RME pathway would enable enhanced cellular accumulation of RF directed macromolecules in breast cancer (unpublished data). Serum levels of riboflavin carrier protein (RCP), a soluble plasma RF binding protein, are significantly increased in plasma obtained from breast cancer patients [18] and may serve as a molecular marker for mammary tumors.
2.1.1.2 Cell Adhesion Molecule Mediated Endocytosis
Although numerous mechanisms for endocytosis exist, all forming intracellular vesicles for the cytosolic incorporation of macromolecules, their exact mechanism of cell entry still remains elusive. Clathrin-dependent endocytosis, the most prevalent form of vesicular internalization, is shared among an array of plasma membrane receptors. For many receptors, a sequence within the carboxy terminus of the protein allows for binding to the adaptin AP2, causing lateral movement along the membrane. A subfamily of immunoglobulins (IGs), namely cell adhesion molecules (CAMs) or L1, found in endothelial and neuronal cells, contain a signaling sequence, ArgSerLeuGlu, on the cytosolic domain which localizes these proteins to clathrin-coated pits. Cell adhesion receptors, including integrin and cadherin, will enter into the cell in this manner after binding cell adhesion peptides. Conformational changes to integrin result in activation and ligand binding which signals the complex to the coated pits. Peptides such as Arg-Gly-Asp (RGD) specifically bind to a cell adhesion receptor and have recently been used in targeted drug delivery systems for the treatment of cancer and autoimmune diseases [19]. In addition, targeted subcellular delivery to the lysosomes is achieved by carbohydrate-mediated binding and internalization via glycoreceptors allowing for intracellular delivery of recombinant enzymes in an effort to restore deficient lysosomal hydrolases [20]. In the presence of a receptor recycling component, the intracellular accumulation of these targeted systems can offer prolonged drug effects for conjugated delivery systems [21]. Further insight into the mechanism of internalization and trafficking for CAMs would permit additional opportunities for effectively targeting neuronal and endothelial diseases.
2.1.2 Fluid Phase Endocytosis
FPE is a nonspecific adsorptive pinocytic mechanism which allows for the cellular incorporation of molecules contained in the extracellular fluid. Molecules absorbed via this pathway avoid direct binding with membrane constituents but often possess nonspecific charge and hydrophobicity membrane interactions. This process accompanies receptor-mediated absorption, internalizing not only the receptor-ligand complexes concentrated in these pits but also extracellular fluid which is proportional to the internal volume of the endosomes, approximately 100 nm in diameter. Fluid entry occurs mainly in clathrin-coated vesicles following the intracellular trafficking scheme explained above. The major difference in FPE and RME, other than indirect and direct membrane interactions, is the kinetics of transport through the endolysosomal pathway [22]. FPE occurs at a significantly slower rate compared to the high affinity RME process.
Cationic molecules naturally reside near the negatively charged membrane and internalize through FPE. Macromolecular drug delivery systems have been modified to take advantage of this characteristic. Positively charged hydroxypropyl methacrylamide (HPMA) polymers [23] and poly(l-lysine) conjugates [24] show greater accumulation in cells than negatively charged polymers. One well-characterized FPE-directed drug delivery system utilizes dextran conjugates to successfully deliver superoxide dismutase in hepatic tissue following modification of saccharides resulting in nonspecific electrostatic interactions with the cell surface [25]. In addition, horse radish peroxidase (HRP) acts as a model protein for nonspecific bulk phase endocytosis and has also been implicated in targeted therapeutic carriers to cancer tissue [26]. These approaches do not rely on protein interactions for internalization, which strongly varies across different cell types.
2.2 Trafficking Mechanisms for Clathrin-Independent Endosomal Transport
Endocytic internalization is a highly coordinated and active process allowing whereby a cell internalizes an area equivalent to its entire plasma membrane every hour [27]. The cellular mechanics associated with endocytic internalization allows for several different forms of vesicle formation including nonspecific adsorptive endocytosis and clathrin-dependent (CME) and independent RME. All approaches involve the invagination of lipid rich membrane regions which lead to intracellular release of membrane bound vesicles housing extracellular fluid and substances including nutrients [28], macromolecules [5], and receptors. Vesicle acidification is a hallmark feature of CME, which may lead to the inadvertent hydrolysis of pH-sensitive macromolecules. On the other hand, clathrin-independent internalization enables the intracellular accumulation of materials along a less destructive path as compared to CME. The two major pathways include caveolae-mediated endocytosis (CvME) and the less characterized lipid raft internalization. Both methods share a variety of common features making it difficult to form a common distinction [29]; however, both may serve as targets for a less destructive form of targeted drug delivery.
2.2.1 Caveolin Assisted Receptor Mediated Endocytosis
Although clathrin-dependent endocytosis is the predominant endocytosis pathway in most cells, alternative, parallel uptake pathways have been recently identified, such as CvME. Caveolae are flask-shaped invaginations, ranging from 50 to 100 nm in diameter, making up more than 10% of the plasma membrane for endothelial cells. Caveolar morphology differs dramatically between cell types making it complicated to definitively infer on common structural features. These invaginations are held in place by underlying actin filaments in the cytoskeleton, where certain membrane proteins are found to concentrate. Unlike clathrin-mediated endocytosis, caveolae-assisted endocytosis is a triggered process that involves subsequent complex signaling.
Molecular based approaches investigating caveolae-mediated endocytosis have aided in the recognition of membrane proteins associated with this version of cellular entry. The most prevalent protein associated with caveolae structures is caveolin-1, which is pertinent to caveolae formation in the membrane and subsequent vesicular production as well as stabilization from a sub-membrane raft-dependent endocytic process. Simian Virus 40 (SV40) has been examined as a prototypical ligand for caveolar endocytosis. After binding to the plasma membrane, particles move along the membrane to caveolar invaginations where they are contained prior to internalization bound to an unidentified receptor. Caveolae-coated vesicles move through the cytoplasm with the help of an activated signaling transduction cascade. Vesicular cargo is then transferred to more complex tubular organelles, or caveosomes. Antibodies directed at clathrin-associated endocytic components, such as endosomes, lysosomes, and trans golgi network (TGN), do not participate in the post-internalization trafficking of these endosomes. From this point, contents are delivered to subcellular (non-lysosomal) compartments [30]. For example, for SV40, the vesicular contents are transported to the endoplasmic reticulum where the virus remains until it is transported to the nucleus [31] (Figure 1). This pathway could be utilized for targeted macromolecular delivery into cells through the binding of caveolae-associated membrane receptors.
Figure 1.
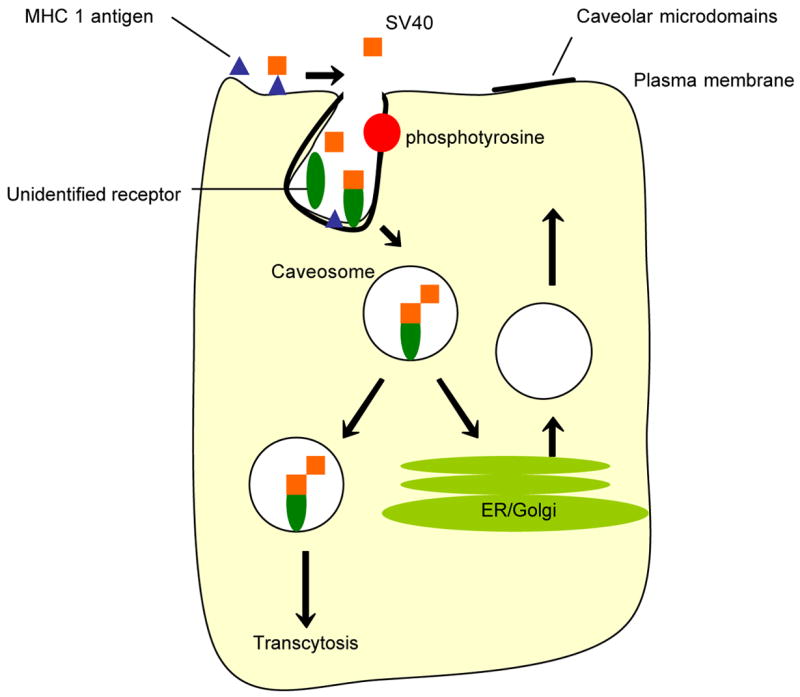
Caveolae-assisted internalization of Simian Virus 40 (SV40). Caveolae are specialized lipid rafts that allow for SV40 internalization and subsequent intracellular signaling. After binding to major histocompatibility class 1 antigens (MHC 1), SV40 moves along the plasma membrane and concentrates in caveolae structures. MHC 1 is not endocytosed, therefore it is suggested an unidentified receptor is responsible for SV40 high affinity internalization. A signaling transduction cascade is induced upon the phosphorylation of tyrosine residues resulting in the depolymerization of actin fibers and invagination of the membrane. Caveosomes are transported intracellularly along microtubular networks to either be transcytosed to the opposite membrane domain or transported to the endoplasmic reticulum (ER) from which SV40 will travel to the nucleus [31].
Ligands known to be internalized through receptor-dependent caveolae-mediated endocytosis include, folic acid [32], albumin, and cholesterol. Folic acid, or vitamin B9, appears an especially attractive target for targeted drug delivery. For example, folate-targeted poly(ethylene glycol) (PEG)-coated nanoparticles are found to bind to folate receptors allowing for caveolae-assisted endocytosis, followed by the formation of intracellular vesicles which can be visualized by confocal microscopy [33]. In addition, albumin, which interacts with endothelium by binding to albondin (gp60) receptor, is used in vascular targeting through its caveolae-mediated uptake mechanism [34]. Caveolae-directed systems have been used to target chemotherapeutics to a nondegradative pathway, where pH sensitive bonds have allowed for drug release followed by diffusion across the endosomal membrane directed to the nucleus [35]. Characterizing the proteins and receptors pertinent to caveolar transport will aid in the design of targeted drug carriers which bypass the harsh environment of the lysosomes to render this trafficking pathway less harmful to drug complexes [36]
2.2.2 A Third Receptor-Specific Endocytic Mechanism?
In general, molecules taken up by a clathrin-independent mechanism are localized to lipid rafts [37]. Short-interfering RNA (siRNA) strategies aimed at investigating the importance of caveolin-1 for clathrin-independent internalization illustrated that this protein is not mandatory for internalization through lipid-rich membrane regions [38]. This data suggested an alternative mode of cellular internalization. Specific ligands, including cholera toxin B (CTB), will bind receptors that are contained in these lipid-rich areas and internalize via a mechanism similar to caveolae-mediated endocytosis. The exact mechanism of CTB internalization remains unclear and difficult to distinguish from caveolin-1 assisted uptake. CTB uptake was unaffected by a clathrin inhibitor, while 33% uptake remains after treatment with a specific caveolae inhibitor. This data suggests that CTB is absorbed into cells via two different clathrin-independent mechanisms, where one does not contain specialized caveolae morphological structures [39]. From theses studies, the term lipid raft originated to explain flat areas of the plasma membrane that are highly enriched with the similar lipid constituents found in caveolae, including cholesterol, phospholipids, glycophospholipids, and sphingomyelin, etc. These lipid rich, detergent-resistant, areas of the membrane serve as a meeting place for interacting molecules destined to the same intracellular compartment [40]. A clear distinction between caveolae and lipid raft endocytic internalization cannot be made at this point. It is also important to note that certain types of raft receptors will migrate to clathrin-coated pits for invagination, leading to an alternative intracellular trafficking pattern.
Currently, CTB serves as a lipid raft marker, and has been utilized in targeted delivery of antigens through its conjugation to liposome carriers [41]. Coating macromolecules with lipid raft-associated ligands have allowed for cellular internalization and vesicular trafficking to non-lysosomal subcellular compartments, making this trafficking mechanism attractive to nondegradative intracellular drug delivery.
3. Diseases Targeted by Endocytically Delivered Therapeutics
Diseases occurring in endosomes and lysosomes have limited exposure to cellular traffic and are highly regulated at the molecular level. Macromolecular drug delivery systems may be targeted along the endolysosomal system to directly associate with diseases occurring in endosomes and lysosomes, such as Lysosomal Storage Disease (LSD), Alzheimer’s , and potentially cancer [2].
3.1 Lysosomal Storage Diseases
LSDs encompass a class of more than forty genetic disorders resulting from a single or multi enzyme deficiency. These enzymes are pertinent to the disassembly of macromolecules including lipids, carbohydrates, and proteins, following their endocytic internalization [42]. In the absence of biologically pertinent enzymes, toxic accumulation results from the build-up of undegraded protein complexes. Enzyme deficiencies present themselves as neurodegenerative disorders and are usually severe or life-threatening conditions [43]. Traditional treatments for LSDs include bone marrow transplantation, substrate inhibition, gene therapy, and enzyme replacement therapy (ERT), where ERT has been proven to be the most effective therapy. One limiting factor associated with traditional enzyme delivery is the rapid removal of these proteins from the bloodstream minimizing their bioavailability and reducing the amount of enzyme available to reach the target site [44]. Provoking membrane recognition and intracellular incorporation via the endolysosomal system increases lysosomal accumulation of targeted lysosomal enzyme complexes.
Understanding the cellular mechanisms that allow for site specific delivery have enabled the development of more successful mechanisms of treatment for lysosomally associated diseases. Clathrin-dependent RME is the major regulatory mechanism for cargo to this organelle. Receptors known to internalize in this manner are enable the binding and internalization of transferrin [17], riboflavin [45], and epidermal growth factor [46]. M6P containing residues are specifically recognized by carbohydrate receptors common to a variety of tissues, including the brain, which permits the transport of M6P targeted protein conjugates to the BBB [4]. Currently, only four enzyme replacement therapies exist in the clinical setting; therefore, targeting enzyme complexes to clathrin mediated RME internalization may offer greater success in the treatment of all forms of LSDs.
3.2 Neuronal Endolysosomal System in Alzheimer’s Disease
Endocytic processes of the neuronal system have been implicated in the pathophysiology of Alzheimer’s Disease (AD). Proteins related to this disease such as amyloid precursor protein (APP), β-amyloid protein (Aβ), and apolipoprotein E (ApoE), are internalized through the endolysosomal trafficking pathway. After entering the cell, these contents are sorted to either recycling endosomes, when sortilin-related receptor L(DLR class) (SORL1) is present, or late endosomes in the absence of SORL1. Aβ, a toxic compound that destroys neurons and regulates AD progression, results when APP is not sufficiently degraded in endosomes [47]. Recent studies have targeted macromolecular complexes including lysosomal cathepsins to the clathrin-dependent endocytic machinery containing upregulated amounts of endosomal Aβ to regulate apoptosis [48]. Also, nerve growth factor (NGF) is upregulated in cholinergic basal forebrain (CBF) neurons which are severely degraded in AD; therefore, the NGF serves a potential targeting ligand by its high affinity binding to two classes of cell surface receptors, TrkA and p75NTR, both of which are produced by these neurons [49]. Neuron specific proteins, including those mentioned here, are currently being investigated for site specific treatment of AD by targeting enzymes and drugs to the endosomes of diseased cells.
3.3 Can Cancer Be Modulated via Lysosomes?
Lysosomes function in post-translational maturation of proteins, degradation of macromolecules, and cellular release of enzymes; thereby, these organelles work as regulators of cellular homeostasis. When lysosomal functions are hampered, tissues would experience uncontrollable cell growth, improper regulation of cell death, and the development of chemoresistance [2]. In addition, inhibition of lysosomal enzymatic activity results in the buildup of undegraded molecules in the cytosol which induces apoptosis [50]. Consequently, lysosomes may have a pertinent role in cancer cell progression. Traditional small molecular weight neoplastic drugs are taken up by all rapidly dividing cell systems, including but not limited to cancer cells [51]. Gene therapy targeted specifically to cancer tissue limits the cytotoxicity and increases cancer cell accumulation of genes; however, lysosomal degradation of the genes prevents their nuclear localization. One approach to avoid lysosomal degradation is to preload lysosomes with photosensitizers prior to the endocytosis and lysosomal accumulation of bioactive macromolecules. Upon irradiation of the cells, the photosensitizer is activated resulting in a reactive byproduct which oxidizes the endosomal membrane releasing the therapeutic macromolecule into the cytosol to exert its effect [52]. Combination therapies such as this offer a less destructive intracellular environment for the transport of anticancer drugs.
4. Modalities of Macromolecular Therapeutic Delivery
A variety of macromolecular drug delivery systems utilizing endocytic internalization mechanisms have been developed to combat limitations associated with previously established therapies. These systems are very versatile as they are able to incorporate targeting molecules or ligands, imaging agents, and therapeutics moieties. Bioengineering macromolecules or modifying previously existing biomolecules to include targeting molecules enables cell-specific delivery of therapeutics. A variety of natural and synthetic macromolecules have been used as drug carriers, including protein-conjugates and polymer-based systems. Many ligands have been used to direct macromolecular therapeutics to the endolysosomal system of particular cell populations (Table 1). Degradative or nondegradative transport mechanisms, such as caveolar or clathrin-assisted RME, may serve as pathways for intracellular drug delivery.
4.1 Nondegradative Directed Systems
Endosomal trafficking following caveolae assisted uptake of macromolecules acts to avoid the abrasive environment of lysosomes. To achieve this, therapeutics are conjugated to macromolecular carriers which are targeted to caveolae-assisted endocytic mechanisms using ligands known to internalize in this manner. Drugs must be able to be released from the delivery system in a time dependent manner as endocytic internalization occurs rapidly. Bonds which are pH sensitive are typically used for quick release within endosomes. The pH sensitive hydrazone (HYD) bond allows for up to 43% release of doxorubicin from a polymeric carrier within 24 hours at the characteristic endosomal pH 5, unlike that amount released, 16%, at pH 7.4 in the blood [53]. Similarly, photochemical disruption of the endosomal membrane enables the cystolic release of internalized macromolecules improving activity both in-vitro and in-vivo [54].
4.2 Targeted Complexes for Lysosomal Delivery
Although most delivery systems are aimed at avoiding lysosomal localization, specific targeting to these organelles may benefit drug delivery strategies aimed at replacing deficient lysosomal enzymes. Clathrin- assisted RME selectively internalizes targeted complexes for distribution to endosomes and lysosomes. A variety of high affinity ligands are capable of navigating these complexes along this pathway (Table 1). TF has been extensively investigated for its ability to deliver chemotherapeutics [55], genes [56], and central nervous system (CNS) bound drugs [57] to lysosomes. Additionally, synthetic approaches such as terminal modification to protein, or enzyme, sequences with a M6P receptor recognized peptide sequence promotes lysosomal delivery for the treatment of lysosomal storage diseases [58]. Following accumulation in these organelles, bioactive molecules must be released in order to transverse the lysosomal membrane and travel to the cytosol and other organelles, such as the nucleus, to exert their effects. In the lysosomes, complexes act as substrates for a variety of acid hydrolases, each with specific amino acid sequences to which they actively bind and cleave. Macromolecular conjugates containing active enzymes sites have been designed to directly link drug molecules so that upon enzyme interaction they are released intact. For example, the tetrapeptide GlyPheLeuGly (GFLG) has served as a stable linkage between polymer carriers and drugs, where the enzyme cathepsin B is utilized to cleave and release active drug moieties at the terminal glycine [59]. Using this combination approach, the cellular recognition, internalization, and intracellular fate can be controlled to provide an efficient means of enzyme replacement.
5. Conclusions and Future Directions
The advances in cellular biology over the past decade have provided highly useful insight into the translocation and subcellular trafficking of macromolecules using endocytic pathways. Drug delivery scientists have capitalized on this knowledge by using receptors and ligands undergoing endocytosis as targeting moieties for specific cellular organelle targeting. However, our knowledge of endosomal regulation and vesicular trafficking is not complete, leading to several issues that remain to be addressed. For RME targeted systems, such as those used to deliver complexes to the endolysosomal system, two major issues exist. First, the cell membrane is so dynamic that receptor expression levels may constantly change. Differences in cellular protein expression levels are exemplified through the folate receptor, a popular target for directed drug delivery, where downregulation of the receptor can occur following the development of tolerance to methotrexate [60]. In addition, changes in membrane recycling patterns could greatly affect the number of receptors available for internalization of targeted complexes and their cargo [61]. Further insight into receptor regulation will lead to reduced experimental variability and stable therapeutic endpoints. One example of addressing variability in receptor expression is the use of artificial receptors, engineered to associate within clathrin-rich regions of the plasma membrane. This strategy enables control over variables such as membrane concentration and ligand affinities. This approach is termed synthetic receptor targeting (SRT) and currently adapted for high affinity binding of targeting molecules and clathrin-dependent uptake. SRT would allow for the cellular accumulation of membrane impermeable ligands and drugs. With our advancing knowledge of cell biology, other approaches will present themselves with additional understanding of the cellular mechanisms that govern internalization and intracellular trafficking.
Figure 2.
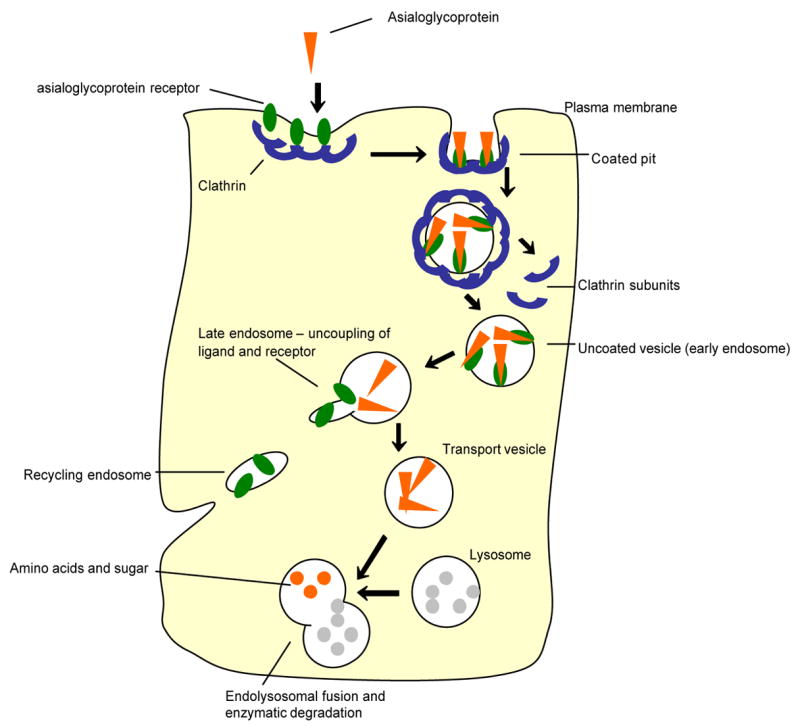
Representative fate of clathrin mediated RME of asialoglycoprotein (ASGP) and its receptor (ASGPr) upon endocytosis. After binding of ASGP to its receptor, the receptor-ligand complex is internalized in a clathrin-coated pit that pinches off to become a coated vesicle. The clathrin coat then depolymerizes to triskelions, resulting in an early endosome. This endosome fuses with a sorting vesicle (late endosome). Lowering of pH causes ASGP dissociation from ASGPr. A receptor-rich region buds off to form a separate vesicle that recycles multiple ASGPr back to the plasma membrane. ASGP-containing vesicles ultimately fuse with lysosomes, wherein it is degraded to amino acids and sugar [72].
Acknowledgments
This work was supported in part by a grant from the Susan G. Komen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juliano RL, Astriab-Fisher A, Falke D. Macromolecular therapeutics: emerging strategies for drug discovery in the postgenome era. Mol Interv. 2001;1:40–53. [PubMed] [Google Scholar]
- 2.Castino R, Demoz M, Isidoro C. Destination 'lysosome': a target organelle for tumour cell killing? J Mol Recognit. 2003;16:337–48. doi: 10.1002/jmr.643. [DOI] [PubMed] [Google Scholar]
- 3.Tate BA, Mathews PM. Targeting the role of the endosome in the pathophysiology of Alzheimer's disease: a strategy for treatment. Sci Aging Knowledge Environ. 2006;2006:re2. doi: 10.1126/sageke.2006.10.re2. [DOI] [PubMed] [Google Scholar]
- 4.Sly WS, Vogler C. Brain-directed gene therapy for lysosomal storage disease: going well beyond the blood-brain barrier. Proc Natl Acad Sci U S A. 2002;99:5760–2. doi: 10.1073/pnas.102175599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–93. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 6.Wong HL, Bendayan R, Rauth AM, Xue HY, Babakhanian K, Wu XY. A mechanistic study of enhanced doxorubicin uptake and retention in multidrug resistant breast cancer cells using a polymer-lipid hybrid nanoparticle system. J Pharmacol Exp Ther. 2006;317:1372–81. doi: 10.1124/jpet.106.101154. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96:1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qaddoumi MG, Gukasyan HJ, Davda J, Labhasetwar V, Kim KJ, Lee VH. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis. Mol Vis. 2003;9:559–68. [PubMed] [Google Scholar]
- 9.D'Souza VM, Bareford LM, Ray A, Swaan PW. Cytoskeletal scaffolds regulate riboflavin endocytosis and recycling in placental trophoblasts. J Nutr Biochem. 2006;17:821–9. doi: 10.1016/j.jnutbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J. Structure of the LDL receptor extracellular domain at endosomal pH. Science. 2002;298:2353–8. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
- 11.Diebold SS, Plank C, Cotten M, Wagner E, Zenke M. Mannose receptor-mediated gene delivery into antigen presenting dendritic cells. Somat Cell Mol Genet. 2002;27:65–74. doi: 10.1023/a:1022975705406. [DOI] [PubMed] [Google Scholar]
- 12.Biessen EA, Vietsch H, Rump ET, Fluiter K, Kuiper J, Bijsterbosch MK, van Berkel TJ. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem J. 1999;340( Pt 3):783–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–61. [PubMed] [Google Scholar]
- 14.Biragyn A, Ruffini PA, Coscia M, Harvey LK, Neelapu SS, Baskar S, Wang JM, Kwak LW. Chemokine receptor-mediated delivery directs self-tumor antigen efficiently into the class II processing pathway in vitro and induces protective immunity in vivo. Blood. 2004;104:1961–9. doi: 10.1182/blood-2004-02-0637. [DOI] [PubMed] [Google Scholar]
- 15.Foraker AB, Khantwal CM, Swaan PW. Current perspectives on the cellular uptake and trafficking of riboflavin. Adv Drug Deliv Rev. 2003;55:1467–83. doi: 10.1016/j.addr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Mishra V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K, Vyas SP. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Target. 2006;14:45–53. doi: 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- 17.Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–87. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 18.Rao PN, Levine E, Myers MO, Prakash V, Watson J, Stolier A, Kopicko JJ, Kissinger P, Raj SG, Raj MH. Elevation of serum riboflavin carrier protein in breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:985–90. [PubMed] [Google Scholar]
- 19.Dunehoo AL, Anderson M, Majumdar S, Kobayashi N, Berkland C, Siahaan TJ. Cell adhesion molecules for targeted drug delivery. J Pharm Sci. 2006;95:1856–72. doi: 10.1002/jps.20676. [DOI] [PubMed] [Google Scholar]
- 20.Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13:135–41. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 21.Muro S, Gajewski C, Koval M, Muzykantov VR. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood. 2005;105:650–8. doi: 10.1182/blood-2004-05-1714. [DOI] [PubMed] [Google Scholar]
- 22.Stromhaug PE, Berg TO, Gjoen T, Seglen PO. Differences between fluid-phase endocytosis (pinocytosis) and receptor-mediated endocytosis in isolated rat hepatocytes. Eur J Cell Biol. 1997;73:28–39. [PubMed] [Google Scholar]
- 23.Mitra A, Nan A, Ghandehari H, McNeill E, Mulholland J, Line BR. Technetium-99m-Labeled N-(2-hydroxypropyl) methacrylamide copolymers: synthesis, characterization, and in vivo biodistribution. Pharm Res. 2004;21:1153–9. doi: 10.1023/b:pham.0000033001.49737.b7. [DOI] [PubMed] [Google Scholar]
- 24.Persiani S, Shen WC. Increase of poly(L-lysine) uptake but not fluid phase endocytosis in neuraminidase pretreated Madin-Darby canine kidney (MDCK) cells. Life Sci. 1989;45:2605–10. doi: 10.1016/0024-3205(89)90245-2. [DOI] [PubMed] [Google Scholar]
- 25.Fujita T, Nishikawa M, Tamaki C, Takakura Y, Hashida M, Sezaki H. Targeted delivery of human recombinant superoxide dismutase by chemical modification with mono- and polysaccharide derivatives. J Pharmacol Exp Ther. 1992;263:971–8. [PubMed] [Google Scholar]
- 26.Wardman P. Indole-3-acetic acids and horseradish peroxidase: a new prodrug/enzyme combination for targeted cancer therapy. Curr Pharm Des. 2002;8:1363–74. doi: 10.2174/1381612023394610. [DOI] [PubMed] [Google Scholar]
- 27.Renau-Piqueras J, Miragall F, Cervera J. Endocytosis of cationized ferritin in human peripheral blood by resting T-lymphocytes. Cell Tissue Res. 1985;240:743–6. doi: 10.1007/BF00216363. [DOI] [PubMed] [Google Scholar]
- 28.Huang SN, Swaan PW. Involvement of a receptor-mediated component in cellular translocation of riboflavin. J Pharmacol Exp Ther. 2000;294:117–25. [PubMed] [Google Scholar]
- 29.Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7:661–71. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 30.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 31.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang WJ, Rothberg KG, Kamen BA, Anderson RG. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–9. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dauty E, Remy JS, Zuber G, Behr JP. Intracellular delivery of nanometric DNA particles via the folate receptor. Bioconjug Chem. 2002;13:831–9. doi: 10.1021/bc0255182. [DOI] [PubMed] [Google Scholar]
- 34.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev. 2001;49:265–80. doi: 10.1016/s0169-409x(01)00141-7. [DOI] [PubMed] [Google Scholar]
- 35.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J Control Release. 2003;89:365–74. doi: 10.1016/s0168-3659(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 36.Bathori G, Cervenak L, Karadi I. Caveolae–an alternative endocytotic pathway for targeted drug delivery. Crit Rev Ther Drug Carrier Syst. 2004;21:67–95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. [DOI] [PubMed] [Google Scholar]
- 37.Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–12. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- 38.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–8. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 39.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–15. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 41.Harokopakis E, Childers NK, Michalek SM, Zhang SS, Tomasi M. Conjugation of cholera toxin or its B subunit to liposomes for targeted delivery of antigens. J Immunol Methods. 1995;185:31–42. doi: 10.1016/0022-1759(95)00102-g. [DOI] [PubMed] [Google Scholar]
- 42.Kudo M, Bao M, D'Souza A, Ying F, Pan H, Roe BA, Canfield WM. The alpha- and beta-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J Biol Chem. 2005;280:36141–9. doi: 10.1074/jbc.M509008200. [DOI] [PubMed] [Google Scholar]
- 43.Kiselyov K, Jennigs JJ, Jr, Rbaibi Y, Chu CT. Autophagy, Mitochondria and Cell Death in Lysosomal Storage Diseases. Autophagy. 2007;3 doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, Pavloff N, Sly WS. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A. 2005;102:14777–82. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Souza VM, Foraker AB, Free RB, Ray A, Shapiro PS, Swaan PW. cAMP-Coupled riboflavin trafficking in placental trophoblasts: a dynamic and ordered process. Biochemistry. 2006;45:6095–104. doi: 10.1021/bi060138f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanover JA, Beguinot L, Willingham MC, Pastan IH. Transit of receptors for epidermal growth factor and transferrin through clathrin-coated pits. Analysis of the kinetics of receptor entry. J Biol Chem. 1985;260:15938–45. [PubMed] [Google Scholar]
- 47.Beyreuther K, Masters CL. Alzheimer's disease. The ins and outs of amyloid-beta. Nature. 1997;389:677–8. doi: 10.1038/39479. [DOI] [PubMed] [Google Scholar]
- 48.Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer's disease pathogenesis: a review. Neurochem Res. 2000;25:1161–72. doi: 10.1023/a:1007675508413. [DOI] [PubMed] [Google Scholar]
- 49.Lad SP, Neet KE, Mufson EJ. Nerve growth factor: structure, function and therapeutic implications for Alzheimer's disease. Curr Drug Targets CNS Neurol Disord. 2003;2:315–34. doi: 10.2174/1568007033482724. [DOI] [PubMed] [Google Scholar]
- 50.Castino R, Pace D, Demoz M, Gargiulo M, Ariatta C, Raiteri E, Isidoro C. Lysosomal proteases as potential targets for the induction of apoptotic cell death in human neuroblastomas. Int J Cancer. 2002;97:775–9. doi: 10.1002/ijc.10139. [DOI] [PubMed] [Google Scholar]
- 51.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111:923–5. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 52.Selbo PK, Hogset A, Prasmickaite L, Berg K. Photochemical internalisation: a novel drug delivery system. Tumour Biol. 2002;23:103–12. doi: 10.1159/000059713. [DOI] [PubMed] [Google Scholar]
- 53.Hruby M, Konak C, Ulbrich K. Polymeric micellar pH-sensitive drug delivery system for doxorubicin. J Control Release. 2005;103:137–48. doi: 10.1016/j.jconrel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Prasmickaite L, Hogset A, Selbo PK, Engesaeter BO, Hellum M, Berg K. Photochemical disruption of endocytic vesicles before delivery of drugs: a new strategy for cancer therapy. Br J Cancer. 2002;86:652–7. doi: 10.1038/sj.bjc.6600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim CJ, Shen WC. Transferrin-oligomers as potential carriers in anticancer drug delivery. Pharm Res. 2004;21:1985–92. doi: 10.1023/b:pham.0000048188.69785.94. [DOI] [PubMed] [Google Scholar]
- 56.Wagner E, Zenke M, Cotten M, Beug H, Birnstiel ML. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990;87:3410–4. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu D, Song BW, Vinters HV, Pardridge WM. Pharmacokinetics and brain uptake of biotinylated basic fibroblast growth factor conjugated to a blood-brain barrier drug delivery system. J Drug Target. 2002;10:239–45. doi: 10.1080/10611860290022679. [DOI] [PubMed] [Google Scholar]
- 58.Sato Y, Beutler E. Binding, internalization, and degradation of mannose-terminated glucocerebrosidase by macrophages. J Clin Invest. 1993;91:1909–17. doi: 10.1172/JCI116409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krinick NL, Sun Y, Joyner D, Spikes JD, Straight RC, Kopecek J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J Biomater Sci Polym Ed. 1994;5:303–24. doi: 10.1163/156856294x00040. [DOI] [PubMed] [Google Scholar]
- 60.Saikawa Y, Knight CB, Saikawa T, Page ST, Chabner BA, Elwood PC. Decreased expression of the human folate receptor mediates transport-defective methotrexate resistance in KB cells. J Biol Chem. 1993;268:5293–301. [PubMed] [Google Scholar]
- 61.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–14. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 62.Theti DS, Bavetsias V, Skelton LA, Titley J, Gibbs D, Jansen G, Jackman AL. Selective delivery of CB300638, a cyclopenta[g]quinazoline-based thymidylate synthase inhibitor into human tumor cell lines overexpressing the alpha-isoform of the folate receptor. Cancer Res. 2003;63:3612–8. [PubMed] [Google Scholar]
- 63.Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Muller JF, Barberi-Heyob M. Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorg Med Chem. 2005;13:2799–808. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Zheng G, Chen J, Li H, Glickson JD. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc Natl Acad Sci U S A. 2005;102:17757–62. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harokopakis E, Hajishengallis G, Michalek SM. Effectiveness of liposomes possessing surface-linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infect Immun. 1998;66:4299–304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greupink R, Bakker HI, Reker-Smit C, van Loenen-Weemaes AM, Kok RJ, Meijer DK, Beljaars L, Poelstra K. Studies on the targeted delivery of the antifibrogenic compound mycophenolic acid to the hepatic stellate cell. J Hepatol. 2005;43:884–92. doi: 10.1016/j.jhep.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Eavarone DA, Yu X, Bellamkonda RV. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J Biomed Mater Res. 2000;51:10–4. doi: 10.1002/(sici)1097-4636(200007)51:1<10::aid-jbm2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 68.Dufes C, Muller JM, Couet W, Olivier JC, Uchegbu IF, Schatzlein AG. Anticancer drug delivery with transferrin targeted polymeric chitosan vesicles. Pharm Res. 2004;21:101–7. doi: 10.1023/b:pham.0000012156.65125.01. [DOI] [PubMed] [Google Scholar]
- 69.Holladay SR, Yang Z, Kennedy MD, Leamon CP, Lee RJ, Jayamani M, Mason T, Low PS. Riboflavin-mediated delivery of a macromolecule into cultured human cells. Biochim Biophys Acta. 1999;1426:195–204. doi: 10.1016/s0304-4165(98)00147-0. [DOI] [PubMed] [Google Scholar]
- 70.Mitra A, Nan A, Line BR, Ghandehari H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr Pharm Des. 2006;12:4729–49. doi: 10.2174/138161206779026317. [DOI] [PubMed] [Google Scholar]
- 71.Quick G, van Zyl J, Hawtrey A, Ariatti M. Effect of nicotinic acid conjugated to DNA-transfecting complexes targeted at the transferrin receptor of HeLa cells. Drug Deliv. 2000;7:231–6. doi: 10.1080/107175400455164. [DOI] [PubMed] [Google Scholar]
- 72.Dalton SR, Wiegert RL, Casey CA. Receptor-mediated endocytosis by the asialoglycoprotein receptor: effect of ethanol administration on endosomal distribution of receptor and ligand. Liver Int. 2003;23:484–91. doi: 10.1111/j.1478-3231.2003.00874.x. [DOI] [PubMed] [Google Scholar]


