Several animal models have exhibited thresholds for petechial hemorrhage in lung within the current output of diagnostic ultrasound systems. In addition, thresholds for damage in the mouse intestine due to diagnostic pulses of ultrasound have been explored. The implications for human lung and intestinal exposure to clinical diagnostic ultrasound have not yet been determined. In this section, the data supporting the thresholds of petechial hemorrhage in these organ systems and the morphological observations will be reviewed. The potential mechanical mechanisms of damage to these organs due to diagnostic ultrasound also will be reviewed. Special attention will be given to the occurrence of inertial cavitation both in vitro and in vivo. The effects of ultrasound parameters, age, and species on the threshold for damage in animal models will be explored.
4.1 Mechanical Mechanisms of Damage
Although no adverse effects on patients exposed to acoustic output levels typical of currently available diagnostic ultrasound systems have been established to date, several animal models have exhibited a threshold for petechial hemorrhage in lung and intestine. The exact mechanical mechanism that induces this damage in biological tissues has not yet been identified. It appears that gas bodies, either macroscopic or microscopic bubbles, are required in the tissue to elicit the effect (Hartman et al, 1990). It is useful to consider the various physical mechanisms that might lead to tissue damage at the lung surface. Inertial cavitation has been discussed as a possible damage mechanism, though other acousto-mechanical effects that act directly on the lung surface could be important. For a discussion of acoustic cavitation and its definition, see Section 2.
For cavitation to play a role in tissue damage, it is important that an isolated gas bubble or nucleation site be available to undergo the energy concentration process, i.e., the bubble growth and implosive collapse, that enables cavitation to induce mechanical damage. However, it seems logical to expect that if some damage to the lung’s surface, or visceral pleura, is induced, and this area is continually insonated with ultrasound, some small pockets of gas would be broken away from the lung parenchyma and distributed into the surrounding pleural fluid, where they could act as cavitation nuclei. If cavitation were to occur in the pleural fluid, then the strong mechanical forces associated with cavitation could induce interfacial tissue damage in the visceral pleura.
In the animal studies demonstrating lung damage from exposure to pulses of diagnostic ultrasound, which will be described below, cavitation could be taking place in the gas-rich environment of the capillaries adjacent to the alveoli. The conditions under which bubbles may develop are not well known, although it has been shown that when gas bodies are present they may be driven into oscillation by an acoustic field, with the potential to produce local stresses on neighboring cells (Flynn, 1964; Apfel, 1982; Vivino et al, 1985; MW Miller et al, 1996; Everbach et al, 1997). In addition, the damage mechanism might involve effects associated with reflection of ultrasound energy at the alveolar epithelial cell membrane interface, which can be considered as an acoustic pressure release surface (Holland et al, 1994). Local reflection of the ultrasound pulses could cause the waveform to strengthen or weaken locally. Relative displacement, or shearing, of the air-tissue interface could also lead to mechanical disruption and damage (Howard and Sturtevant, 1997).
Another plausible mechanism for lung damage is a variation of the well-known spallation effect (Chaussy et al, 1980; Chaussy, 1982; Delius et al, 1988a). When a shock wave strikes the surface of a pressure release interface, such as between water and air, there is a rapid ejection of liquid into the lower density space (the air). This effect occurs because the compressive portion of the sound field cannot be sustained when it strikes the pressure release interface. The rapid release of the pressure into the less dense medium (especially if it is air) can result in large velocities of the interface. Thus, this “spallation” effect could result in mechanical damage to the lung surface, or visceral pleura. Related to spallation is the parametric excitation of surface waves, which can occur over a wide frequency range and at lower amplitudes (Eisenmenger, 1959; Brand and Nyborg, 1965). Ultrasonic humidifiers operate by parametric excitation of surface waves that form jets and droplets (Hansen, 1970). As subsequent acoustic pulses continue to strike a particular location, there would be a gradual fatiguing that would result in damage that is dependent on the strength of the tissues lining that interface. Indeed, the different responses for small animals, which have thin pleura (perhaps weak interfacial tissue), and large animals, which have thick pleura (presumably stronger interfacial tissue), could be explained by this mechanism.
4.2 Evidence of Cavitation In Vivo From Lithotripters
It is possible to generate bubbles in vivo using the short-duration, high-amplitude acoustic pulses employed by extracorporeal shock wave lithotripters (ESWL). The peak positive pressure for lithotripsy pulses can be as much as 100 MPa and the negative pressure around 20 MPa. Finite amplitude distortion causes high frequencies to appear in high-amplitude diagnostic ultrasound fields. Although ESWL pulses have significant energy at high frequencies, a large portion of the energy is actually in the 100 kHz frequency range, much lower than frequencies in diagnostic scanners. The lower frequency makes cavitation more likely. There is evidence to indicate that collapsing bubbles (such as the one shown in Fig. 4-1) may play a role in stone disruption (Coleman et al, 1987; Delius et al, 1988a; Williams et al, 1989; Vakil and Everbach, 1993; Stonehill et al, 1998). When cavitation due to application of ESWL occurs next to a solid surface, a high-velocity liquid jet may form during collapse generating a sufficient impulse to produce observable damage on metal surfaces (Coleman et al, 1987). The impact is sufficient to pit even solid brass and aluminum plates (Fig. 4-2). Clearly, lithotripsy and diagnostic ultrasound differ in the acoustic power generated and are not comparable in the bioeffects produced. Yet some diagnostic devices produce peak rarefactional pressures greater than 3 MPa, which is in the lower range of lithotripter outputs (Duck et al, 1985, 1987). Interestingly, lung damage and surface petechiae have been noted as side effects of ESWL in clinical cases (Chaussy, 1982). Inertial cavitation was suspected as the cause of this damage, and these results prompted several researchers to study the effects of diagnostic ultrasound exposure on lung parenchyma.
Figure 4-1.

Collapsing bubble near a boundary. When cavitation is produced near boundaries, a liquid jet may form through the center of a bubble and strike the boundary surface. (Photo courtesy of Lawrence A. Crum, PhD.)
Figure 4-2.

Pitting caused by cavitation on a brass plate due to exposure to a lithotripter. (Photo courtesy of Lawrence A. Crum, PhD.)
4.3 Ultrasound-Induced Effects on Lung
Lung has proven to be an important tissue to investigate the bioeffects from diagnostic ultrasound. The published literature on the topic is summarized in Table 4-1. The presence of air in the alveolar spaces constitutes a significant source of gas bodies. Child et al (1990) measured threshold pressures for hemorrhage in mouse lung exposed to 1 MHz to 4 MHz short-pulse diagnostic ultrasound (i.e., 1 to 10 μs pulse durations). The threshold of damage in murine lung at 4 MHz was established to be 1.4 MPa peak rarefactional pressure, which is below the currently allowed peak output of clinical ultrasound scanners.
Table 4-1.
Summary of Publications on Ultrasound-Induced Lung Damage at Diagnostic Frequencies
| Species | Frequency [MHz] | pr† [MPa] | Derating scheme (dB) | Pulse Duration [μs] | PRF [Hz] | Exposure Duration [s] | Total On-Time [ms] | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Suprathreshold Observations | |||||||||
| Adult mouse | 6.0 | 3.0 | 0.4* | 1.2 | 1 | 1,250 | 300 | 375 | O’Brien and Zachary (1997) |
| 5.6 | 2.2 | ||||||||
| Adult mouse | 1.1 | 0.8 | 1.5 | 0.6 | 10 | 100 | 180 | 180 | Penney et al (1993)§ |
| Adult rabbit | 3.0 | 2.7–3.8 | 1.3* | 1.3–1.9 | 1 | 1,250 | 300 | 375 | O’Brien and Zachary (1997) |
| 6.0 | 2.6–6.6 | 1.8–2.2* | 0.8–2.2 | ||||||
| Adult pig | 2.0 | 3.3 | 3.1 | 1.6 | 4.0 | 2,000 | 600 | 4,800 | Harrison et al (1995) |
| Adult monkey | 4.0 | 4.2 | 1.7* | 1.6 | 0.7 | 1,515 | 300 | 295 | Tarantal and Canfield (1994) |
| 4.5 | 1.9 | ||||||||
| Threshold Observations | |||||||||
| Neonate mouse (Bath temperature = 10°C) | 1.0 | 0.4 | 0.0 | 0.4 | 10 | 100 | 180 | 164 | Frizzell et al (1994) |
| 1.5 | 1.5 | 1,000 | 2.4 | 22 | |||||
| Neonate mouse (Bath temperature = 37°C) | 1.2 | 0.6 | 0.0 | 0.6 | 10 | 100 | 180 | 180 | Dalecki et al (1997a) |
| Juvenile mouse | 1.2 | 0.9 | 0.4 | 0.8 | 10 | 100 | 180 | 180 | Dalecki et al (1997a) |
| Adult mouse | 1.2 | 0.8 | 1.5 | 0.7 | 10 | 100 | 180 | 180 | Dalecki et al (1997a) |
| Adult mouse | 1.1 | 0.5 | 1.5 | 0.4 | 10 | 100 | 180 | 180 | Child et al (1990) |
| 1.2 | 0.8 | 1.5 | 0.6 | 10 | 100 | 180 | 180 | ||
| 1.2 | 0.8 | 1.5 | 0.6 | 10 | 10 | 180 | 18 | ||
| 2.3 | 0.8 | 2.0 | 0.4 | 10 | 100 | 180 | 180 | ||
| 3.5 | 1.7 | 2.5 | 0.7 | 10 | 100 | 180 | 180 | ||
| 3.7 | 1.4 | 2.6 | 0.5 | 10 | 100 | 180 | 180 | ||
| 3.7 | 1.9 | 2.6 | 0.7 | 1 | 1,000 | 180 | 180 | ||
| Adult mouse | 1.1 | 1.1 | 1.9 | 0.8 | 10 | 17 | 180 | 30 | Raeman et al (1993)§ |
| 1,000 | 3 | 30 | |||||||
| Adult mouse | 2.3 | 1.0 | 1.5 | 0.6 | 10 | 100 | 20 | 20 | Raeman et al (1996) |
| 0.9 | 0.5 | 10 | 100 | 180 | 180 | ||||
| Adult rat | 4.0 | 2.5 | 1.1* | 1.1 | 1.0 | 400 | 90 | 36 | Holland et al (1996) |
| 2.0 | 0.9* | 0.9 | 1.0 | 1,250 | 90 | 113 | |||
| Neonate pig | 2.3 | 1.1 | 2.0 | 0.6 | 10 | 100 | 960 | 960 | Baggs et al (1996)§ |
| Young pig | 2.3 | 1.3 | 4.0 | 0.5 | 10 | 100 | 2,160 | 2,160 | Dalecki et al (1997c) |
| Observations of No Damage Attributable to Ultrasound | |||||||||
| Neonate mouse (Bath temperature = 10°C) | 1.2 | 0.5–2.0 | 0 dB | 0.5–1.9 | 10 | 100 | 180 | 180 | Dalecki et al (1997a) |
| Adult pig | 3.0 | 4.1 | 2.0* | 1.9 | 1 | 1,250 | 300 | 375 | O’Brien and Zachary (1997) |
| 6.0 | 8.0–8.3 | 3.4* | 2.2–2.3 | ||||||
Assumed 0.3 dB/cm-MHz deration scheme.
Those studies that reported in situ values only were extrapolated to obtain water values based on the deration scheme reported.
The authors were contacted in order to supply some of the data that did not appear in the publication.
Pathological features of this damage included extravasation of blood cells into the alveolar spaces (Penney et al, 1993). Findings suggested that the damage evoked by ultrasound included effects on the microvasculature, or capillaries, only. It was hypothesized that cavitation, originating from gas-filled alveoli, was responsible for the damage. Their data were the first to provide direct evidence that clinically relevant pulsed ultrasound exposures produce deleterious effects in mammalian tissue in the absence of significant heating. Hemorrhagic foci induced by 4 MHz pulsed Doppler ultrasound exposure have also been reported in the monkey (Tarantal and Canfield, 1994). Damage in the monkey lung was of a significantly lesser degree than that in the mouse. Subsequently, thresholds for lung damage from pulsed diagnostic ultrasound have been assessed in neonatal mice (Frizzell et al, 1994; Dalecki et al, 1997a), neonatal pig (Baggs et al, 1996), rat (Holland et al, 1996), rabbit and pig (Zachary and O’Brien, 1995; Dalecki et al, 1997c).
The photos in Figures 4-3 through 4-6 demonstrate typical hemorrhagic lesions produced by exposure to Doppler ultrasound on the surface of rat and monkey lung. The threshold of ultrasound-induced lung damage is plotted as a function of frequency for the mouse, rat, and pig in Figure 4-7. Note that individual investigators reporting these thresholds (Table 4-1) have utilized different definitions for the onset of damage and have employed different statistical treatments of their data. These threshold data were fit to a model of the form,
Figure 4-3.

Arrow marks typical hemorrhagic lesion produced by a 1.5 min exposure to 4 MHz pulsed Doppler ultrasound on the surface of rat lung. The pulse duration was 1.0 μs, and the in situ exposure level was 3.2 MPa, corresponding to an MI of 1.6. (Reprinted by permission of Elsevier Science, from Holland CK, et al: Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med Biol 22:917, 1996.)
Figure 4-6.

Histological appearance of lung tissue from bonnet macaque exposed to 4 MHz pulsed diagnostic ultrasound. Note intra-alveolar hemorrhage (arrowheads). Bar scale = 0.2 mm. (Reprinted by permission of Elsevier Science, from Tarantal AF, Canfield DR: Ultrasound-induced lung hemorrhage in the monkey. Ultrasound Med Biol 20:65, 1994.)
Figure 4-7.

Threshold of ultrasound-induced damage in mouse, rat, and pig lung as a function of center frequency.
| (4-1) |
where Pr is the derated rarefactional threshold pressure, f is the center frequency of the insonifying pulse, and m1 and m2 are constants.
The least squares fit is also plotted in Figure 4-7. The least squares values for m1 and m2 are 0.63 and 0.54, respectively. Alternatively, a linear regression analysis was performed on log(Pr) versus log(f). The slope of the linear regression is equal to m2 in equation 4–1. The significance of this regression analysis was P = 0.003 with R = 0.611.
In all of these animal systems, pockets of gas have been proposed to be involved in the production of ultrasonic tissue injury. The majority of this experimental work was conducted with commercial diagnostic ultrasound equipment. In these studies, it was impossible to show categorically that these effects were induced by bubbles because the cavitation-induced bubbles were not observed. Further studies are required to determine the relevance of these findings to the human. In particular, the questions of paramount clinical significance are (1) Does diagnostic ultrasound produce lung damage in humans and, if so, (2) what are the clinical or physiological implications of such damage, and (3) under what exposure conditions does the damage become significant?
4.3.1 Pulse Repetition Frequency and Total Exposure Effects
There is conflicting evidence regarding the effect of pulse repetition frequency (PRF) and total exposure duration (on-time plus quiescent period) on the threshold of lung damage. The exposure conditions for lung hemorrhage in mouse neonates were determined at 10°C with pulsed ultrasound of 10 μs pulse duration (Frizzell et al, 1994). Specimens were sonicated for 2.4 s at a PRF of 1 kHz and for 180 s at a PRF of 100 Hz. The percentage of specimens exhibiting hemorrhage was determined versus ISPPA, based on a linear extrapolation. The data were fit to a second order polynomial using least squares analysis. Extrapolation of this fit to the value for percentage hemorrhage observed for the sham sonicated specimens (approximately 32%) gave the threshold values for ISPPA of 95 and 4 W/cm2 for the 2.4 and 180 s exposure durations, respectively. The corresponding values for the peak rarefactional pressure were 1.5 and 0.37 MPa, respectively. The results showed that the threshold exposure conditions for lung hemorrhage are much lower than the conditions for cavitational or other effects reported for tissues that do not contain well-defined gas bodies, confirming the results of Child et al (1990). In addition, the results showed an inverse relation between exposure level and either exposure duration or sound on-time for equivalent damage, suggesting that time is an important parameter associated with lung damage.
In contrast, several studies suggest that the total ultrasound on-time and the exposure duration only weakly influence the threshold for lung hemorrhage (Raeman et al, 1993, 1996). For a constant total on-time of 0.03 s of ultrasound, the damage threshold for a 3 min exposure period (10 μs pulse duration, 17 Hz PRF) was approximately equal to the damage threshold for a 3 s exposure (10 μs pulse duration, 1 kHz PRF) (Raeman et al, 1993). Also, no statistically significant difference in the threshold for murine lung hemorrhage was found at 2.3 MHz (10 μs pulse duration, 100 Hz PRF) for a 20 s exposure duration (on-time of 0.02 s) or 3 min exposure duration (on-time of 0.18 s) (Raeman et al, 1996). Raeman et al (1996) assert that the threshold for lung hemorrhage is relatively insensitive to total exposure time even though the extent and degree of suprathreshold damage increases with time. It is these shorter exposure durations and sound on-times that may be most relevant clinically.
4.3.2 Species-Dependent Effects Due to Pulsed Ultrasound Exposure
There is conflicting evidence regarding the effect of species on the lung damage threshold due to pulsed ultrasound exposure. In a series of studies, the thresholds for lung hemorrhage in mice (Child et al, 1990; Dalecki et al, 1997a) and in swine (Baggs et al, 1996; Dalecki et al, 1997c) have been determined. These authors found that the threshold for lung hemorrhage is not dependent on the species. The extent of the damage relative to the size of the lung, however, was found to be dependent on species. Whereas a suprathreshold exposure in a mouse may damage a large percentage of the lung, the same exposure would produce a localized lesion in the pig lung.
In contrast, the lungs of six adult pigs were exposed to the output of a clinical diagnostic ultrasound scanner with 3 and 6 MHz center frequencies at MI exposure levels between 1.9 and 2.3 in another study (O’Brien and Zachary, 1997). These in situ MI values at the pleural surface were estimated by applying a 1 dB/cm-MHz deration to peak rarefactional pressures measured in water (corresponding to 1.8 to 2.2 MPa). Some of the exposure levels utilized in this study exceeded the current limit allowable by the Food and Drug Administration (FDA) for clinical examinations. None of the six pig lungs exhibited any observable damage. Forty-seven adult male rabbits and three mice were also exposed to pulsed ultrasound with 3 and 6 MHz center frequencies over a range of exposure levels. As in the pig, the MI values at the pleural surface of the rabbit were estimated by applying a 1 dB/cm-MHz deration to peak rarefactional pressures measured in water (corresponding to 1.0 to 3.4 MPa). Output levels that clearly produced lung damage in rabbits did not have an adverse effect on pig lungs. Likewise, the three mouse exposures produced greater lung damage than comparable rabbit exposures. Thus, a species-dependent effect was observed in these pulsed ultrasound studies.
4.3.3 Age-Dependent Effects
Results of studies with nonhuman primates (Tarantal and Canfield, 1994) suggested that animals ranging in age from 3 months to 5 years (mean age of 2.5 yr) had a greater propensity for the occurrence of multiple, well-demarcated, circular hemorrhagic foci (0.1–1.0 cm) after exposure to a clinical scanner under maximum output (5 MHz scanned imaging pulses interleaved with 4 MHz pulsed and color Doppler at an MI of 1.8), as compared with older animals (up to 16 yr). These authors proposed that neonates and infants were most likely more susceptible to the effects of exposure because of age-dependent features. However, it was not possible to produce lung damage in 10 to 12 week old crossbred pigs even at MI values as high as 2.1 (O’Brien and Zachary, 1997).
Studies of neonatal swine (Baggs et al, 1996) and of young swine (Dalecki et al, 1997c) indicate that the threshold for lung hemorrhage is not dependent on age within a time frame of 10 d. A similar study in the mouse (Dalecki et al, 1997a) also suggested an age-independent lung-damage threshold for animals ranging in age from 24 hr to 10 wk. But at suprathreshold exposure levels, these researchers observed a greater extent of damage in older animals compared with the younger mice. These findings may be related to the size of the experimental animals rather than age. Note that in young mice, age and size are correlated.
Due to the developmental state of human infants born prematurely, it is possible that certain structural features might make them more susceptible to ultrasound-induced bioeffects. These features include the relative thickness of skin, muscle, fat, connective tissue, and pleura, as well as overall lung maturity, which could increase the risk of lung damage by ultrasound. This is a reasonable concern because ultrasound imaging is frequently used for cardiac applications (echocardiography) in order to diagnose complications of prematurity, such as patent ductus arteriosus and pulmonary artery hypertension. Further data are needed in relevant animal models to address this important issue.
4.3.4 Morphological Observations
Morphological studies describing the microscopic lesions induced by pulsed ultrasound in lung tissue have been documented in monkeys (Tarantal and Canfield, 1994), mice (Penney et al, 1993; Zachary and O’Brien, 1995), rabbits (Zachary and O’Brien, 1995), and in neonatal pigs (Baggs et al, 1996). Studies using juvenile to adult pigs demonstrated that pulmonary hemorrhage could not be induced following exposure to pulsed ultrasound at MI levels as high as 2.1 (Zachary and O’Brien, 1995). In all affected species, lesions were similar and characterized by alveolar hemorrhage and congestion of alveolar capillaries. Lesions in mice and rabbits, and generally in monkeys, were contiguous with the visceral pleural surface of the lung and thus appeared to arise at this site and spread centrally into lung parenchyma (Tarantal and Canfield, 1994; Zachary and O’Brien, 1995). Morphological evaluations of affected lung tissue indicate that pulmonary hemorrhage does not appear to arise from damage to arterioles, venules, respiratory bronchioles, or distal airways. An ultrastructural evaluation by Penney et al (1993) suggested that there was a direct effect of pulsed ultrasound on capillary endothelial and alveolar epithelial cells that was lytic in nature; however, the site of vascular damage could not be identified. Overall, evaluation of both light and electron microscopic studies suggested that lung lesions appeared to originate from the microvascular system located within alveolar septa at the level of the blood-gas barrier and that further investigation was warranted.
The gross organization and cellular composition of the lung is similar in mammals, although there are significant physiological and anatomical differences related to organization of the distal airways, alveolar morphology, and blood supply (Tyler and Julian, 1992). Although the numbers of alveoli within the lung acinus (tissue distal to the terminal bronchioles) are relatively constant among species, there is a distinct difference in the structure and arrangement of the bronchioles supplying the gas-exchange area including the type of terminal bronchioles and extent of smooth muscle incorporated along the length of the alveolar ducts (Mercer and Crapo, 1992). For human and nonhuman primates, smooth muscle fibers extend along the terminal generations of the alveolar ducts, whereas in the mouse, these fibers do not extend past the bronchiole-alveolar duct junction. This finding may suggest that there is a greater potential for hemorrhage and tissue destruction in the mouse lung, particularly where the terminal airways are shorter, thinner, and perhaps less distensible (Tarantal and Canfield, 1994).
Morphological studies demonstrate that capillaries within the alveolar septa of most mammals are arranged as a single layer separated from the air spaces by a thin cellular barrier. The thickness of the alveolar epithelium plus the capillary endothelium is about 100 nm. It is plausible that these regions are more susceptible to conditions under which bubble oscillation and rupture may occur, with a greater degree of damage possible when lung tissues have less ability to expand. Due to this anatomical configuration, Tarantal and Canfield (1994) hypothesized that these regions are more susceptible to conditions under which bubble oscillation and rupture may occur. These authors also noted that an important factor specific to the lung may be the monolayer of surfactant within the alveoli. Surfactant, the fluid lining the alveoli, is responsible for modifying surface tension in order to promote lung expansion and prevent lung collapse (Hawgood and Shiffer, 1991). It is possible that, during exposure to ultrasound, small microbubbles are created within the edges of the surfactant-rich alveolus (location of Type II alveolar epithelial cells). These microbubbles may oscillate and collapse, causing localized disruption of the epithelial-endothelial barrier and subsequent extravasation of red blood cells into the alveolar space. Holland and Apfel (1989; 1990) have previously shown a direct correlation between a reduction in the cavitation threshold and reduced host fluid surface tension. Surfactant therapy administered to premature infants with respiratory distress syndrome could potentially exacerbate cavitation-mediated damage in lungs if they were exposed to high levels of ultrasound (Tarantal, unpublished). Therefore the effect of such exogenous surfactants on ultrasound-induced lung damage is of particular importance.
Physiological properties of lung of various animal species are shown in Section 3, Table 3–1. The critical structural features that can be used to separate species into distinct groups, shown in Section 3, Table 3–2, are pleural thickness (thin versus thick), pleural blood supply (pulmonary artery versus bronchial artery), septal or interlobular connective tissue (scant versus abundant), and relative thickness of the chest wall (thin or thick), which refers to the relative amounts of skin, muscle, fat, and connective tissue. There are significant interspecies structural differences of the lungs, even when lungs and associated structures are evaluated at a sub-gross level (Plopper and Pinkerton, 1992). While all common mammalian species have parietal and visceral pleural surfaces, the thickness, vascularity, and organization of the mesothelium, submesothelial connective tissue, and vascular system forming, in particular, the visceral pleura, vary among species, as do the extent and distribution of secondary septation with the lung and the distribution of connective tissue elements (Tyler and Julian, 1992). In addition, the source of arterial blood to the visceral pleura varies among species (shown in Section 3, Table 3–2), and this distribution is linked to the thinness or thickness of the visceral pleura. On the basis of these comparisons, human beings clearly can be grouped with pigs (the “thick” group). Therefore, pigs are an important animal model for assessing potential lung injury in human beings in order to understand the pathogenesis of ultrasound-induced lung damage and hemorrhage, as well as the physiological implications. The group that possesses a “thin” visceral pleura includes rats and rabbits. The animals in the “thin” group serve as a critical structural biological control group to test the hypothesis that structural differences are important components when considering the potential for damage in humans. More research is necessary to establish a correlation of the thinness of the visceral pleura and the potential for lung damage.
4.3.5 MI versus Intensity as a Better Indicator of Mechanical Bioeffect Risk
O’Brien and Zachary (1997) compared the performance of the MI (Apfel and Holland, 1991; AIUM/NEMA, 1992b) to the derated spatial peak, pulse average intensity (ISPPA.3) in tracking ultrasound-induced lung hemorrhage in a rabbit model. The principal consideration of the study was to assess experimentally the question: “Is the MI an equivalent or better indicator of nonthermal bioeffect risk than ISPPA.3?” This question was motivated by the adoption of the MI in place of ISPPA.3 as the acoustic output quantity regulated by the FDA (1994). Currently, the maximum allowed limit for the MI is 1.9. Forty-seven adult New Zealand white male rabbits were exposed to a range of ultrasound amplitudes at center frequencies of 3 and 6 MHz for 5 min. A calibrated, commercial diagnostic ultrasound system was used as the source with output levels exceeding, in some cases, permissible FDA levels. The MI was shown to be at least an equivalent, and in some cases a better, indicator of rabbit lung damage than either the ISPPA.3 or derated peak rarefactional pressure. The assessment of the correlation of MI with a bioeffect such as lung hemorrhage is needed over a greater frequency range (e.g., 2 to 10 MHz) to determine clinical relevance.
4.3.6 Detection of Inertial Cavitation In Vivo
The high amplitude shock waves employed in ESWL can generate cavitation in animal tissues (Delius et al, 1990; Coleman et al, 1992; Huber et al 1994). The physical evidence that cavitation also occurs in human tissue during routine clinical ESWL comes from the appearance of well-defined hyperechoic regions in ultrasound images during therapy that are attributed to scatter from newly generated bubbles. In addition, Coleman et al (1996) have employed a 1 MHz, focused piezo-ceramic hydrophone, first described by Coakley (1970), to monitor the 1 MHz component of the broadband acoustic emission that accompanies bubble dynamics induced by ESWL. This evidence indicates that the acoustic emission arises from inertial cavitation in human tissue during clinical application of ESWL.
Although a proven phenomenon in vitro (Holland et al, 1992), the occurrence of cavitation in vivo due to diagnostic ultrasound has been difficult to document in mammalian systems primarily due to the transient nature of its occurrence (i.e., on a μs time scale) and the localized character of the resultant effects (i.e., < 10 μm). To explore the hypothesis of cavitation-based bioeffects from diagnostic ultrasound, Holland et al (1996) determined the thresholds of damage in rat lungs exposed to 4.0 MHz pulsed Doppler and color Doppler ultrasound. In this study, a 30 MHz acoustic detection scheme developed by Roy et al (1990) was employed to provide the first direct evidence of cavitation from diagnostic ultrasound pulses. Damage was observed with histological features consistent with those seen in mice and monkeys due to diagnostic ultrasound exposures. However, in this limited study, bubble activity was not correlated with histological damage. The question remains: Does cavitation, if produced by diagnostic ultrasound in vivo, cause significant biological effects?
4.3.7 In Vivo Effects in Mice Exposed to 30 kHz Continuous Wave Ultrasound
Studies have been performed under continuous-wave (CW) conditions at 30 kHz using mice (O’Brien and Zachary, 1994b) with the goal of assessing the frequency dependence of the threshold pressure levels as compared with those reported under pulsed wave (PW) conditions at 1 to 4 MHz by Child et al (1990). The acoustic pressure threshold levels necessary to produce mouse lung damage in vivo were evaluated to assess the safety of a commercial, whole-body ultrasound cleaner for geriatric patients in a bathtub environment. The same mouse strain and anesthetic agents were used in both experimental protocols. The photos in Figures 4-8 through 4-10 demonstrate the typical features of a sham-exposed control and CW ultrasound-induced lung damage in the mouse with comparative histological sections.
Figure 4-8.

Sham-exposed control mouse lung exhibiting no lesions (fixed tissue). Left: dorsal-ventral view. Right: ventral-dorsal view. (Photo courtesy of James F. Zachary, DVM, PhD.)
Figure 4-10.
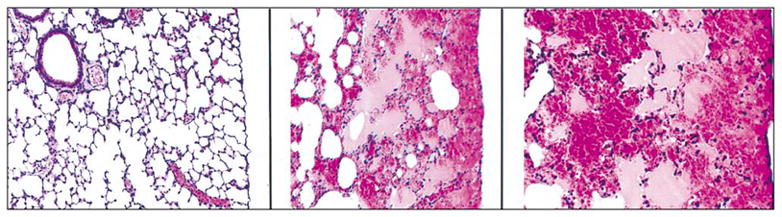
Left: Sham exposed control mouse lung (low magnification) exhibiting no lesions (H&E stain). Center: Mouse lung exposed to 30 kHz, CW ultrasound for 5 min (low magnification). Alveoli are contiguous with the visceral pleura and are filled with blood. The hemorrhage spreads to a finite limit into adjacent lung parenchyma (H&E stain). Right: CW exposed mouse lung (high magnification) exhibiting alveolar hemorrhage (H&E stain). The visceral pleura and contiguous alveolar septa are intact. (Photo courtesy of James F. Zachary, DVM, PhD.)
Linear regression analysis of lung damage scores versus exposure acoustic pressure levels yielded highly significant regressions with slightly increasing slopes and non-zero intercepts as exposure duration increased. The acoustic pressure threshold for lung damage was between 80 and 100 kPa at 30 kHz, or values between 0.46 and 0.58, where p is the acoustic pressure threshold in MPa and f is the ultrasound frequency in MHz (O’Brien and Zachary, 1994b). It is important to note that this quantity is similar to the MI, which is equal to , where pr.3 is the peak rarefactional pressure that has been derated by 0.3 dB cm−1MHz−1 at the location of the maximum pulse intensity integral, and f is the center frequency in MHz. The results of Child et al (1990) from experiments conducted between 1.1 and 3.7 MHz yielded values between 0.4 and 0.7, thus suggesting a frequency dependency for ultrasound-induced lung damage for the mouse.
Lung damage produced by 30 kHz CW ultrasound did not appear to be a strong function of exposure duration (O’Brien and Zachary, 1994b). In general, these findings showed that the mechanism leading to hemorrhage occurred early (within the first 5 min) in the exposure period. As sonication progressed, the degree of lung damage only increased slightly. The mechanisms responsible for the damage from the 30 kHz CW ultrasound and 1–4 MHz pulsed diagnostic ultrasound could be different. Further research is necessary to determine the mechanism(s) of ultrasound-induced damage in lung in animals exposed to either pulsed or CW ultrasound. Therefore, caution should be made when interpreting the 30 kHz results within the pulsed diagnostic ultrasound context.
4.3.8 Species-Dependent Effects Due to 30 kHz CW Exposures
Lung damage in mice, rabbits, and pigs was studied at 30 kHz under CW conditions (O’Brien and Zachary, 1994a, 1994b, 1996). Suprathreshold exposure levels were utilized to compare the extent of hemorrhage in pigs and rabbits to the mouse. The study design did not determine threshold levels. O’Brien and Zachary (1994a) also evaluated the hypothesis that the mouse may not be a suitable animal model for studies that examine ultrasound-induced effects on lung tissue for purposes of extrapolating or estimating the degree of potential damage in other large mammalian species, particularly the human. To test this hypothesis, the rabbit was selected for comparison to the mouse because the rabbit exhibits sufficient physiological and morphological differences from the mouse. Extensive lung hemorrhage and death occurred in the mouse at 100 and 145 kPa, whereas considerably less hemorrhage was noted for the rabbit and even less hemorrhage for the pig. Neither rabbits nor pigs were killed due to lung injury following exposure of the lung to ultrasound at similar exposure levels.
A clear difference between mouse and rabbit sensitivities to lung damage at each of the exposure pressure levels was observed, with the rabbit being much less sensitive to damage than the mouse (O’Brien and Zachary, 1994a). In this study, the lung damage score, which increased with the amount of lung damage, was plotted against the insonifying peak rarefactional pressure. Linear regression analyses of the lung damage scores versus acoustic pressure exposure levels for the two species were also performed. Both regressions were highly significant (P < 0.0001 and P = 0.009, respectively) with a much greater slope for mice compared with that for rabbits by a factor of 3.6 (0.069/0.019), demonstrating a difference in degree of sensitivity to lung damage between these two species.
This study was extended to pigs to explore the species dependence of the degree of sensitivity to ultrasound-induced lung damage at an ultrasonic frequency of 30 kHz under CW exposure conditions (O’Brien and Zachary, 1996). Eighteen mice were used as positive controls (10 min duration at 145 kPa). Because pig lung has numerous physiological and anatomical similarities to human lung, it was selected as the animal model for these studies. Linear regression analyses of lung damage scores versus acoustic pressure levels for the two species, rabbit and pig, were performed. Both regressions were highly significant (both P < 0.0001) with a much greater slope for rabbits compared with pigs by a factor of 3.8 (0.0069/0.0018) demonstrating the difference in degree of sensitivity to lung damage between these two species.
The mean and standard deviation for the combined (by species) lung damage scores for 43 mice, 22 rabbits, and 16 pigs from all three studies at an acoustic pressure level of 145 kPa and an exposure duration of 10 min (see Fig. 4-11) suggest that ultrasound-induced lung damage is a strong function of species (O’Brien and Zachary, 1994a, 1994b, 1996). Also, linear regression analyses of lung damage scores versus all of the acoustic pressure levels for the three species from all three studies were performed. All three of these regressions were highly significant (all P < 0.0001) with a much greater slope for mice compared with rabbits by a factor of 3.9 (0.026/0.0066), with a much greater slope for rabbits compared with pigs by a factor of 3.7 (0.0066/0.0018), and with a much greater slope for mice compared with pigs by a factor of 14.4 (0.026/0.0018), demonstrating the difference of sensitivity to lung damage among these three species, in the order mouse > rabbit > pig.
Figure 4-11.
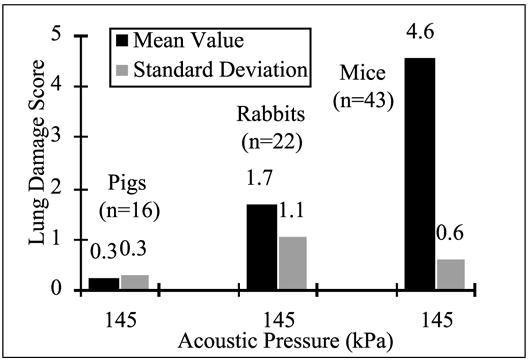
Lung damage score at an acoustic pressure amplitude of 145 kPa for mice, rabbits, and pigs exposed to 30 kHz continuous wave ultrasound. (Adapted from and reprinted by permission of Elsevier Science, from O’Brien WD Jr., Zachary JF: Comparison of mouse and rabbit lung damage exposure to 30 kHz ultrasound. Ultrasound Med Biol 20:299, 1994a; and O’Brien WD Jr., Zachary JF: Mouse lung damage from exposure to 30 kHz ultrasound. Ultrasound Med Biol 20:287, 1994b; and O’Brien WD Jr., Zachary JF: Rabbit and pig lung damage from exposure to CW 30-kHz ultrasound. Ultrasound Med Biol 22:345, 1996.)
4.4 Clinical Implications of Lung Damage
Humans and animals that suffer trauma to the lungs or pulmonary hemorrhage can exhibit abnormalities of pulmonary function (Prentice and Ahrens, 1994; Kirton et al, 1995). The degree of abnormal function is dependent upon the severity of injury and the proportion of total lung volume damaged. Because of the very large reserve volume of the lungs, which is only required during exhaustive exercise, patients can accommodate rather severe lung damage and still survive. There exists abundant peer-reviewed published scientific literature that clearly and convincingly documents that ultrasound at commercial diagnostic levels can produce lung damage and focal hemorrhage in a variety of mammalian species (see Table 4-1). The degree to which this is a clinically significant problem in humans is not known.
Given the thresholds for lung hemorrhage in laboratory animals, what can be inferred about the sensitivity of human lung to diagnostic ultrasound? It is unlikely that an ethically acceptable protocol can be devised that will directly determine the threshold for hemorrhage in human subjects. On the one hand, the observations by Meltzer and colleagues (1998) suggest that human lung is not markedly more susceptible to ultrasound exposure than that of animals that have been used in systematic threshold determinations. On the other hand, in view of the thresholds for damage in various laboratory animals, it would not be responsible to assume that the threshold for hemorrhage in human lung was significantly lower.
4.5 Ultrasound-Induced Effects on Intestine
In addition to effects in the lung, ultrasonically induced petechial hemorrhage has also been observed in the mouse intestine. Lehmann and Herrick (1953) reported the production of petechial hemorrhage in the abdomen of mice exposed to 1 MHz therapeutic ultrasound. Typical therapeutic exposures involved unfocused transducers generating CW or long burst-mode ultrasound at spatial peak intensities of a few watts/cm2. The mechanism of damage was attributed to cavitation by these authors because the damage appeared to be mechanical upon histological examination. Intestinal hemorrhage has also been reported in rats due to therapeutic ultrasound exposure (Cowden and Abell, 1963). Because of the long pulse durations utilized in therapeutic ultrasound exposures, heating as a mechanism for damage cannot be ruled out. Indeed, some investigators (Miller DL and Thomas, 1994) observed both hyperemia and petechial hemorrhage in mouse intestine exposed to 1.0 MHz pulsed and CW ultrasound. Pulsed exposure conditions included a 1 ms pulse duration and 250 or 500 Hz PRFs. Histologically, the petechiae appeared to be localized mostly to the lamina propria of the mucosa. In addition, both hyperemia and petechial hemorrhage were observed in mouse intestine exposed to local heating from radio-frequency diathermy at 10.35 MHz. Therefore, these authors (Miller DL and Thomas, 1994) concluded that the ultrasonically induced petechiae appeared to be attributable to heating and not cavitation.
Petechial hemorrhage can be attributed unambiguously to mechanical mechanisms from exposure to ESWL. The relatively low 1-Hz PRF yields low time-average intensities and no significant heating. Petechial hemorrhage from lithotripter shockwaves has been produced in rat intestine (Chaussy, 1982) and mouse intestine (Raeman et al, 1994; Miller DL and Thomas 1995a; Dalecki et al, 1995c). Hemorrhage has also been observed in liver and kidney from lithotripter shockwaves (Chaussy, 1982; Delius et al, 1988b, 1990), but not from pulsed diagnostic ultrasound in these organs (Carstensen et al, 1990b). As evidence for cavitation involvement in the damage, lithotripter shock waves generate hyperechoic regions attributed to bubbles, which are observed on ultrasonic images (Kuwahara et al, 1989; Delius et al, 1990). In addition, mechanical inversion of the lithotripter shockwave via the use of a novel pressure release reflector inhibits the formation of cavitation and simultaneously decreases the effectiveness in renal calculi destruction (Bailey et al, 1998).
One group (Miller DL and Thomas, 1995a) observed that the number of intestinal lesions increased with increasing number of shockwaves and with increasing amplitude. The threshold for intestinal damage in the mouse was roughly between 1.6 and 4.0 MPa peak positive pressure and between 1.4 and 3.5 MPa peak negative pressure. Dalecki et al (1995c) also observed that the extent of intestinal damage in the mouse increased with the output level of the lithotripter once the threshold level was exceeded. In another study in mice, Dalecki et al (1996) demonstrated that the presence of gas in the intestine vastly increased the potential for intestinal hemorrhage. Pregnant mice were exposed to lithotripter shockwaves with 10 MPa peak positive and 2.5 MPa peak rarefactional pressure amplitudes. All maternal intestines showed hemorrhagic regions extending several centimeters in length. In contrast, the exposed fetuses exhibited minimal intestinal hemorrhage. The results of these authors support a bubble activation or cavitation mechanism for the production of intestinal hemorrhage by exposure to acoustic fields.
Thresholds for damage in the mouse intestine due to diagnostic pulses of ultrasound have also been explored (Dalecki et al, 1995b). Pulses with a duration of 10 μs and a PRF of 100 Hz at center frequencies of 0.7, 1.1, 2.4, and 3.6 MHz yielded thresholds for intestinal hemorrhage listed in Table 4-2. The tabulated values were calculated by utilizing insertion loss measurements of Dalecki et al (1995b) rather than derating the peak rarefactional pressure by 0.3 dB/cm-MHz. Thermocouple measurements of the temperature rise during ultrasound exposure revealed temperature elevations of 2°C or less even at the highest exposure levels. Near the threshold, heating was much less and clearly too small to support a thermal mechanism for damage. The frequency dependence of the production of intestinal hemorrhage is consistent with a cavitational, or gas body activation, mechanism of damage.
Table 4-2.
Thresholds for Murine Intestinal Hemorrhage as a Function of Frequencya
| Threshold Levels
|
||||
|---|---|---|---|---|
| Frequency (MHz) | p+ (MPa) | P− (MPa) | ISPPA (W/cm2) | |
| 0.7 | 0.9 | 0.9 | 16 | 1.1 |
| 1.1 | 1.3 | 1.5 | 57 | 1.4 |
| 2.4 | 11.5 | 3.9 | 1075 | 2.5 |
| 3.6 | 7.8 | 2.8 | 505 | 1.5 |
Figure 4-4.

Subpleural hemorrhage in rat lung parenchyma exposed to 4 MHz pulsed diagnostic ultrasound. (Reprinted by permission of Elsevier Science, from Holland CK, et al: Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med Biol 22:917, 1996.)
Figure 4-5.

Lung tissue from bonnet macaque exposed to 4 MHz diagnostic ultrasound at an MI of 1.8 for 10 min (5 min cranial plus 5 min caudal lung lobe exposures). Note multiple hemorrhagic foci. (Reprinted by permission of Elsevier Science, from Tarantal AF, Canfield DR: Ultrasound-induced lung hemorrhage in the monkey. Ultrasound Med Biol 20:65, 1994.)
Figure 4-9.

Mouse lung exposed to 30 kHz, CW ultrasound for 5 min exhibiting severe diffuse intraparenchymal hemorrhage (fixed tissue). Left: dorsal-ventral view. Right: ventral-dorsal view. (Reprinted by permission of Elsevier Science, from O’Brien WD Jr., Zachary JF: Mouse lung damage from exposure to 30 kHz ultrasound. Ultrasound Med Biol 20:287, 1994b.)
References
- Abramowicz JS. Ultrasound contrast media and their use in obstetrics and gynecology. Ultrasound Med Biol. 1997;23:1287. doi: 10.1016/s0301-5629(97)00201-9. [DOI] [PubMed] [Google Scholar]
- Abramowicz JS, Phillips DB, Jessee LN, et al. Enhanced blood flow visualization in the perfused human placenta by Albunex®, an ultrasound contrast medium. Placenta. 1996;17:A.21. doi: 10.1002/(sici)1097-0096(199911/12)27:9<513::aid-jcu5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Achiron R, Lipitz S, Sivan E, et al. Sonohysterography for ultrasonographic evaluation of tamoxifen-associated cystic thickened endometrium. J Ultrasound Med. 1995;14:685. doi: 10.7863/jum.1995.14.9.685. [DOI] [PubMed] [Google Scholar]
- Allahbadia GN. Fallopian tubes and ultrasonography: The Sion experience. Fertil Steril. 1992;58:901. doi: 10.1016/s0015-0282(16)55432-6. [DOI] [PubMed] [Google Scholar]
- AIUM American Insitute of Ultrasound in Medicine Bioeffects Committee. Bioeffects and Safety of Diagnostic Ultrasound. Laurel, MD: American Institute of Ultrasound in Medicine; 1993. [Google Scholar]
- AIUM/NEMA: American Institute of Ultrasound in Medicine/National Electrical Manufacturers Association. Acoustic Output Measurement and Labeling Standard for Diagnostic Ultrasound Equipment. Laurel, MD: American Institute of Ultrasound in Medicine; 1992a. [Google Scholar]
- AIUM/NEMA: American Institute of Ultrasound in Medicine/National Electrical Manufacturers Association. Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. [Google Scholar]
- AIUM/NEMA: American Insitute of Ultrasound in Medicine/National Electrical Manufacturers Association. Standard for the Real-Time Display of Thermal and Mechanical Acoustic Output Indices on Diagnostic Ultrasound Equipment. Laurel, MD: American Institute of Ultrasound in Medicine; 1992b. rev 1996. [Google Scholar]
- Anderson AL, Hampton LD. Acoustics of gas-bearing sediments: I. Background. J Acoust Soc Am. 1980;67:1865. [Google Scholar]
- Apfel RE. Acoustic cavitation: A possible consequence of biomedical uses of ultrasound. Br J Cancer. 1982;45:140. [PMC free article] [PubMed] [Google Scholar]
- Apfel RE. Acoustic cavitation prediction. J Acoust Soc Am. 1981;69:1624. [Google Scholar]
- Apfel RE. Possibility of microcavitation from diagnostic ultrasound. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1986;32:19. doi: 10.1109/t-uffc.1986.26805. [DOI] [PubMed] [Google Scholar]
- Apfel RE. The role of the impurities in the cavitation-threshold determination. J Acoust Soc Am. 1970;48:1179. [Google Scholar]
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short pulse, low duty cycle diagnostic ultrasound. Ultrasound Med Biol. 1991;17:179. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- Ariani M, Fishbein MC, Chae IS, et al. Dissolution of peripheral arterial thrombi by ultrasound. Circulation. 1991;84:1680. doi: 10.1161/01.cir.84.4.1680. [DOI] [PubMed] [Google Scholar]
- Aris A, Solanes H, Camara ML, et al. Arterial line filtration during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1986;91:526. [PubMed] [Google Scholar]
- Armour EL, Corry PM. Cytotoxic effects of ultrasound in vitro: Dependence on gas content, frequency, radical scavengers, and attachment. Radiat Res. 1982;89:369. [PubMed] [Google Scholar]
- Armstrong WF, Mueller TM, Kinney EL, et al. Assessment of myocardial perfusion abnormalities with contrast-enhanced two-dimensional echocardiography. Circulation. 1982;66:166. doi: 10.1161/01.cir.66.1.166. [DOI] [PubMed] [Google Scholar]
- Aronson S, Roth R, Fernandez A, et al. Assessment of regional renal blood flow in the dog with FS069, a novel intravenous ultrasound contrast agent. Anesth Analg. 1996;82:510. [Google Scholar]
- Atchley AA, Frizzell LA, Apfel RE, et al. Thresholds for cavitation produced in water by pulsed ultrasound. Ultrasonics. 1988;26:280. doi: 10.1016/0041-624x(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Atchley AA, Prosperetri A. The crevice model of bubble nucleation. J Acoust Soc Am. 1989;86:1065. [Google Scholar]
- Averkiou M. Nonlinear propagation modeling with KZK equation and comparison with measurements. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Ayme EJ, Carstensen EL. Cavitation induced by asymmetric, distorted pulses of ultrasound: A biological test. Ultrasound Med Biol. 1989;15:61. doi: 10.1016/0301-5629(89)90133-6. [DOI] [PubMed] [Google Scholar]
- Azadniv M, Doida Y, Miller MW, et al. Temporality in ultrasound-induced cell lysis in vitro. Echocardiography. 1995;13:45. doi: 10.1111/j.1540-8175.1996.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Baggs R, Penney DP, Cox C, et al. Thresholds for ultrasonically induced lung hemorrhage in neonatal swine. Ultrasound Med Biol. 1996;22:119. doi: 10.1016/0301-5629(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Bailey MR, Blackstock DT, Cleveland RO, et al. Comparison of electrohydraulic lithotripters with rigid and pressure-release ellipsoidal reflectors: I. Acoustic fields. J Acoust Soc Am. 1998;104:2517. doi: 10.1121/1.423758. [DOI] [PubMed] [Google Scholar]
- Bailey MR, Dalecki D, Child SZ, et al. Bioeffects of positive and negative acoustic pressures in vivo. J Acoust Soc Am. 1996;100:3941. doi: 10.1121/1.417340. [DOI] [PubMed] [Google Scholar]
- Balen FG, Allen CM, Lees WR. Ultrasound contrast agents. Clin Radiol. 1994;49:77. doi: 10.1016/s0009-9260(05)83446-4. [DOI] [PubMed] [Google Scholar]
- Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol. 1997;23:953. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- Barbarese E, Ho SY, D’Arrigo JS, et al. Internalization of microbubbles by tumor cells in vivo and in vitro. J Neurooncol. 1995;26:25. doi: 10.1007/BF01054766. [DOI] [PubMed] [Google Scholar]
- Barnett SB, Miller MW, Cox C, et al. Increased sister chromatid exchanges in Chinese hamster ovary cells exposed to high intensity pulsed ultrasound. Ultrasound Med Biol. 1988;14:397. doi: 10.1016/0301-5629(88)90075-0. [DOI] [PubMed] [Google Scholar]
- Barnhart J, Levene H, Villanando E, et al. Air-filled albumin microspheres for echocardiography contrast enhancement. Invest Radiol. 1990;25:S162. doi: 10.1097/00004424-199009001-00070. [DOI] [PubMed] [Google Scholar]
- Bell E. The action of ultrasound on mouse liver. Journal of Cellular and Comparative Physiology. 1957;50:83. doi: 10.1002/jcp.1030500107. [DOI] [PubMed] [Google Scholar]
- Belz GG, Breithaupt K, Butzer R, et al. Image quality and safety following intravenous BY963, a new transpulmonary echo contrast media in man. J Am Coll Cardiol. 1994;23:A24. [Google Scholar]
- Bender LF, Janes JM, Herrick JF. Histologic studies following exposure of bone to ultrasound. Arch Phys Med Rehabil. 1954;35:555. [PubMed] [Google Scholar]
- Beranek LL. Acoustical Measurements. New York, NY: American Institute of Physics; 1988. p. 33. [Google Scholar]
- Blackshear PL, Blackshear GL. Mechanical hemolysis. In: Shalak R, Chien S, editors. Handbook of Bioengineering. New York, NY: McGraw-Hill; 1987. pp. 15.1–15.9. [Google Scholar]
- Bleeker H. Master of Science thesis in bioengineering. University Park, PA: Pennsylvania State University; 1990a. Physical and Ultrasonic Characterization of Ultrasonic Contrast Agents. [Google Scholar]
- Bleeker HJ, Shung KK, Barnhart JL. Ultrasonic characterization of Albunex®, a new contrast agent. J Acoust Soc Am. 1990b;87:1792. [Google Scholar]
- Blinc A, Francis CW, Trudnowski JL, et al. Characterization of ultrasound-potentiated fibrinolysis in vitro. Blood. 1993;81:2636. [PubMed] [Google Scholar]
- Bonilla-Musoles F, Simon C, Serra V, et al. An assessment of hysterosalpingosonography (HSSG) as a diagnostic tool for uterine cavity defects and tubal patency. J Clin Ultrasound. 1992;20:175. doi: 10.1002/jcu.1870200303. [DOI] [PubMed] [Google Scholar]
- Boussuges A, Abdellaoui S, Gardette B, et al. Circulating bubbles and breath-hold underwater fishing divers: A two-dimensional echocardiography and continuous wave Doppler study. Undersea Hyperb Med. 1997;24:309. [PubMed] [Google Scholar]
- Boussuges A, Carturan D, Ambrosi P, et al. Decompression induced venous gas emboli in sport diving: Detection with 2D echocardiography and pulsed Doppler. Int J Sports Med. 1998;19:7. doi: 10.1055/s-2007-971871. [DOI] [PubMed] [Google Scholar]
- Brand RP, Nyborg WL. Parametrically excited surface waves. J Acoust Soc Am. 1965;37:509. [Google Scholar]
- Brayman AA, Azadniv M, Cox C, et al. Hemolysis of Albunex®-supplemented, 40% hematocrit human erythrocytes in vitro by 1 MHz pulsed ultrasound: Acoustic pressure and pulse length dependence. Ultrasound Med Biol. 1996a;22:927. doi: 10.1016/0301-5629(96)00108-1. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Azadniv M, Makin IRS, et al. Effect of a stabilized microbubble contrast agent on hemolysis of human erythrocytes exposed to high-intensity pulsed ultrasound. Echocardiography. 1995;12:13. [Google Scholar]
- Brayman AA, Church CC, Miller MW. Re-evaluation of the concept that high cell concentrations “protect” cells in vitro from ultrasonically-induced lysis. Ultrasound Med Biol. 1996b;22:497. doi: 10.1016/0301-5629(95)02078-0. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Doida Y, Miller MW. Apparent contribution of respiratory gas exchange to the in vitro “cell density effect” in ultrasonic cell lysis. Ultrasound Med Biol. 1992;18:701. doi: 10.1016/0301-5629(92)90121-p. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Miller MW. Acoustic cavitation nuclei survive the apparent ultrasonic destruction of Albunex® microspheres. Ultrasound Med Biol. 1997a;23:793. doi: 10.1016/s0301-5629(97)00008-2. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Miller MW. Ultrasonic cell lysis in vitro upon fractional, discontinuous exposure vessel rotation. J Acoust Soc Am. 1994;95:3666. doi: 10.1121/1.409937. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Strickler PS, Luan H, et al. Hemolysis of 40% hematocrit, Albunex®-supplemented human erythrocyte suspensions by intense pulsed ultrasound: Frequency, duty factor, pulse length and sample rotation dependence. Ultrasound Med Biol. 1997b;23:1237. doi: 10.1016/s0301-5629(97)00126-9. [DOI] [PubMed] [Google Scholar]
- Brown J, Alderman J, Quedens-Case C, et al. Enhancement demonstration of neovascularity in a VX2 carcinoma by ultrasonic contrast (FS069, MBI, Inc) J Ultrasound Med. 1996;15:518. [Google Scholar]
- Burns PN. Ultrasound contrast agents in radiological diagnosis. Radiol Med. 1994a;87:71. [PubMed] [Google Scholar]
- Burns PN, Powers JE, Hope-Simpson D, et al. Harmonic power mode Doppler using microbubble contrast agents: An improved method for small vessel flow imaging. Proc IEEE Ultrasononics Symp. 1994b;3:1547. [Google Scholar]
- Camarano G, Ragosta M, Gimple W, et al. Identification of viable myocardium with contrast echocardiography in patients with poor left ventricular systolic function caused by recent or remote myocardial infarction. Am J Cardiol. 1995;75:215. doi: 10.1016/0002-9149(95)80022-k. [DOI] [PubMed] [Google Scholar]
- Campbell S, Bourne TH, Tan SL. Hysterosalpingo contrast sonography (HyCoSy) and its future role within the investigation of infertility in Europe. Ultrasound Obstet Gynecol. 1994;4:245. doi: 10.1046/j.1469-0705.1994.04030245.x. [DOI] [PubMed] [Google Scholar]
- Carmichael AJ, Mossoba MM, Riesz P, et al. Free radical production in aqueous solutions exposed to simulated ultrasonic diagnostic conditions. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1986;33:148. doi: 10.1109/t-uffc.1986.26807. [DOI] [PubMed] [Google Scholar]
- Carstensen EL, Campbell DS, Hoffman D, et al. Killing of Drosophila larvae by the fields of an electrohydraulic Iithotripter. Ultrasound Med Biol. 1990a;16:687. doi: 10.1016/0301-5629(90)90102-i. [DOI] [PubMed] [Google Scholar]
- Carstensen EL, Dalecki D, Gracewski S, et al. Nonlinear propagation and the mechanical index scanners. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Abstracts and Handouts: Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Carstensen EL, Duck FA, Meltzer RS, et al. Bioeffects in echocardiography. Echocardiography. 1992;6:605. doi: 10.1111/j.1540-8175.1992.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Carstensen EL, Hartman C, Child SZ, et al. Test for kidney hemorrhage following exposure to intense, pulsed ultrasound. Ultrasound Med Biol. 1990b;16:681. doi: 10.1016/0301-5629(90)90101-h. [DOI] [PubMed] [Google Scholar]
- Carstensen EL, Kelly P, Church C, et al. Lysis of erythrocytes by exposure to CW ultrasound. Ultrasound Med Biol. 1993;19:147. doi: 10.1016/0301-5629(93)90007-b. [DOI] [PubMed] [Google Scholar]
- Cennamo G, Rosa N, Vallone GF, et al. First experience with a new echographic contrast agent. Br J Ophthalmol. 1994;78:823. doi: 10.1136/bjo.78.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PH, Shung KK. Attenuation and backscatter measurements on Albunex®. Proc IEEE Ultrasonics Symp. 1993;2:913. [Google Scholar]
- Chang PH, Shung KK, Levene HB. Quantitative measurements of second harmonic Doppler using ultrasound contrast agents. Ultrasound Med Biol. 1996;22:1205. doi: 10.1016/s0301-5629(96)00146-9. [DOI] [PubMed] [Google Scholar]
- Chang PH, Shung KK, Levene HB. Second harmonic imaging and harmonic Doppler measurements with Albunex®. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1995;42:1020. [Google Scholar]
- Chapelon JY, Dupenloup F, Cohen H, et al. Reduction of cavitation using pseudorandom signals. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1996;43:623. [Google Scholar]
- Chaussy C. Extracorporeal Shock Wave Lithotripsy: New Aspects in the Treatment of Kidney Stone Disease. Basel; Karger: 1982. [Google Scholar]
- Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2:1265. doi: 10.1016/s0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- Chiang HT, Lin M. Pericardiocentesis guided by two-dimensional contrast echocardiography. Echocardiography. 1993;10:465. doi: 10.1111/j.1540-8175.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Child SZ, Hartman CL, Schery LA, et al. Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol. 1990;16:817. doi: 10.1016/0301-5629(90)90046-f. [DOI] [PubMed] [Google Scholar]
- Christenson HK, Claesson PM. Cavitation and the interaction between macroscopic hydrophobic surfaces. Science. 1988;236:390. doi: 10.1126/science.239.4838.390. [DOI] [PubMed] [Google Scholar]
- Christiansen C, Kryvi H, Sontum PC, et al. Physical and biochemical characterization of Albunex®, a new ultrasound contrast agent consisting of air-filled albumin microspheres suspended in a solution of human albumin. Appl Biochern Biotechnol. 1994;19:307. [PubMed] [Google Scholar]
- Christopher T. A hopefully complete nonlinear model for predictiong in vivo fields of clinical scanners. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Abstracts and Handouts: Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Church CC. The effects of an elastic solid surface layer on the radial pulsations of gas bubbles. J Acoust Soc Am. 1995;97:1510. [Google Scholar]
- Church CC, Flynn HG, Miller MW, et al. The exposure vessel as a factor in uttrasonically induced mammalian cell lysis II. Ultrasound Med Biol. 1982;8:299. doi: 10.1016/0301-5629(82)90036-9. [DOI] [PubMed] [Google Scholar]
- Ciaravino V, Brulfert A, Miller MW, et al. Diagnostic ultrasound and sister chromatid exchanges: Failure to reproduce positive results. Science. 1985;227:1349. doi: 10.1126/science.3883487. [DOI] [PubMed] [Google Scholar]
- Ciaravino V, Miller MW, Carstensen EL. Sister chromatid exchanges in human lymphocytes exposed in vitro to therapeutic ultrasound. Mutat Res. 1986;172:185. doi: 10.1016/0165-1218(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Ciaravino V, Miller MW, Kaufman GE. The effect of 1 MHz ultrasound on the proliferation of synchronized Chinese hamster V-79 cells. Ultrasound Med Biol. 1981;7:175. doi: 10.1016/0301-5629(81)90007-7. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, Romano F, Anastasio PS, et al. Sonohysterography versus hysteroscopy in the diagnosis of endouterine polyps. Gynecol Obstet Invest. 1994;38:266. doi: 10.1159/000292494. [DOI] [PubMed] [Google Scholar]
- Cicinelli E, Romano F, Anastasio PS, et al. Transabdominal sonohysterography, transvaginal sonography and hysteroscopy in the evaluation of submucous myomas. Obstet Gynecol. 1995;85:42. doi: 10.1016/0029-7844(94)00298-r. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Hill CR. Physical and chemical aspects of ultrasonic disruption of cells. J Acoust Soc Am. 1970;47:649. doi: 10.1121/1.1911940. [DOI] [PubMed] [Google Scholar]
- Coakley WT. Acoustical detection of single cavitation events in a focused field in water at 1 MHz. J Acoust Soc Am. 1970;49:792. [Google Scholar]
- Coakley WT, Nyborg WL. Cavitation; dynamics of gas bubbles; applications. In: Fry FJ, editor. Ultrasound: Its Application in Medicine and Biology. New York, NY: Elsevier; 1978. pp. 77–159. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Revised. New York, NY: Academic Press; 1977. [Google Scholar]
- Coleman AJ, Chio MJ, Saunders JE, et al. Acoustic emission and sonoluminescence due to cavitation at the beam focus of an electrohydraulic shockwave lithotripter. Ultrasound Med Biol. 1992;18:267. doi: 10.1016/0301-5629(92)90096-s. [DOI] [PubMed] [Google Scholar]
- Coleman AJ, Chio MJ, Saunders JE, et al. Detection of acoustic cavitation in tissue during clinical extracorporeal lithotripsy. Ultrasound Med Biol. 1996;22:1079. doi: 10.1016/s0301-5629(96)00118-4. [DOI] [PubMed] [Google Scholar]
- Coleman AJ, Saunders JE. A review of the physical properties and biological effects of the high amplitude acoustic fields used in extracorporeal lithotripsy. Ultrasonics. 1993;31:75. doi: 10.1016/0041-624x(93)90037-z. [DOI] [PubMed] [Google Scholar]
- Coleman AJ, Saunders JE, Crum LA, et al. Acoustic cavitation generated by an extracorporeal shock-wave lithotripter. Ultrasound Med Biol. 1987;13:69. doi: 10.1016/0301-5629(87)90076-7. [DOI] [PubMed] [Google Scholar]
- Coley BD, Trambert MA, Mattrey RF. Perfluorocarbon-enhanced sonography: Value in detecting acute venous thrombosis in rabbits. Am J Roentgenol. 1994;163:961. doi: 10.2214/ajr.163.4.8092043. [DOI] [PubMed] [Google Scholar]
- Correas JM, Quay SA. EchoGen™ emulsion: A new ultrasound contrast agent based on phase shift colloids. Clin Radiol. 1996;51(S1):11. [PubMed] [Google Scholar]
- Cotter B, Keramati S, Kwan OL, et al. Myocardial opacification with low dose activated EchoGen™ in patients: Initial experience. Circulation. 1995;92:1. [Google Scholar]
- Cowden JW, Abell MR. Some effects of ultrasonic radiation on normal tissues. Exp Mol Pathol. 1963;2:367. doi: 10.1016/0014-4800(63)90017-0. [DOI] [PubMed] [Google Scholar]
- Crequat J, Pennehouat G, Cornier E, et al. Evaluation of intra-uterine pathology and tubal patency by contrast echography. Contraception, Fertilite, Sexualite (French) 1993;21:861. [PubMed] [Google Scholar]
- Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol (London) 1961;158:1. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch EC, Parks WC. Comparative biochemistry and molecular biology of pulmonary connective tissues. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. p. 451. [Google Scholar]
- Crouse LJ, Cheirif J, Hanly DE, et al. Opacification and border delineation improvement in patients with suboptimal endocardial border definition in routine echocardiography: Results of the Phase III Albunex® Multicenter Trial. J Am Coll Cardiol. 1993;22:1494. doi: 10.1016/0735-1097(93)90562-f. [DOI] [PubMed] [Google Scholar]
- Crum LA. Tensile strength of water. Nature. 1979;278:148. [Google Scholar]
- Crum LA, Fowlkes JB. Acoustic cavitation generated by microsecond pulses of ultrasound. Nature. 1986;319:52. [Google Scholar]
- Crum LA, Roy RA, Dino MA, et al. Acoustic cavitation produced by microsecond pulses of ultrasound: A discussion of some selected results. J Acoust Soc Am. 1992;91:1113. doi: 10.1121/1.402638. [DOI] [PubMed] [Google Scholar]
- Cullinan JA, Fleischer AC, Kepple DM, et al. Sonohysterography: A technique for endometrial evaluation. Radiographics. 1995;15:501. doi: 10.1148/radiographics.15.3.7624559. [DOI] [PubMed] [Google Scholar]
- Curtis JC. Action of intense ultrasound on the intact mouse liver. In: Kelly E, editor. Ultrasonic Energy. Champaign-Urbana, IL: University of Illinois Press; 1965. p. 85. [Google Scholar]
- Dalecki D, Carstensen EL, Parker KJ, et al. Absorption of finite amplitude focused ultrasound. J Acoust Soc Am. 1991;89:2435. doi: 10.1121/1.400976. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Child SZ, Raeman CH, et al. Age dependence of ultrasonically induced lung hemorrhage in mice. Ultrasound Med Biol. 1997a;23:767. doi: 10.1016/s0301-5629(97)00071-9. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Child SZ, Raeman CH, et al. Tactile perception of ultrasound. J Acoust Soc Am. 1995a;97:3165. doi: 10.1121/1.411877. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Child SZ, Raeman CH, et al. Thresholds for fetal hemorrhages produced by a piezoelectric lithotriper. Ultrasound Med Biol. 1997b;23:287. doi: 10.1016/s0301-5629(96)00212-8. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Child SZ, Raeman CH, et al. Ultrasonically induced lung hemorrhage in young swine. Ultrasound Med Biol. 1997c;23:777. doi: 10.1016/s0301-5629(97)00070-7. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Keller BB, Raeman CH, et al. Effects of pulsed ultrasound on the frog heart: I. Thresholds for changes in cardiac rhythm and aortic pressure. Ultrasound Med Biol. 1993a;19:385. doi: 10.1016/0301-5629(93)90057-u. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Carstensen EL. Effects of pulsed ultrasound on the frog heart: II. An investigation of heating as a potential mechanism. Ultrasound Med Biol. 1993b;19:391. doi: 10.1016/0301-5629(93)90058-v. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. A test for cavitation as a mechanism for intestinal hemorrhage in mice exposed to a piezoelectric lithotripter. Ultrasound Med Biol. 1996;22:493. doi: 10.1016/0301-5629(96)00033-6. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. Hemolysis in vivo from exposure to pulsed ultrasound. Ultrasound Med Biol. 1997d;23:301. doi: 10.1016/s0301-5629(96)00203-7. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. Intestinal hemorrhage from exposure to pulsed ultrasound. Ultrasound Med Biol. 1995b;21:1067. doi: 10.1016/0301-5629(95)00041-o. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. Remnants of Albunex® nucleate acoustic cavitation. Ultrasound Med Biol. 1997e;23:1405. doi: 10.1016/s0301-5629(97)00142-7. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. The influence of contrast agents on hemorrhage produced by lithotripter fields. Ultrasound Med Biol. 1997f;23:1435. doi: 10.1016/s0301-5629(97)00151-8. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. Effects of pulsed ultrasound on the frog heart: III. The radiation force mechanism. Ultrasound Med Biol. 1997g;23:275. doi: 10.1016/s0301-5629(96)00209-8. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, et al. Thresholds for intestinal hemorrhage in mice exposed to a piezo-electric lithotripter. Ultrasound Med Biol. 1995c;21:1239. doi: 10.1016/0301-5629(95)02014-4. [DOI] [PubMed] [Google Scholar]
- D’Arrigo JS, Imae T. Physical characteristics of ultra-stable lipid-coated microbubbles. J Colloid Interface Sci. 1992;149:592. [Google Scholar]
- Degenhardt E, Jibril S, Gohde M, et al. Ambulatory contrast hysterosonography as a possibility for assessing tubal patency. (German) Geburtshilfe Frauenheilkd. 1995;55:143. doi: 10.1055/s-2007-1022793. [DOI] [PubMed] [Google Scholar]
- Deichert V, Schlief R, van de Sandt M, et al. Transvaginal hystero-contrast-sonography (Hy-Co-Sy) compared with conventional tubal diagnostics. Hum Reprod. 1989;4:418. doi: 10.1093/oxfordjournals.humrep.a136920. [DOI] [PubMed] [Google Scholar]
- Deichert V, van de Sandt M, Lauth G, et al. Transvaginal hysterosonography. A new diagnostic procedure for differentiating intrauterine and myometrial findings. Geburstshilfe Frauenheilkd. 1988;48:835. doi: 10.1055/s-2008-1026637. [DOI] [PubMed] [Google Scholar]
- de Jong N. Improvements in ultrasound contrast agents. IEEE Eng Med Biol Mag. 1996;15:72. [Google Scholar]
- de Jong N, Hoff L, Skotland T, et al. Absorption and scatter of encapsulated gas filled microspheres: Theoretical considerations and some measurements. Ultrasonics. 1992;30:95. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- de Jong N, Hoff L. Ultrasound scattering properties of Albunex® microspheres. Ultrasonics. 1993;31:175. doi: 10.1016/0041-624x(93)90004-j. [DOI] [PubMed] [Google Scholar]
- de Jong N, Ten Cate FJ, Lancee CT, et al. Principles and recent developments in ultrasound contrast agents. Ultrasonics. 1991;29:324. doi: 10.1016/0041-624x(91)90030-c. [DOI] [PubMed] [Google Scholar]
- Delius M, Brendel W, Heine G. A mechanism of gall-stone destruction by extracorporeal shock wave. Naturwissenschaften. 1988a;75:200. doi: 10.1007/BF00735580. [DOI] [PubMed] [Google Scholar]
- Delius M, Denk R, Berding C, et al. Biological effects of shock waves: Cavitation by shock waves in piglet liver. Ultrasound Med Biol. 1990;16:467. doi: 10.1016/0301-5629(90)90169-d. [DOI] [PubMed] [Google Scholar]
- Delius M, Draenert K, Aldiek Y, et al. Biological effects of shock-waves—In vivo effects of high-energy pulses on rabbit bone. Ultrasound Med Biol. 1995;21:1219. doi: 10.1016/0301-5629(95)00030-5. [DOI] [PubMed] [Google Scholar]
- Delius M, Enders G, Xuan Z, et al. Biological effects of shock waves: Kidney damage by shock waves in dogs—dose dependence. Ultrasound Med Biol. 1988b;14:117. doi: 10.1016/0301-5629(88)90178-0. [DOI] [PubMed] [Google Scholar]
- Delius M, Hoffman E, Steinbeck G, et al. Biological effects of shock waves—Induction of arrhythmia in piglet hearts. Ultrasound Med Biol. 1994;20:279. doi: 10.1016/0301-5629(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Delius M, Jordan M, Eizenhoefer H, et al. Biological effects of shock waves: Kidney hemorrhage by shock waves in dogs—administration rate dependence. Ultrasound Med Biol. 1988c;14:689. doi: 10.1016/0301-5629(88)90025-7. [DOI] [PubMed] [Google Scholar]
- Denbow ML, Blomley MJK, Cosgrove DO, et al. Ultrasound microbubble contrast angiography in monochorionic twins. Lancet. 1997;349:773. doi: 10.1016/S0140-6736(97)24011-0. [DOI] [PubMed] [Google Scholar]
- Deng CX, Xu Q, Apfel RE, et al. In vitro measurements of inertial cavitation thresholds in human blood. Ultrasound Med Biol. 1996;22:939. doi: 10.1016/0301-5629(96)00104-4. [DOI] [PubMed] [Google Scholar]
- Devin C. Survey of thermal, radiation, and viscous damping of pulsating air bubbles in water. J Acoust Soc Am. 1959;31:1654. [Google Scholar]
- Dittrich HC, Bales GL, Hunt RM, et al. Multiple organ tissue perfusion by intravenously (IV) administered novel ultrasound contrast agents in dogs. J Ultrasound Med. 1994;13:S9. [Google Scholar]
- Dittrich HC, Bales GL, Kuvelas T, et al. Myocardial contrast echocardiography in experimental coronary artery occlusion with a new intravenously administered contrast agent. J Am Soc Echocardiogr. 1995a;8:465. doi: 10.1016/s0894-7317(05)80333-5. [DOI] [PubMed] [Google Scholar]
- Dittrich HC, Kuvelas T, Dadd K, et al. Safety and efficacy of the ultrasound contrast agent FS069 in normal humans: Results of a phase one trial. Circulation. 1995b;92:1. [Google Scholar]
- Doida Y, Miller MW. Failure to confirm an increase in unscheduled DNA synthesis in sonicated mammalian cells in vitro. Ultrasonics. 1992;30:35. doi: 10.1016/0041-624x(92)90029-l. [DOI] [PubMed] [Google Scholar]
- Doida Y, Miller MW, Cox C, et al. Confirmation of an ultrasound-induced mutation in two in vitro mammalian cell lines. Ultrasound Med Biol. 1990;16:699. doi: 10.1016/0301-5629(90)90103-j. [DOI] [PubMed] [Google Scholar]
- Dooley DA, Sacks PG, Miller MW. Production of thymine base damage in ultrasound exposed EMT6 mouse mammary sarcoma cells. Radiat Res. 1984;97:71. [PubMed] [Google Scholar]
- Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1989;101:153. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- Duck F. Estimating in situ exposure in the presence of acoustic nonlinearities. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Duck FA, Martin K. Trends in diagnostic ultrasound exposure. Phys Med Biol. 1991;36:1423. doi: 10.1088/0031-9155/36/11/002. [DOI] [PubMed] [Google Scholar]
- Duck FA, Starritt HC, Aindow JD, et al. The output of pulse-echo ultrasound equipment: A survey of powers, pressures and intensities. Br J Radiol. 1985;58:989. doi: 10.1259/0007-1285-58-694-989. [DOI] [PubMed] [Google Scholar]
- Duck FA, Starritt HC, Anderson SP. A survey of the acoustic output of ultrasonic, Doppler equipment. Clinical Physics and Physiological Measurement. 1987;8:39. doi: 10.1088/0143-0815/8/1/002. [DOI] [PubMed] [Google Scholar]
- Düjic Z, Eterovic D, Denoble P, et al. Effect of a single air dive on pulmonary diffusing capacity in professional divers. J Appl Physiol. 1993;74:55. doi: 10.1152/jappl.1993.74.1.55. [DOI] [PubMed] [Google Scholar]
- Dunnill MS. Postnatal growth of the lung. Thorax. 1962;17:329. [Google Scholar]
- Dyson M, Brookes M. Stimulation of bone repair by ultrasound. Ultrasound: 1982 Proceedings of the Third Meeting of the World Federation of Ultrasound in Medicine and Biology, Brighton England, July 1982. Ultrasound Med Biol. 1982;8(Supplement 1):50. [Google Scholar]
- Dyson M, Woodward B, Pond JB. Flow of red blood cells stopped by ultrasound. Nature (London) 1971;232:572. doi: 10.1038/232572a0. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Olstad CS, Carrod G. Human dose-response relationship for decompression and endogenous bubble formation. J Appl Physiol. 1990;69:914. doi: 10.1152/jappl.1990.69.3.914. [DOI] [PubMed] [Google Scholar]
- Edmonds P. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Eisenmenger W. Dynamic properties of the surface tension of water and aqueous solutions of surface active agents with standing capillary waves in the frequency range from 10 kc/s to 1.5 Mc/s. Acustica. 1959;9:327. [Google Scholar]
- Ellwart JW, Brettel H, Kober LO. Cell membrane damage by ultrasound at different cell concentrations. Ultrasound Med Biol. 1988;14:43. doi: 10.1016/0301-5629(88)90162-7. [DOI] [PubMed] [Google Scholar]
- Endl E, Steinbach P, Hofstadter F. Flow cytometric analysis of cell suspensions exposed to shock waves in the presence of the radical sensitve dye hydroethidine. Ultrasound Med Biol. 1995;21:569. doi: 10.1016/0301-5629(94)00133-x. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Makin IRS, Azadniv M, et al. Correlation of ultrasound-induced hemolysis with cavitation detector output in vitro. Ultrasound Med Biol. 1997;23:619. doi: 10.1016/s0301-5629(97)00039-2. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Makin IRS, Francis C, et al. Effect of acoustic cavitation on platelets in the presence of an echo-contrast agent. Ultrasound Med Biol. 1996a;24:125. doi: 10.1016/s0301-5629(97)00233-0. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Makin IRS, Porter TR, et al. Acoustic emissions of a fluorocarbon echo-contrast agent undergoing inertial cavitation. Circulation. 1996b;94:1. [Google Scholar]
- Feinstein SB, Cheirif J, Ten Cate FJ, et al. Safety and efficacy of a new transpulmonary ultrasound contrast agent: Initial multicenter clinical results. J Am Coll Cardiol. 1990;16:316. doi: 10.1016/0735-1097(90)90580-i. [DOI] [PubMed] [Google Scholar]
- Feinstein SB, Keller MW, Kerber RE, et al. Sonicated echocardiographic contrast agents: Reproducibility studies. J Am Soc Echocardiogr. 1989;2:125. doi: 10.1016/s0894-7317(89)80075-6. [DOI] [PubMed] [Google Scholar]
- Fischer JE, Acuff-Smith K-D, Schilling MA, et al. Teratologic evaluation of rats prenatally exposed to pulsed-wave ultrasound. Teratology. 1994;49:150. doi: 10.1002/tera.1420490211. [DOI] [PubMed] [Google Scholar]
- Flynn HG. Cavitation dynamics, I. A mathematical formulation. J Acoust Soc Am. 1975;57:1379. [Google Scholar]
- Flynn HG. Generation of transient cavities in liquids by microsecond pulses of ultrasound. J Acoust Soc Am. 1982;72:1926. [Google Scholar]
- Flynn HG. Physics of acoustic cavitation in liquids. In: Mason WP, editor. Physical Acoustics. 1B. New York, New York: Academic Press; 1964. p. 57. [Google Scholar]
- Flynn H, Church CC. Transient pulsations of small gas bubbles in water. J Acoust Soc Am. 1988;84:985. doi: 10.1121/1.397253. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Diagnostic Ultrasound Guidance for 1993 [February 17, 1993, Revised 510(k)] Rockville, MD: Center for Devices and Radiological Health, Food and Drug Administration, US Department of Health and Human Services; 1993. [Google Scholar]
- Food and Drug Administration. Use of Mechanical Index in Place of Spatial Peak, Pulse Average Intensity in Determining Substantial Equivalence. Rockville, MD: Center for Devices and Radiological Health, Food and Drug Administration, US Department of Health and Human Services; 1994. [Google Scholar]
- Forsberg F, Liu JB, Merton DA, et al. Contrast injection with and without hypobaric activation. J Ultrasound Med. 1996;15:S19. [Google Scholar]
- Forsberg F, Liu JB, Merton DA, et al. Parenchymal enhancement and tumor visualization using a new sonographic contrast agent. J Ultrasound Med. 1995;14:949. doi: 10.7863/jum.1995.14.12.949. [DOI] [PubMed] [Google Scholar]
- Fowlkes JB, Crum LA. Cavitation threshold measurements for microsecond pulses of ultrasound. J Acoust Soc Am. 1988;83:2190. doi: 10.1121/1.396347. [DOI] [PubMed] [Google Scholar]
- Fox FE, Herzfield KF. Gas bubbles with organic skin as cavitation nuclei. J Acoust Soc Am. 1954;26:984. [Google Scholar]
- Francis CW, Önundarson PT, Carstensen EL, et al. Enhancement of fibrinolysis in vitro by ultrasound. J Clin Invest. 1992;90:2063. doi: 10.1172/JCI116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch T, Hilmann J, Kämpfe M, et al. SHU 508, a transpulmonary echocontrast agent. Invest Radiol. 1990;25:S160. doi: 10.1097/00004424-199009001-00069. [DOI] [PubMed] [Google Scholar]
- Fritzsch T, Schartl M, Siegert J. Preclinical and clinical results with an ultrasonic contrast agent. Invest Radiol. 1988;23:S302. doi: 10.1097/00004424-198809001-00067. [DOI] [PubMed] [Google Scholar]
- Frizzell LA. Threshold dosages for damage to mammalian liver by high intensity focused ultrasound. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1988;35:578. doi: 10.1109/58.8036. [DOI] [PubMed] [Google Scholar]
- Frizzell LA, Carstensen EL, Dyro J. Shear properties of mammalian tissues at low megaHertz frequencies. J Acoust Soc Am. 1976;60:1409. doi: 10.1121/1.381236. [DOI] [PubMed] [Google Scholar]
- Frizzell LA, Chen E, Lee C. Effects of pulsed ultrasound on the mouse neonate: Hind limb paralysis and lung hemorrhage. Ultrasound Med Biol. 1994;20:53. doi: 10.1016/0301-5629(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Frizzell LA, Lee CS, Aschenbach PD, et al. Involvement of ultrasonically induced cavitation in the production of hind limb paralysis of the mouse neonate. J Acoust Soc Am. 1983;74:1062. doi: 10.1121/1.389941. [DOI] [PubMed] [Google Scholar]
- Fry FJ, Kossof G, Eggleton RC, et al. Threshold ultrasonic dosages for structural changes in mammalian brain. J Acoust Soc Am. 1970;48:1413. doi: 10.1121/1.1912301. [DOI] [PubMed] [Google Scholar]
- Fry WJ. Action of ultrasound on nerve tissue—A review. J Acoust Soc Am. 1953;25:1. [Google Scholar]
- Fu Y-K, Kaufman GE, Miller MW, et al. Modification by cysteamine of ultrasound lethality to Chinese hamster V-79 cells. Radiat Res. 1979;80:575. [PubMed] [Google Scholar]
- Fu Y-K, Miller MW, Griffiths TD, et al. Ultrasound lethality to synchronous and asynchronous Chinese hamster V-79 cells. Ultrasound Med Biol. 1980;6:39. doi: 10.1016/0301-5629(80)90062-9. [DOI] [PubMed] [Google Scholar]
- Fujiwaki S, Saito J, Horikoshi H, et al. Diagnosis of intrauterine disorders by sonohysterography. (Japanese) Acta Obstet Gynaecol Jpn. 1995;47:437. [PubMed] [Google Scholar]
- Galiuto L, Marchese A, Cavallari D, et al. Evaluation of postinfarction viable myocardium at jeopardy by dobutamine echocardiography and myocardial contrast echocardiography. Echocardiography. 1994;11:337. [Google Scholar]
- Ganong WF, editor. Review of Medical Physiology. Los Altos, CA: Lange Medical Publications; 1967. Respiration; p. 514. [Google Scholar]
- Gaucherand P, Piacenza JM, Salle B, et al. Sonohysterography of the uterine cavity: Preliminary investigations. J Clin Ultrasound. 1995;23:339. doi: 10.1002/jcu.1870230603. [DOI] [PubMed] [Google Scholar]
- Gavrilov LR, Tsirulnikov EM, Davies I. Application of focused ultrasound for the stimulation of neural structures. Ultrasound Med Biol. 1996;22:179. doi: 10.1016/0301-5629(96)83782-3. [DOI] [PubMed] [Google Scholar]
- Geldhof AA, DeVoogt HJ, Rao BR. High energy shock waves do not affect either primary tumor growth or metastasis of prostate carcinoma, R-3327-MatLyLu. Urol Res. 1989;17:9. doi: 10.1007/BF00261041. [DOI] [PubMed] [Google Scholar]
- Georgiadis D, Baumgartner RW, Karatschai R, et al. Further evidence of gaseous embolic material in patients with artificial heart valves. J Thorac Cardiovasc Surg. 1998;115:808. doi: 10.1016/S0022-5223(98)70359-9. [DOI] [PubMed] [Google Scholar]
- Glen SK, Georgiadis D, Grosset DG, et al. Transcranial Doppler ultrasound in commercial air divers: A field study including cases with right-to-left shunting. Undersea Hyperb Med. 1995;22:129. [PubMed] [Google Scholar]
- Goldberg BB. Ultrasound contrast agents. Clin Diagn Ultrasound. 1993a;28:35. [PubMed] [Google Scholar]
- Goldberg BB, Liu JB, Burns PN, et al. Galactose-based intravenous sonographic contrast agent: Experimental studies. J Ultrasound Med. 1993b;12:463. doi: 10.7863/jum.1993.12.8.463. [DOI] [PubMed] [Google Scholar]
- Goldberg BB, Liu JB, Forsberg F. Ultrasound contrast agents: A review. Ultrasound Med Biol. 1994;20:319. doi: 10.1016/0301-5629(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Goldstein SR. Saline infusion sonohysterography. Clin Obstet Gynaecol. 1996;39:248. doi: 10.1097/00003081-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3:356. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- Grauer SE, Xu J, Gong Z, et al. Aerosomes™ MRX115 produces myocardial opacification without hemo-dynamic effects after intravenous injections in primates. Circulation. 1995;92:1. [Google Scholar]
- Grauer SE, Xu J, Pantely GA, et al. MRX 115, an echocardiographic contrast agent, produces myocardial opacification after intravenous injection in primates: Studies before and after occlusion of the left anterior descending coronary artery. Acad Radiol. 1996;3:S405. doi: 10.1016/s1076-6332(96)80598-8. [DOI] [PubMed] [Google Scholar]
- Grayburn PA, Erickson JM, Escobar J, et al. Peripheral intravenous myocardial contrast echocardiography using a 2% dodecafluoropentane emulsion: Identification of myocardial risk area and infarct size in the canine model of ischemia. J Am Coll Cardiol. 1995;26:1340. doi: 10.1016/0735-1097(95)00306-1. [DOI] [PubMed] [Google Scholar]
- Hansen BW. High-speed photographic studies of droplet formation at 20 kHz ultrasonic atomization of oil. Ultrasonics. 1970;8:97. [Google Scholar]
- Harpaz D, Chen X, Francis CW, et al. Ultrasound enhancement of thrombolysis and reperfusion in vitro, J Am Coll Cardiol. 1993;21:1507. doi: 10.1016/0735-1097(93)90331-t. [DOI] [PubMed] [Google Scholar]
- Harrison GH, Balcer-Kubiczek EK, Eddy HA. Potentiation of chemotherapy by low-level ultrasound. Int J Radiat Biol. 1991;59:1453. doi: 10.1080/09553009114551301. [DOI] [PubMed] [Google Scholar]
- Harrison GH, Eddy HA, Wang JP, et al. Microscopic lung alterations and reduction of respiration rate in insonated anesthetized swine. Ultrasound Med Biol. 1995;21:981. doi: 10.1016/0301-5629(95)97511-q. [DOI] [PubMed] [Google Scholar]
- Hartman C, Child SZ, Mayer R, et al. Lung damage from exposure to the fields of an electrohydraulic lithotripter. Ultrasound Med Biol. 1990;16:675. doi: 10.1016/0301-5629(90)90100-q. [DOI] [PubMed] [Google Scholar]
- Harvey EN, Barnes DK, McElroy WD, et al. Bubble formation in animals I: Physical factors. J Cell Comp Physiol. 1944;24:1. [Google Scholar]
- Harvey EN, Loomis L. High frequency sound waves of small intensity and their biological effects. Nature. 1928;121:622. [Google Scholar]
- Harvey W, Dyson M, Pond JB, et al. The in vitro stimulation of protein synthesis in human fibroblasts by therapeutic levels of ultrasound. In: Kazner E, de Vlieger M, Muller HR, McCready VE, editors. Proceedings of the Second European Congress on Ultrasonics in Medicine. Excerpta Medica International Congress series no. 363; Amsterdam, Excerpta Medica Foundation. 1982. pp. 10–21. [Google Scholar]
- Hawgood S, Shiffer K. Structures and properties of the surfactant-associated proteins. Annu Rev Physiol. 1991;53:375. doi: 10.1146/annurev.ph.53.030191.002111. [DOI] [PubMed] [Google Scholar]
- Heckman JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg [Am] 1994;76:26. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Henglein A. Sonochemistry: Historical developments and modern aspects. Ultrasonics. 1987;25:6. [Google Scholar]
- Henglein A, Kormann C. Scavenging of OH radicals produced in the sonolysis of water. Int J Radiat Biol. 1985;48:251. doi: 10.1080/09553008514551241. [DOI] [PubMed] [Google Scholar]
- Herbertz J. Spontaneous cavitation in liquids free of nuclei. Fortschritte der Akustik DAGA. 1988;14:439. [Google Scholar]
- Hildebrandt J, Young AC. Anatomy and physics of respiration. In: Ruch TC, Patton HD, editors. Physiology and Biophysics. 19. Philadelphia, PA: W.B. Saunders; 1966. p. 733. [Google Scholar]
- Hislop A, Howard S, Fairweather DV. Morphometric studies on the structural development of the lung in Macaca fasicularis during fetal and postnatal life. J Anat. 1984;138:95. [PMC free article] [PubMed] [Google Scholar]
- Holland CK, Apfel RE. An improved theory for the prediction of microcavitation thresholds. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1989;36:204. doi: 10.1109/58.19152. [DOI] [PubMed] [Google Scholar]
- Holland CK, Apfel RE. Thresholds for transient cavitation in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- Holland CK, Deng CX, Apfel RE, et al. Direct evidence of cavitation in vivo from diagnostic ultrasound. Ultrasound Med Biol. 1996;22:917. doi: 10.1016/0301-5629(96)00083-x. [DOI] [PubMed] [Google Scholar]
- Holland CK, Roy RA, Apfel RE, et al. In vitro detection of cavitation induced by a diagnostic ultrasound system. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1992;39:95. doi: 10.1109/58.166815. [DOI] [PubMed] [Google Scholar]
- Holland CK, Sandstrom K, Zheng X, et al. The acoustic field of a pulsed Doppler diagnostic ultrasound system near a pressure-release surface. J Acoust Soc Am. 1994;95:2855. [Google Scholar]
- Hong AS, Chae JS, Dublin SB, et al. Ultrasonic clot dissolution: An in vitro study. Am Heart J. 1990;2:418. doi: 10.1016/0002-8703(90)90088-f. [DOI] [PubMed] [Google Scholar]
- Hoshi S, Orikasa S, Kuwahara M-A, et al. High energy shock wave treatment on implanted urinary bladder cancer in rabbits. J Urol. 1991;146:439. doi: 10.1016/s0022-5347(17)37820-5. [DOI] [PubMed] [Google Scholar]
- Howard D, Sturtevant B. In vitro study of the mechanical effects of shock-wave lithotripsy. Ultrasound Med Biol. 1997;23:1107. doi: 10.1016/s0301-5629(97)00081-1. [DOI] [PubMed] [Google Scholar]
- Huber P, Debus P, Peschke P, et al. In vivo detection of ultrasonically induced cavitation by a fibre-optic technique. Ultrasound Med Biol. 1994;20:811. doi: 10.1016/0301-5629(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Hug O, Pape R. Nachweis der Ultraschallkavitation in Gewebe. Strahlentherapies. 1954;94:79. [PubMed] [Google Scholar]
- Hynynen K. The threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound Med Biol. 1991;17:157. doi: 10.1016/0301-5629(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Hynynen K, Chung AH, Colucci V, et al. Potential adverse effects of high-intensity ultrasound exposure on blood vessels in vivo. Ultrasound Med Biol. 1996;22:193. doi: 10.1016/0301-5629(95)02044-6. [DOI] [PubMed] [Google Scholar]
- Inoue M, Church CC, Brayman AA, et al. Confirmation of the protective effect of cysteamine in in vitro ultrasound exposure. Ultrasonics. 1989;27:362. doi: 10.1016/0041-624x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Inoue M, Miller MW, Church CC. An alternative explanation for a postulated non-thermal non-cavitational ultrasound mechanism of action on in vitro cells at hyperthermic temperature. Ultrasonics. 1990;28:185. [Google Scholar]
- Ishimaru A. Wave Propagation and Scattering in Random Media. New York, NY: Academic Press; 1978. [Google Scholar]
- Ito H, Tomooka T, Sakai N, et al. Lack of myocardial perfusion immediately after successful thrombolysis: A predictor of poor recovery of left ventricular function in anterior myocardial infarction. Circulation. 1992;85:1699. doi: 10.1161/01.cir.85.5.1699. [DOI] [PubMed] [Google Scholar]
- Ivey JA, Gardner EA, Fowlkes JB, et al. Acoustic generation of intra-arterial contrast boluses. Ultrasound Med Biol. 1995;21:757. doi: 10.1016/0301-5629(95)00015-j. [DOI] [PubMed] [Google Scholar]
- Jakobsen JÅ, Egge TS, Abildgaard A, et al. Infusion in vascular and renal sonography. Acad Radiol. 1996;3:S322. doi: 10.1016/s1076-6332(96)80571-x. [DOI] [PubMed] [Google Scholar]
- Jayaweera A, Edwards N, Glasheen WP, et al. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994;74:1157. doi: 10.1161/01.res.74.6.1157. [DOI] [PubMed] [Google Scholar]
- Jeffers RJ, Feng RQ, Fowlkes JB, et al. Dimethyl-foramide as an enhancer of cavitation-induced cell lysis in vitro, J Acoust Soc Am. 1995;97:669. doi: 10.1121/1.412289. [DOI] [PubMed] [Google Scholar]
- Jones HW, Hoerr NL, Osol A, editors. Blakiston’s New Gould Medical Dictionary. 3. NY, New York: McGraw Hill; 1972. [Google Scholar]
- Jones JH, Longworth KE. Gas exchange at rest and during exercise in mammals. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 271–308. [Google Scholar]
- Kaps M, Schaffer P, Beller KD, et al. Phase I: Transcranial echo contrast studies in healthy volunteers. Stroke. 1995;26:2048. doi: 10.1161/01.str.26.11.2048. [DOI] [PubMed] [Google Scholar]
- Kaufman GE. Mutagenicity of ultrasound in cultured mammalian cells. Ultrasound Med Biol. 1985;11:497. doi: 10.1016/0301-5629(85)90162-0. [DOI] [PubMed] [Google Scholar]
- Kaufman GE, Miller MW, Griffiths TD, et al. Lysis and viability of cultured mammalian ceils exposed to 1 MHz ultrasound. Ultrasound Med Biol. 1977;3:21. doi: 10.1016/0301-5629(77)90117-x. [DOI] [PubMed] [Google Scholar]
- Kaufman GE, Miller MW. Growth retardation in Chinese hamster V-79 cells exposed to 1 MHz ultrasound. Ultrasound Med Biol. 1978;4:139. doi: 10.1016/0301-5629(78)90039-x. [DOI] [PubMed] [Google Scholar]
- Kaul S, Pandian N, Okada RD, et al. Contrast echocardiography in acute myocardial ischemia: I. In vivo determination of total left ventricular: Area at risk. J Am Coil Cardiol. 1984;4:1272. doi: 10.1016/s0735-1097(84)80149-7. [DOI] [PubMed] [Google Scholar]
- Kay JM. Blood vessels of the lung. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 163–174. [Google Scholar]
- Kedar RP, Cosgrove D, McCready VR, et al. Microbubble contrast agent for color Doppler US: Effect on breast masses. Work in progress Radiology. 1996;198:679. doi: 10.1148/radiology.198.3.8628854. [DOI] [PubMed] [Google Scholar]
- Keller MW, Feinstein SB, Briller RA, et al. Automated production and analysis of echo contrast agents. J Ultrasound Med. 1986;5:493. doi: 10.7863/jum.1986.5.9.493. [DOI] [PubMed] [Google Scholar]
- Keller MW, Feinstein SB, Watson DD. Successful left ventricular opacification following peripheral venous injection of sonicated contrast agent: An experimental evaluation. Am Heart J. 1987;114:570. doi: 10.1016/0002-8703(87)90754-x. [DOI] [PubMed] [Google Scholar]
- Kessel D, Jeffers RJ, Fowlkes JB, et al. Porphyrin-induced enhancement of ultrasound cytotoxicity. Int J Radiat Biol. 1994;66:221. doi: 10.1080/09553009414551131. [DOI] [PubMed] [Google Scholar]
- Killam AL, Greener Y, McFerran BA, et al. Lack of bio-effects of ultrasound energy after intravenous administration of FS069 (Optison™) in the anesthetized rabbit. J Ultrasound Med. 1998;17:349. doi: 10.7863/jum.1998.17.6.349. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Greenleaf JF, Kinnick RR, et al. Ultrasound-mediated transfection of mammalian cells. Hum Gene Ther. 1996;7:1339. doi: 10.1089/hum.1996.7.11-1339. [DOI] [PubMed] [Google Scholar]
- Kirton OC, DeHaven CB, Morgan JP, et al. Elevated imposed work of breathing masquerading as ventilator weaning intolerance. Chest. 1995;108:1021. doi: 10.1378/chest.108.4.1021. [DOI] [PubMed] [Google Scholar]
- Klibanov AL, Ferrara KW, Hughes MS, et al. Direct video microscopic observation of the dynamic effects of medical ultrasound on ultrasound contrast microspheres. Investigative Radiology. 1998;33:863. doi: 10.1097/00004424-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Kodama T, Takayama K. Dynamic behavior of bubbles during extracorporeal shock-wave lithotripsy. Ultrasound Med Biol. 1998;24:723. doi: 10.1016/s0301-5629(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Kondo T, Gamson J, Mitchell JB, et al. Free radical formation and cell lysis induced by ultrasound in the presence of different rare gases. Int J Radiat Biol. 1988a;54:955. doi: 10.1080/09553008814552351. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kano E. Effect of free radicals induced by ultrasonic cavitation on cell killing. Int J Radiat Biol. 1988b;54:475. doi: 10.1080/09553008814551841. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kano E. Enhancement of hyperthermic cell killing by non-thermal effect of ultrasound. Int J Radiat Biol. 1987;51:157. doi: 10.1080/09553008714550591. [DOI] [PubMed] [Google Scholar]
- Kondo T, Lodaira T, Kano E. Free radical formation induced by ultrasound and its effects on strand breakage in DNA of cultured FM3A cells. Free Radic Res Common. 1993;19:S193. doi: 10.3109/10715769309056s193. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Meltzer RS, Chernine A, et al. Does external ultrasound accelerate thrombolysis? Results from a rabbit model. Circulation. 1994;89:339. doi: 10.1161/01.cir.89.1.339. [DOI] [PubMed] [Google Scholar]
- Kremkau FW. Mechanical index rationale (Abstract) J Ultrasound Med. 1991;10:S32. [Google Scholar]
- Krishna PD, Newhouse VL. Second harmonic characteristics of the ultrasound contrast agents Albunex® and FS069. Ultrasound Med Biol. 1997;23:453. doi: 10.1016/s0301-5629(96)00217-7. [DOI] [PubMed] [Google Scholar]
- Kristiansen TK. The effect of low power specifically programmed ultrasound on the healing time of fresh fractures using a Colles’ model. J Orthop Trauma. 1990;4:227. [Google Scholar]
- Kudo S, Kinoshita H, Hirohashi K, et al. Preoperative localization of hepatomas by sonography with microbubbles of carbon dioxide. Am J Roentgenol. 1994;163:1405. doi: 10.2214/ajr.163.6.7992737. [DOI] [PubMed] [Google Scholar]
- Kudo M, Tomita S, Tochio H, et al. Small hepatocellular carcinoma: Diagnosis with US angiography with intraarterial CO2 microbubbles. Radiology. 1992a;182:155. doi: 10.1148/radiology.182.1.1309216. [DOI] [PubMed] [Google Scholar]
- Kudo M, Tomita S, Tochio H, et al. Sonography with intraarterial infusion of carbon dioxide microbubbles (sonographic angiography): Value in differential diagnosis of hepatic tumors. Am J Roentgenol. 1992b;158:65. doi: 10.2214/ajr.158.1.1309220. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Ioritani N, Kanube K, et al. Hyperechoic region induced by focused shock waves in vitro and in vivo: Possibility of acoustic cavitation bubbles. J Lithotr Stone Dis. 1989;1:282. [Google Scholar]
- Kwak HY, Panton RL. Tensile-strength of simple liquids predicted by a model of molecular-interactions. J Phys D Appl Phys. 1985;18:647. [Google Scholar]
- Lafay V, Boussuges A, Ambrosi P, et al. Doppler-echocardiography study of cardiac function during a 36 arm (3,650 kPa) human dive. Undersea Hyperb Med. 1997;24:67. [PubMed] [Google Scholar]
- Lanza GM, Wallace KD, Scott MJ, et al. Thrombi/arteries: A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996;94:3334. doi: 10.1161/01.cir.94.12.3334. [DOI] [PubMed] [Google Scholar]
- Lauer CG, Burge R, Tang DB, et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation. 1992;86:1257. doi: 10.1161/01.cir.86.4.1257. [DOI] [PubMed] [Google Scholar]
- Lee CS, Frizzell LA. Exposure levels for ultrasonic cavitation in the mouse neonate. Ultrasound Med Biol. 1988;14:735. doi: 10.1016/0301-5629(88)90029-4. [DOI] [PubMed] [Google Scholar]
- Lehmann JF, Herrick JF. Biologic reactions to cavitation, a consideration for ultrasonic therapy. Arch Phys Med Rehabil. 1953;34:86. [PubMed] [Google Scholar]
- Liebeskind D, Bases R, Elequin F, et al. Diagnostic ultrasound: Effects on the DNA and growth patterns of animal cells. Radiology. 1979a;131:177. doi: 10.1148/131.1.177. [DOI] [PubMed] [Google Scholar]
- Liebeskind D, Bases R, Mendez F, et al. Sister chromatid exchanges in human lymphocytes after exposure to diagnostic ultrasound. Science. 1979b;205:1273. doi: 10.1126/science.472742. [DOI] [PubMed] [Google Scholar]
- Liebeskind D, Padawer J, Wolley, et al. Diagnostic ultrasound time-lapse and transmission electron microscopic studies of cells insonated in vitro. Br J Cancer Suppl. 1982;45:176. [PMC free article] [PubMed] [Google Scholar]
- Litscher G, Schwarz G, Lenhard H, et al. Biomed Tech (Berl) Suppl. Vol. 42. 1997. Embolism detection in transcranial Doppler ultrasound-Most recent technical developments; p. 515. [DOI] [PubMed] [Google Scholar]
- Loverock P, ter Haar G, Omerod MG, et al. The effect of ultrasound on the cytotoxicity of adriamycin. Br J Radiol. 1990;63:542. doi: 10.1259/0007-1285-63-751-542. [DOI] [PubMed] [Google Scholar]
- Luo H, Steffen W, Cercek B, et al. Enhancement of thrombolysis by external ultrasound. Am Heart J. 1993;125:1564. doi: 10.1016/0002-8703(93)90741-q. [DOI] [PubMed] [Google Scholar]
- Macdonald M, Madsen E. Nonlinear propagation in a liquid tissue-mimicking medium. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- MacRobbie AG, Raeman CH, Child SZ, et al. Thresholds for premature contractions in murine hearts exposed to pulsed ultrasound. Ultrasound Med Biol. 1997;23:761. doi: 10.1016/s0301-5629(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Madanshetty SI, Roy RA, Apfel RE. Acoustic microcavitation: Its active and passive acoustic detection. J Acoust Soc Am. 1991;90:1515. doi: 10.1121/1.401891. [DOI] [PubMed] [Google Scholar]
- Makin IRS, Everbach EC, Porter T, et al. Comparison of cavitational activity of echocontrast agents filled with different gases. Circulation. 1995;92 (Suppl I):1. [Google Scholar]
- Markus H. Transcranial Doppler detection of circulating cerebral emboli. A Review Stroke. 1993;24:1246. doi: 10.1161/01.str.24.8.1246. [DOI] [PubMed] [Google Scholar]
- Maroulis GB, Parsons AK, Yeko TR. Hydrogynecography: A new technique enables vaginal sonography to visualize pelvic adhesions and other pelvic structures. Fertil Steril. 1992;58:1073. doi: 10.1016/s0015-0282(16)55465-x. [DOI] [PubMed] [Google Scholar]
- Marsh JN, Hall CS, Hughes MS, et al. Broadband through transmission signal loss measurements of Albunex® at concentrations approaching in vivo doses. J Acoust Soc Am. 1997;101:1155. [Google Scholar]
- Mayer R, Schenk E, Child SZ, et al. Pressure threshold for shock wave induced renal hemorrhage. J Urol. 1990;144:1505. doi: 10.1016/s0022-5347(17)39787-2. [DOI] [PubMed] [Google Scholar]
- Medwin H. Counting bubbles acoustically: A review. Ultrasonics. 1977;15:7. [Google Scholar]
- Meerbaum S, Meltzer RS, editors. Echocardiography. Boston, MA: Kluwer/Martinus Nijhoff; 1989. Myocardial Contrast Two-Dimensional. [Google Scholar]
- Meltzer RS, Roelandt J, editors. Contrast Echocardiography. The Hague, The Netherlands: Martinus Nijhoff; 1982a. [Google Scholar]
- Meltzer RS, Adsumelli R, Risher W, et al. Lack of lung hemorrhage in humans after intraoperative trans-esophageal echocardiography with ultrasound conditions similar to those causing lung hemorrhage in laboratory animals. J Am Soc Echocardiogr. 1998;11:57. doi: 10.1016/s0894-7317(98)70120-8. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Porder JB, Porder K. Ultrasound bioeffects, mechanisms, and safety. In: Siegel R, editor. Ultrasound Angioplasty. Boston, MA: Kluwer Academic Publishers; 1986. [Google Scholar]
- Meltzer RS, Schwarz KQ, Mottley JG, et al. Therapeutic cardiac ultrasound. Am J Cardiol. 1991;67:422. doi: 10.1016/0002-9149(91)90053-n. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Vermeulen HWJ, Valk NK, et al. New echocardiographic contrast agents: Transmission through the lungs and myocardial perfusion imaging. J Cardiovasc Ultrasonogr. 1982b;1:277. [Google Scholar]
- Mercer RR, Crapo JP. Architecture of the acinus. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 109–119. [Google Scholar]
- Metzger-Rose C, Wright WH, Baker MR, et al. Effects of phospholipid-coated microbubbles (MRX-115) on the detection of testicular ischemia in dogs. Acad Radiol. 1996;3:S314. doi: 10.1016/s1076-6332(96)80568-x. [DOI] [PubMed] [Google Scholar]
- Meza M, Greener Y, Hunt R, et al. Myocardial contrast echocardiography: Reliable, safe, and efficacious myocardial perfusion assessment after intravenous injections of a new echocardiographic contrast agent. Am Heart J. 1996;132:871. doi: 10.1016/s0002-8703(96)90324-5. [DOI] [PubMed] [Google Scholar]
- Mihran RT, Barnes FS, Wachtel H. Temporally specific modification of myelinated axon excitability in vitro following a single ultrasound pulse. Ultrasound Med Biol. 1990;16:297. doi: 10.1016/0301-5629(90)90008-z. [DOI] [PubMed] [Google Scholar]
- Miller DL. The influence of hematocrit on hemolysis by ultrasonically activated gas-filled micropores. Ultrasound Med Biol. 1988;14:293. doi: 10.1016/0301-5629(88)90095-6. [DOI] [PubMed] [Google Scholar]
- Miller DL, Bao S. The relationship of scattered subharmonic, 3.3 MHz fundamental and second harmonic signals to damage of monolayer cells by ultrasonically activated Albunex®. J Acoust Soc Am. 1998a;103:1183. doi: 10.1121/1.421250. [DOI] [PubMed] [Google Scholar]
- Miller DL, Geis RA. Enhancement of ultrasonically induced hemolysis by perfluorocarbon-based compared to air-based echo contrast agents. Ultrasound Med Biol. 1998b;24:285. doi: 10.1016/s0301-5629(97)00267-6. [DOI] [PubMed] [Google Scholar]
- Miller DL, Geis RA. Gas-body-based contrast agent enhances vascular bioeffects of 1.09 MHz ultrasound on mouse intestine. Ultrasound Med Biol. 1998c;24:1201. doi: 10.1016/s0301-5629(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Miller DL, Geis RA. The interaction of ultrasonic heating and cavitation in vascular bioeffects on mouse intestine. Ultrasound Med Biol. 1998d;24:123. doi: 10.1016/s0301-5629(97)00209-3. [DOI] [PubMed] [Google Scholar]
- Miller DL, Geis RA, Chrisler WB. Ultrasonicaliy induced hemolysis at high cell and gas body concentrations in a thin-disc exposure chamber. Ultrasound Med Biol. 1997;23:625. doi: 10.1016/s0301-5629(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Miller DL, Reese JA, Frazier ME. Single strand DNA breaks in human lymphocytes induced by ultrasound in vitro. Ultrasound Med Biol. 1989;15:765. doi: 10.1016/0301-5629(89)90117-8. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Contrast agent gas-bodies enhance hemolysis induced by lithotripter shockwaves and high-intensity focused ultrasound in whole blood. Ultrasound Med Biol. 1996a;22:1089. doi: 10.1016/s0301-5629(96)00126-3. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Frequency dependence of cavitation activity in a rotating tube exposure system compared to the Mechanical Index. J Acoust Soc. 1993;93:3475. doi: 10.1121/1.405677. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Heating as a mechanism for ultrasonically induced petechial hemorrhages in mouse intestine. Ultrasound Med Biol. 1994;20:493. doi: 10.1016/0301-5629(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Thresholds for hemorrhages in mouse skin and in intestine induced by lithotripter shock waves. Ultrasound Med Biol. 1995a;21:249. doi: 10.1016/s0301-5629(94)00112-x. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. The role of cavitation in the induction of cellular DNA damage by ultrasound and lithotripter Shockwaves in vitro. Ultrasound Med Biol. 1996b;22:681. doi: 10.1016/0301-5629(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM. Ultrasound contrast agents nucleate inertial cavitation in vitro. Ultrasound Med Biol. 1995b;21:1059. doi: 10.1016/0301-5629(95)93252-u. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, Buschbom RL. Comet assay reveals DNA strand breaks induced by ultrasonic cavitation in vitro. Ultrasound Med Biol. 1995c;21:841. doi: 10.1016/0301-5629(95)00017-l. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, Frazier ME. Single strand breaks in CHO cell DNA induced by ultrasonic cavitation In vitro. Ultrasound Med Biol. 1991b;17:401. doi: 10.1016/0301-5629(91)90140-r. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, Frazier ME. Ultrasonic cavitation indirectly induces single strand breaks in DNA of viable cells in vitro by the action of residual hydrogen peroxide. Ultrasound Med Biol. 1991 c;17:729. doi: 10.1016/0301-5629(91)90106-7. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, Williams AR. Mechanisms for hemolysis by ultrasonic cavitation in the rotating exposure system. Ultrasound Med Biol. 1991a;17:171. doi: 10.1016/0301-5629(91)90124-f. [DOI] [PubMed] [Google Scholar]
- Miller DL, Williams AR. Bubble cycling as the explanation of the promotion of ultrasonic cavitation in a rotating tube exposure system. Ultrasound Med Biol. 1989;15:641. doi: 10.1016/0301-5629(89)90172-5. [DOI] [PubMed] [Google Scholar]
- Miller MW. Does ultrasound induce sister chromatid exchanges? Ultrasound Med Biol. 1985;11:561. doi: 10.1016/0301-5629(85)90026-2. [DOI] [PubMed] [Google Scholar]
- Miller MW, Azadniv M, Cox C, et al. Lack of induced increase in sister chromatid exchanges in human lymphocytes exposed to in vivo therapeutic ultrasound. Ultrasound Med Biol. 1991;17:81. doi: 10.1016/0301-5629(91)90012-l. [DOI] [PubMed] [Google Scholar]
- Miller MW, Azadniv M, Doida Y, et al. Effect of a stabilized microbubble contrast agent on CW ultrasound-induced red blood cell lysis in vitro. Echocardiography. 1995;12:1. [Google Scholar]
- Miller MW, Azadniv M, Petit SE, et al. Sister chromatid exchanges in Chinese hamster ovary cells exposed to high intensity pulsed ultrasound: Inability to confirm previous positive results. Ultrasound Med Biol. 1989;15:255. doi: 10.1016/0301-5629(89)90069-0. [DOI] [PubMed] [Google Scholar]
- Miller MW, Brayman AA. Comparative sensitivity of human erythrocytes and lymphocytes to sonolysis by 1 MHz ultrasound. Ultrasound Med Biol. 1997;23:635. doi: 10.1016/s0301-5629(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Miller MW, Church CC, Ciaravino V. Time lapse and microscopic examinations of insonated in vitro cells. Ultrasound Med Biol. 1990;16:73. doi: 10.1016/0301-5629(90)90088-t. [DOI] [PubMed] [Google Scholar]
- Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- Mitri FF, Andronikov AD, Perpinyal S, et al. A clinical comparison of sonographic hydrotubation and hysterosalpingography. Br J Obstet Gynaecol. 1991;98:1031. doi: 10.1111/j.1471-0528.1991.tb15342.x. [DOI] [PubMed] [Google Scholar]
- Miyoshi N, Misík V, Reisz P. Sonodynamic toxicity of gallium-porphyrin analogue ATX-70 in human leukemia cells. Radiat Res. 1997;148:43. [PubMed] [Google Scholar]
- Mobley J, Marsh JN, Hall CS, et al. Broadband measurements of phase velocity in Albunex® suspensions. J Acoust Soc Am. 1998;103:2145. doi: 10.1121/1.421360. [DOI] [PubMed] [Google Scholar]
- Mor-Avi V, Robinson K, Shroff S, et al. Stability of Albunex® microspheres under ultrasonic irradiation: An in vitro study. J Am Soc Echocardiogr. 1994;7:S29. [Google Scholar]
- Morgan TR, Laudone VP, Heston WD, et al. Free radical production by high energy shock waves— Comparison with ionizing irradiation. J Urol. 1988;139:186. doi: 10.1016/s0022-5347(17)42350-0. [DOI] [PubMed] [Google Scholar]
- Mulvagh SL, Foley DA, Aeschbacher BC, et al. Second harmonic imaging of an intravenously administered echocardiographic contrast agent: Visualization of coronary arteries and measurement to coronary blood flow. J Am Coll Cardiol. 1996;27:1519. doi: 10.1016/0735-1097(95)00619-2. [DOI] [PubMed] [Google Scholar]
- Nada T, Moriyasu F, Fujimoto M, et al. Gray scale enhancement of liver tumors using proteinacious microspheres (FS069) J Ultrasound Med. 1995;14:S7. [Google Scholar]
- National Council on Radiation Protection and Measurement. Biological Effects of Ultrasound: Mechanisms and Clinical Implications. Report No. 74. Bethesda, MD: National Council on Radiation Protection and Measurement; 1983. [Google Scholar]
- Nilsson A-M, Odselius R, Roijer A. Pro- and antifibrinolytic effects of ultrasound on streptokinase-induced thrombolysis. Ultrasound Med Biol. 1995;21:833. doi: 10.1016/0301-5629(95)00014-i. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Luo H, Fishbein MC, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: An in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Matsuda Y, Yabuuchi I, et al. Hepatocellular carcinoma in adenomatous hyperplasia: Detection with contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1993;187:353. doi: 10.1148/radiology.187.2.8386389. [DOI] [PubMed] [Google Scholar]
- Nyborg WL. Acoustic streaming. In: Hamilton MF, Blackstock DT, editors. Nonlinear Acoustics. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Nyborg WL. Acoustic streaming. In: Mason WP, editor. Physical Acoustics (Volume 1B) New York, NY: Academic Press; 1965. p. 265. [Google Scholar]
- Nyborg WL. Physical principles of ultrasound. In: Fry FJ, editor. Ultrasound: Its Application in Medicine and Biology. New York, NY: Elsevier; 1978. [Google Scholar]
- O’Brien WD, Jr, Zachary JF. Comparison of mouse and rabbit lung damage exposure to 30 kHz ultrasound. Ultrasound Med Biol. 1994a;20:299. doi: 10.1016/0301-5629(94)90070-1. [DOI] [PubMed] [Google Scholar]
- O’Brien WD, Jr, Zachary JF. Lung damage assessment from exposure to pulsed-wave ultrasound in the rabbit, mouse, and pig. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1997;44:473. doi: 10.1109/58.585132. [DOI] [PubMed] [Google Scholar]
- O’Brien WD, Jr, Zachary JF. Mouse lung damage from exposure to 30 kHz ultrasound. Ultrasound Med Biol. 1994b;20:287. doi: 10.1016/0301-5629(94)90069-8. [DOI] [PubMed] [Google Scholar]
- O’Brien WD, Jr, Zachary JF. Rabbit and pig lung damage from exposure to CW 30-kHz ultrasound. Ultrasound Med Biol. 1996;22:345. doi: 10.1016/0301-5629(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Okura H, Yoshikawa J, Yoshida K, et al. Quantitation of left-to-right shunts in secundum atrial septal defect by two-dimensional contrast echocardiography with use of Albunex®. Am J Cardiol. 1995;75:639. doi: 10.1016/s0002-9149(99)80639-0. [DOI] [PubMed] [Google Scholar]
- Olsson SB, Johansson B, Nilsson A-M, et al. Enhancement of thrombolysis by ultrasound. Ultrasound Med Biol. 1994;20:375. doi: 10.1016/0301-5629(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Oosterhof GON, Cornel EB, Smits GAHJ, et al. The influence of high energy shock waves on the development of metastases. Ultrasound Med Biol. 1996;22:339. doi: 10.1016/0301-5629(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Oosterhof GON, Smits GAHJ, de Ruyter AE, et al. In vivo effects of high energy shock waves on urological tumors: An evaluation of treatment modalities. J Urol. 1990;144:785. doi: 10.1016/s0022-5347(17)39592-7. [DOI] [PubMed] [Google Scholar]
- Ophir J, Parker KJ. Contrast agents in diagnostic ultrasound. Ultrasound Med Biol. 1989;15:319. doi: 10.1016/0301-5629(89)90044-6. [DOI] [PubMed] [Google Scholar]
- Panigel M, Abramowicz JS, Miller RK. Techniques: Biophysical methods for assessment of placental function. In: Rama Sastry BV, editor. Placental Pharmacology. Boca Raton: Florida, CRC Press; 1996. [Google Scholar]
- Parsons AK, Cullinan JA, Goldstein SR, et al. Sonohysterography, sonosalpingography, and sonohysterosalpingography. A text-atlas of normal and abnormal findings. In: Fleischer AC, Manning FA, Jeanty P, et al., editors. Sonography in Obstetrics and Gynecology. Principles and Practice. Stamford, CT: Appleton and Lange; 1996. [Google Scholar]
- Parsons AK, Lense JJ. Sonohysterography for endormetrial abnormalities: Preliminary results. J Clin Ultrasound. 1993;21:87. doi: 10.1002/jcu.1870210203. [DOI] [PubMed] [Google Scholar]
- Patton CA, Harris GR, Phillips RA. Output levels and bioeffect indices from diagnostic ultrasound exposure data reported to the FDA. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1994;41:353. [Google Scholar]
- Penney DP, Schenk EA, Maltby K, et al. Morphologic effects of pulsed ultrasound in the lung. Ultrasound Med Biol. 1993;19:127. doi: 10.1016/0301-5629(93)90005-9. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Coulam CB. Hysterosalpingography with color Doppler ultrasonography. Am J Obstet Gynecol. 1991;164:1530. doi: 10.1016/0002-9378(91)91432-v. [DOI] [PubMed] [Google Scholar]
- Pilla AA, Mont MA, Nusser PR. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J Orthop Trauma. 1990;4:246. doi: 10.1097/00005131-199004030-00002. [DOI] [PubMed] [Google Scholar]
- Pilmanis AA, Meissner FW, Olson RM. Left ventricular gas emboli in six cases of altitude-induced decompression sickness. Aviat Space Environ Med. 1996;67:1092. [PubMed] [Google Scholar]
- Pinamonti S, Caruso A, Mazzeo V, et al. DNA damage from pulsed sonications of human leucocytes in vitro. IEEE Trans Ultrasonics, Ferroelectrics, and Frequency Control. 1986;33:179. doi: 10.1109/t-uffc.1986.26812. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Gehr P, Crapo JD. Architecture and cellular composition of the air-blood barrier. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 121–128. [Google Scholar]
- Plopper CG, Pinkerton KE. Structural and cellular diversity of the mammalian respiratory system. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 1–5. [Google Scholar]
- Pohl EE, Rosenfeld EH, Pohl P. Effects of ultrasound on agglutination and aggregation of human erythrocytes in vitro. Ultrasound Med Biol. 1995;21:711. doi: 10.1016/0301-5629(95)00004-b. [DOI] [PubMed] [Google Scholar]
- Pohlhammer J, O’Brien WD., Jr Dependence of the ultrasonic scatter coefficient on collagen concentration in mammalian tissues. J Acoust Soc Am. 1981;69:283. doi: 10.1121/1.385349. [DOI] [PubMed] [Google Scholar]
- Porter TR, Iversen PL, Li S, et al. Interaction of diagnostic ultrasound with synthetic oligonucieotide-labeled perfluorocarbon-exposed sonicated dextrose albumin microbubbles. J Ultrasound Med. 1996a;15:577. doi: 10.7863/jum.1996.15.8.577. [DOI] [PubMed] [Google Scholar]
- Porter TR, Kricsfeld A, Cheatham S, et al. The effect of ultrasound frame rate on perfluorocarbon-exposed sonicated dextrose albumin microbubble size and concentration when insonifying at different flow rates, transducer frequencies, and acoustic outputs. J Am Soc Echocardiogr. 1997;10:593. doi: 10.1016/s0894-7317(97)70021-x. [DOI] [PubMed] [Google Scholar]
- Porter TR, LaVeen RF, Fox R, et al. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J. 1996b;132:964. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- Porter TR, Li S, Everbach EC. Direct in vivo recordings of cavitational activity within the anterior myocardium during intermittent and conventional harmonic imaging following intravenous ultrasound contrast (Abstract) J Am Coll Cardiol. 1998a;31 (Suppl A):400A. [Google Scholar]
- Porter TR, Li S, Hiser W, et al. Simultaneous assessment of wall motion and myocardial perfusion using a rapid acquisition intermittent harmonic imaging pulsing interval of 5–15 Hz following acute myocardial infarction and during stress echocardiography (Abstract) J Am Coll Cardiol. 1998b;31 (Suppl A):123A. [Google Scholar]
- Porter TR, Xie F. Visually discernible myocardial echocardiographic contrast after intravenous injection of sonicated dextrose albumin microbubbles containing high molecular weight, less soluble gases. J Am Coll Cardiol. 1995a;25:509. doi: 10.1016/0735-1097(94)00376-2. [DOI] [PubMed] [Google Scholar]
- Porter TP, Xie F, Kilzer K. A non-invasive method of visually assessing renal perfusion using a newly developed intravenous ultrasound contrast. J Am Coll Cardiol. 1995b;26:246A. doi: 10.1016/0735-1097(95)00132-j. [DOI] [PubMed] [Google Scholar]
- Porter TR, Xie F, Kricsfeld A. Myocardial ultrasound contrast with intravenous perfluoropropane-enhanced sonicated dextrose albumin: Initial clinical experience in humans. J Am Coll Cardiol (Special Issue) 1995c;26:39A. doi: 10.1016/0735-1097(95)00132-j. [DOI] [PubMed] [Google Scholar]
- Porter TR, Xie F, Kricsfeld A, et al. Noninvasive identification of acute myocardial ischemia and reperfusion with contrast ultrasound using intravenous perfluoropropane-exposed sonicated dextrose albumin. J Am Coll Cardiol. 1995d;26:33. doi: 10.1016/0735-1097(95)00132-j. [DOI] [PubMed] [Google Scholar]
- Porter TR, Xie F, Li S, et al. Increased ultrasound contrast and decreased microbubble destruction rates using triggered ultrasound imaging. J Am Soc Echocardiogr. 1996c;9:599. doi: 10.1016/s0894-7317(96)90054-1. [DOI] [PubMed] [Google Scholar]
- Prat F, Chapelon J-Y, el Fadil FA, et al. In vivo effects of cavitation alone or in combination with chemotherapy in a peritoneal carcinomatosis in the rat. Br J Cancer. 1991;68:13. doi: 10.1038/bjc.1993.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat F, Ponchon T, Berger F, et al. Hepatic lesions in the rabbit induced by acoustic cavitation. Gastroenterology. 1991;100:1345. [PubMed] [Google Scholar]
- Prat F, Sibille A, Luccioni C, et al. Increased chemo-cytotoxicity to colon cancer cells by shock wave-induced cavitation. Gastroenterology. 1994;106:937. doi: 10.1016/0016-5085(94)90752-8. [DOI] [PubMed] [Google Scholar]
- Prentice D, Ahrens T. Pulmonary complications of trauma. Crit Care Nurs Q. 1994;17:24. doi: 10.1097/00002727-199408000-00004. [DOI] [PubMed] [Google Scholar]
- Quay SA. Ultrasound contrast agent development: Phase shift colloids. J Ultrasound Med. 1994;13:S9. [Google Scholar]
- Raeman CH, Child SZ, Carstensen EL. Timing of exposures in ultrasonic hemorrhage of murine lung. Ultrasound Med Biol. 1993;19:507. doi: 10.1016/0301-5629(93)90126-9. [DOI] [PubMed] [Google Scholar]
- Raeman CH, Child SZ, Dalecki D, et al. Damage to murine kidney and intestine from exposure to the fields of a piezoelectric lithotripter. Ultrasound Med Biol. 1994;20:589. doi: 10.1016/0301-5629(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Raeman CH, Child SZ, Dalecki D, et al. Exposure-time dependence of the threshold for ultrasonically induced murine lung hemorrhage. Ultrasound Med Biol. 1996;22:139. doi: 10.1016/0301-5629(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Raeman CH, Dalecki D, Child SZ, Meltzer RS, et al. Albunex® does not increase the sensitivity of the lung to pulsed ultrasound. Echocardiography. 1997;14:553. doi: 10.1111/j.1540-8175.1997.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Ramnarine KV, Nassiri DK, McCarthy A, et al. Effects of pulse ultrasound on embryonic development: An in vitro study. Ultrasound Med Biol. 1998;24:575. doi: 10.1016/s0301-5629(98)00007-6. [DOI] [PubMed] [Google Scholar]
- Ramnarine KV, Nassiri DK, Pearce JM, et al. Estimation of in situ ultrasound exposure during obstetric examinations. Ultrasound Med Biol. 1993;19:319. doi: 10.1016/0301-5629(93)90104-v. [DOI] [PubMed] [Google Scholar]
- Reher P, Elbeshir el-NI, Harvey W, et al. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med Biol. 1997;23:1251. doi: 10.1016/s0301-5629(97)00031-8. [DOI] [PubMed] [Google Scholar]
- Richman TS, Viscomi EN, deCherney A. Fallopian tubal patency assessed by ultrasound following fluid injection. Radiology. 1984;152:507. doi: 10.1148/radiology.152.2.6539931. [DOI] [PubMed] [Google Scholar]
- Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. J Free Radic Biol Med. 1992;13:247. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335:1857. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- Rovai D, Lubrano V, Paterni M, et al. Left ventricular and myocardial opacification after intravenous injection of a novel ultrasound contrast agent: BR1. Eur Heart J. 1995;16(S):91. [Google Scholar]
- Roy RA, Atchley AA, Crurn LA, et al. A precise technique for the measurement of acoustic cavitation thresholds, and some preliminary results. J Acoust Soc Am. 1985;78:1799. doi: 10.1121/1.392767. [DOI] [PubMed] [Google Scholar]
- Roy RA, Madanshetty SI, Apfel RE. An acoustic backscattering technique for the detection of transient cavitation produced by microsecond pulses of ultrasound. J Acoust Soc Am. 1990;87:2451. doi: 10.1121/1.399091. [DOI] [PubMed] [Google Scholar]
- Saad AH. PhD thesis. Manchester, England: University of Manchester; 1983. Some Biological Effects of Ultrasound. [Google Scholar]
- Saad AH, Hahn GM. Ultrasound-enhanced drug toxicity on Chinese hamster ovary cells in vitro. Cancer Res. 1989;49:5931. [PubMed] [Google Scholar]
- Saad AH, Hahn GM. Ultrasound-enhanced effects of Adriamycin against murine tumors. Ultrasound Med Biol. 1992;18:715. doi: 10.1016/0301-5629(92)90122-q. [DOI] [PubMed] [Google Scholar]
- Sabia PJ, Powers ER, Jayaweera AR, et al. Functional significance of collateral blood flow in patients with recent acute myocardial infarction: A study using myocardial contrast echocardiography. Circulation. 1992a;85:2080. doi: 10.1161/01.cir.85.6.2080. [DOI] [PubMed] [Google Scholar]
- Sabia PJ, Powers ER, Ragosta M, et al. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992b;327:1825. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- Sacks PG, Miller MW, Sutherland RM. Response of multicell spheroids to 1 MHz ultrasonic irradiation: Cavitation related damage. Radiat Res. 1983;93:545. [PubMed] [Google Scholar]
- Sahebjami H. Aging of the normal lung. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 351–366. [Google Scholar]
- Sandstrom K. Challenges associated with characterizing and predicting acoustic fields in water. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Santoso T, Roelandt J, Mansyoer H, et al. Myocardial perfusion imaging in humans by contrast echocardiography using polygelin colloid solution. J Am Coll Cardiol. 1985;6:612. doi: 10.1016/s0735-1097(85)80121-2. [DOI] [PubMed] [Google Scholar]
- Schaer GN, Koechli OR, Schuessler B, et al. Improvement in perineal sonographic bladder neck imaging with ultrasound contrast medium. Obstet Gynecol. 1995;86:950. doi: 10.1016/0029-7844(95)00301-7. [DOI] [PubMed] [Google Scholar]
- Schlief R, Schurman R, Niendorf HP. Basic properties and results of clinical trials of ultrasound contrast agents based on galactose. Ann Acad Med Singapore. 1993;22:762. [PubMed] [Google Scholar]
- Schlief R, Staks T, Mahler M, et al. Successful opacification of the left heart chambers on echocardiographic examination after intravenous injection of a new saccharide based contrast agent. Echocardiography. 1990;7:61. doi: 10.1111/j.1540-8175.1990.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Schneider M, Arditi M, Barrau MB, et al. BR1: A new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30:451. doi: 10.1097/00004424-199508000-00001. [DOI] [PubMed] [Google Scholar]
- Schneider M, Broillet A, Arditi M, et al. Doppler intensitometry with BR1, a sonographic contrast agent. Acad Radiol. 1996;3:S308. doi: 10.1016/s1076-6332(96)80566-6. [DOI] [PubMed] [Google Scholar]
- Schneider M, Bussat P, Barrau MB, et al. Polymeric microballoons as ultrasound contrast agents. Physical and ultrasonic properties compared with sonicated albumin. Invest Radiol. 1992;27:134. doi: 10.1097/00004424-199202000-00008. [DOI] [PubMed] [Google Scholar]
- Schrope BA, Newhouse VL. Second harmonic ultrasound blood perfusion measurement. Ultrasound Med Biol. 1993;19:567. doi: 10.1016/0301-5629(93)90080-8. [DOI] [PubMed] [Google Scholar]
- Schrope BA, Newhouse VL, Uhlendorf V. Simulated capillary blood flow measurement using a nonlinear ultrasonic contrast agent. Ultrason Imaging. 1992;14:134. doi: 10.1177/016173469201400204. [DOI] [PubMed] [Google Scholar]
- Schurmann R, Schlief R. Saccharide-based contrast agents. Characteristics and diagnostic potential. Radiol Med (Torino) 1994;87(5 Suppl 1):15. [PubMed] [Google Scholar]
- Schwarz KO, Becher H, Schimpfky C, et al. Doppler enhancement with SH U 508A in multiple vascular regions. Radiology. 1994;193:195. doi: 10.1148/radiology.193.1.7916468. [DOI] [PubMed] [Google Scholar]
- Sehgal CM, Arger PH, Pugh CR. Sonographic enhancement of the renal cortex by contrast media. J Ultrasound Med. 1995;14:741. doi: 10.7863/jum.1995.14.10.741. [DOI] [PubMed] [Google Scholar]
- Sehgal CM, LaVeen RF, Shalansky-Goldberg RD. Ultrasound-assisted thrombolysis. Invest Radiol. 1993;28:939. doi: 10.1097/00004424-199310000-00016. [DOI] [PubMed] [Google Scholar]
- Shastri KA, Logue GL, Lundgren CE, et al. Diving decompression fails to activate complement. Undersea Hyperb Med. 1997;24:51. [PubMed] [Google Scholar]
- Shaw PJ, Bates D, Cartilidge NEF, et al. Neurologic and neuropsychological morbidity following major surgery: Comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987;18:700. doi: 10.1161/01.str.18.4.700. [DOI] [PubMed] [Google Scholar]
- Shung KK, Flenniken RR. Time-domain ultrasonic contrast blood flowmetry. Ultrasound Med Biol. 1995;21:71. doi: 10.1016/0301-5629(94)00096-4. [DOI] [PubMed] [Google Scholar]
- Shung KK, Smith MB, Tsui BMW. Principles of Medical Imaging. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Shung KK, Thieme GA. Ultrasonic Scattering in Biological Tissue. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Shung KK, Zipparo M. Ultrasonic transducers and arrays. IEEE Eng Med Biol Mag. 1996;15:20. [Google Scholar]
- Siddiqi TA, O’Brien WD, Jr, Meyer RA, et al. In situ exposimetry: The ovarian ultrasound examination. Ultrasound Med Biol. 1991;17:257. doi: 10.1016/0301-5629(91)90047-z. [DOI] [PubMed] [Google Scholar]
- Simon RH. The biology and biochemistry of pulmonary epithelial cells. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992a. pp. 545–564. [Google Scholar]
- Simon RH, Ho SY, Lange SC, et al. Applications of lipid-coated microbubble contrast to tumor therapy. Ultrasound Med Biol. 1993;19:123. doi: 10.1016/0301-5629(93)90004-8. [DOI] [PubMed] [Google Scholar]
- Simon RH, Ho SY, Perkins CR, et al. Quantitative assessment of tumor enhancement by ultrastable lipid-coated microbubbles as a sonographic contrast agent. Invest Radiol. 1992b;27:29. doi: 10.1097/00004424-199201000-00003. [DOI] [PubMed] [Google Scholar]
- Singh C, Katyal SL. Secretory proteins of clara cells and type ii cells. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 93–108. [Google Scholar]
- Skyba DM, Price RJ, Linka AZ, et al. Microbubble destruction by ultrasound results in capillary rupture: Adverse bioeffects or a possible mechanism for in vivo drug delivery? J Am Soc Echocardiogr. 1998;11:497. [Google Scholar]
- Smith MD, Elion JL, McClure RR, et al. Left heart opacification with peripheral venous injection of a new saccharide echo contrast agent in dogs. J Am Coll Cardiol. 1989;13:1622. doi: 10.1016/0735-1097(89)90357-4. [DOI] [PubMed] [Google Scholar]
- Smith MD, Kwan OL, Reiser J, et al. Superior intensity and reproducibility of SHU-454, a new right’ heart contrast agent. J Am Coll Cardiol. 1984;3:992. doi: 10.1016/s0735-1097(84)80358-7. [DOI] [PubMed] [Google Scholar]
- Smith NB, Vorhees CV, Meyer A. Proceedings of the 1990 IEEE Ultrasonics Symposium. 1990. An automated ultrasonic exposure system to assess the effects of in utero diagnostic ultrasound; pp. 1385–1388. [Google Scholar]
- Smith PL, Treasure T, Newman SP, et al. Cerebral consequences of cardiopulmonary by pass. Lancet. 1986;1:823. doi: 10.1016/s0140-6736(86)90938-4. [DOI] [PubMed] [Google Scholar]
- Solleder P, Beller KD, Linder R. BY963: A sonographic contrast medium. Acad Radiol. 1996;3:S194. doi: 10.1016/s1076-6332(96)80532-0. [DOI] [PubMed] [Google Scholar]
- Sonne HS, Christensen PD, Muan B, et al. Left ventricular opacification after intravenous injection of Albunex®: The effect of different administration procedures. Int J Cardiac Imaging. 1995;11:47. doi: 10.1007/BF01148953. [DOI] [PubMed] [Google Scholar]
- Sotaniemi KA, Mononen H, Hokkanen TE. Long-term cerebral outcome after open-heart surgery. A five-year neuropsychological follow-up study. Stroke. 1986;17:410. doi: 10.1161/01.str.17.3.410. [DOI] [PubMed] [Google Scholar]
- Starritt HC, Duck FA. Quantification of acoustic shock in routine exposure measurement. Ultrasound Med Biol. 1992;18:513. doi: 10.1016/0301-5629(92)90090-w. [DOI] [PubMed] [Google Scholar]
- Starritt HC, Duck FA, Humphrey VF. An experimental investigation of streaming in pulsed diagnostic ultrasound beams. Ultrasound Med Biol. 1989;15:363. doi: 10.1016/0301-5629(89)90048-3. [DOI] [PubMed] [Google Scholar]
- Stella M, Trevison L, Montaldi A, et al. Induction of sister-chromatid exchanges in human lymphocytes exposed in vitro and in vivo to therapeutic ultrasound. Mutat Res. 1984;138:75. doi: 10.1016/0165-1218(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Stevenson D, Walther F, Long W, et al. Controlled trial of a single dose of synthetic surfactant at birth in premature infants weighting 500 to 699 grams. J Pediatr. 1992;120:S3. doi: 10.1016/s0022-3476(05)81226-0. [DOI] [PubMed] [Google Scholar]
- Stonehill MA, Williams JC, Jr, Bailey MR, et al. An acoustically matched high pressure chamber for control of cavitation in shock wave lithotripsy: Mechanisms of shock wave damage in vitro. Methods Cell Science. 1998;19:303. [Google Scholar]
- Suhr D, Brummer F, Irmer U, et al. Disturbance of cellular calcium homeostasis by in vitro application of shock waves. Ultrasound Med Biol. 1996;22:671. doi: 10.1016/0301-5629(96)00044-0. [DOI] [PubMed] [Google Scholar]
- Suhr D, Brummer F, Irmer U, et al. Reduced cavitation-induced cellular damage by the antioxidative effect of vitamin E. Ultrasonics. 1994;32:301. doi: 10.1016/0041-624x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Suneetha N, Kumar RP. Ultrasound-induced enhancement of ACH, ACHE and GABA in fetal brain tissue of mouse. Ultrasound Med Biol. 1993;19:411. doi: 10.1016/0301-5629(93)90060-2. [DOI] [PubMed] [Google Scholar]
- Suren A, Osmers R, Kulenkampff D, et al. Visualization of blood flow in small ovarian tumor vessel by transvaginal color Doppler sonography after echo enhancement with injection of Levovist. Gynecol Obstet Invest. 1994;38:210. doi: 10.1159/000292481. [DOI] [PubMed] [Google Scholar]
- Szabo T, Grossman C, Clougherty F. Tissue loss effects on acoustic output parameters. In: Edmonds P, editor. American Institute of Ultrasound in Medicine Workshop on Effects of Nonlinear Propagation on Output Display Indices (TI and MI), Abstracts and Handouts, Boston, March 20, 1998. Laurel, MD: American Institute of Ultrasound in Medicine; 1998. Chair. [Google Scholar]
- Tachibana K. Enhancement of fibrinolysis with ultrasound energy. J Vasc Interv Radiol. 1992;3:299. doi: 10.1016/s1051-0443(92)72029-6. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Tachibana S. Prototype therapeutic ultrasound emitting catheter for accelerating thrombolysis. J Ultrasound Med. 1997;16:529. doi: 10.7863/jum.1997.16.8.529. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Canfield DR. Ultrasound-induced lung hemorrhage in the monkey. Ultrasound Med Biol. 1994;20:65. doi: 10.1016/0301-5629(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Ecklund K, Dunning PS. Renal cortical perfusion in rabbits: Visualization with color amplitude imaging and an experimental microbubble-based US contrast agent. Radiology. 1996;210:125. doi: 10.1148/radiology.201.1.8816532. [DOI] [PubMed] [Google Scholar]
- Tei C, Sakamaki T, Shah P, et al. Myocardial contrast echocardiography: A reproducible technique of myocardial opacification for identifying regional perfusion deficits. Circulation. 1983;67:585. doi: 10.1161/01.cir.67.3.585. [DOI] [PubMed] [Google Scholar]
- Tenney SM, Remmers JE. Comparative quantitative morphology of the mammalian lung: Diffusing area. Nature. 1963;197:54. doi: 10.1038/197054a0. [DOI] [PubMed] [Google Scholar]
- ter Haar G, Daniels S. Evidence for ultrasonically induced cavitation in vivo. Phys Med Biol. 1981;26:1145. doi: 10.1088/0031-9155/26/6/013. [DOI] [PubMed] [Google Scholar]
- Thorsen E, Risberg J, Segabdal K, et al. Effects of venous gas microemboli on pulmonary gas transfer function. Undersea Hyperb Med. 1995;22:347. [PubMed] [Google Scholar]
- Trenchard PM. Ultrasound-induced orientation of discoid platelets and simultaneous changes in light transmission: Preliminary characterization of the phenomenon. Ultrasound Med Biol. 1987;13:183. doi: 10.1016/0301-5629(87)90120-7. [DOI] [PubMed] [Google Scholar]
- Tsirulnikov EM, Vartanyan IA, Gersurd GV, et al. Use of amplitude-modulated focused ultrasound for diagnosis of hearing disorders. Ultrasound Med Biol. 1988;14:277. doi: 10.1016/0301-5629(88)90093-2. [DOI] [PubMed] [Google Scholar]
- Tufecki EC, Girit S, Bayirli E. Evaluation of tubal patency by transvaginal sonosalpingography. Fertil Steril. 1992;57:336. doi: 10.1016/s0015-0282(16)54841-9. [DOI] [PubMed] [Google Scholar]
- Tyler WS, Julian MD. Gross and subgross anatomy of lungs, pleura, connective tissue septa, distal airways, and structural units. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 37–47. [Google Scholar]
- Uhlendorf V, Hoffmann C. Nonlinear acoustical response of coated microbubbles in diagnostic ultrasound. Cannes, France, IEEE Ultrasonics Symposium Proceedings. 1994;3:1559. [Google Scholar]
- Uhlendorf V, Scholle F-D. Imaging of spatial distribution and flow of microbubbles using nonlinear acoustic properties. Acoustic Imaging. 1996;22:233. [Google Scholar]
- Umemura S, Kawabata K. 1993 IEEE Ultrasonics Symposium Proceedings; Enhancement of sonochemical reactions by second-harmonic superimposition; New York, NY. 1993. p. 917. [Google Scholar]
- Umemura S, Kawabata K, Sasaki K. In vitro and in vivo enhancement of sonodynamically active cavitation by second-harmonic superimposition. J Acoust Soc Am. 1997;101:569. doi: 10.1121/1.418120. [DOI] [PubMed] [Google Scholar]
- Umemura S, Yumita N, Nishigaki R, et al. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res. 1990;81:962. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger E. Liposomes as myocardial imaging ultrasound contrast agents. Atlantic City, NJ. The Leading Edge in Diagnostic Ultrasound 1995 Conference, First Annual International Symposium of Contrast Agents in Diagnostic Ultrasound, sponsored by the Thomas Jefferson University and Jefferson Medical College; 1995a. [Google Scholar]
- Unger E. MRX-115 Aerosomes as ultrasound contrast agents for diagnostic radiology. Chicago, IL: Advances in Echocardiography Symposium; 1995b. [Google Scholar]
- Unger EC, Lund PJ, Shen DK, et al. Nitrogen-filled liposomes as a vascular US contrast agent: Preliminary evaluation. Radiology. 1992;185:453. doi: 10.1148/radiology.185.2.1410353. [DOI] [PubMed] [Google Scholar]
- Vakil N, Everbach EC. Transient acoustic cavitation in gallstone fragmentation: A study of gallstones fragmented in vivo. Ultrasound Med Biol. 1993;19:331. doi: 10.1016/0301-5629(93)90105-w. [DOI] [PubMed] [Google Scholar]
- Van Liew HD, Burkard ME. Behavior of bubbles of slowly permeating gas used for ultrasonic imaging contrast. Invest Radiol. 1995a;30:315. doi: 10.1097/00004424-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Van Liew HD, Burkard ME. Bubbles in circulating blood: Stabilization and simulations of cyclic changes of size and content. J Appl Physiol. 1995b;74:1379. doi: 10.1152/jappl.1995.79.4.1379. [DOI] [PubMed] [Google Scholar]
- Van Roessel J, Wamsteker K, Exalto N. Sonographic investigation of the uterus during artificial uterine cavity distention. J Clin Ultrasound. 1987;15:439. doi: 10.1002/jcu.1870150703. [DOI] [PubMed] [Google Scholar]
- Vandenberg BF, Melton HG. Acoustic lability of albumin microspheres. J Am Soc Echocardiogr. 1994;7:582. doi: 10.1016/s0894-7317(14)80080-1. [DOI] [PubMed] [Google Scholar]
- Veltri A, Capello S, Faissola B, et al. Dynamic contrast-enhanced ultrasound with carbon dioxide microbubbles as adjunct to arteriography of liver tumors. Cardiovasc Intervent Radiol. 1994;17:133. doi: 10.1007/BF00195505. [DOI] [PubMed] [Google Scholar]
- Venezia R, Zangara C. Echohysterosalpingography: New diagnostic possibilities with SHU 450 Echovist. Acta Eur Fertil. 1991;22:279. [PubMed] [Google Scholar]
- Veress E, Vincze J. The haemolysing action of ultrasound on erythrocytes. Acustica. 1977;36:100. [Google Scholar]
- Verrall RE, Seghal CM. Sonoluminescence. In: Suslick KS, editor. Ultrasound: Its Chemical, Physical and Biological Effects. New York, NY: VCH Publishers; 1988. p. 227. [Google Scholar]
- Villanueva F, Glasheen WP, Sklenar J, et al. Assessment of risk area coronary occlusion and infarct size after reperfusion with myocardial contrast echocardiography using left and right atrial injections of contrast. Circulation. 1993;88:596. doi: 10.1161/01.cir.88.2.596. [DOI] [PubMed] [Google Scholar]
- Villanueva F, Glasheen WP, Sklenar J, et al. Successful and reproducible myocardial opacification during two-dimensional echocardiography from right heart injection of contrast. Circulation. 1992;85:1557. doi: 10.1161/01.cir.85.4.1557. [DOI] [PubMed] [Google Scholar]
- Vivino AA, Boraker DK, Miller D, et al. Stable cavitation at low ultrasonic intensities induces cell death and inhibits 3H-TdR incorporation by Con-A-stimulated murine lymphocytes in vitro. Ultrasound Med Biol. 1985;11:751. doi: 10.1016/0301-5629(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Vladimirtseva AL, Manzhos PI. Effect of low-frequency ultrasound on the pleura and adjoining lung tissue. (Russian) Biull Eksp Biol Med. 1986;102:102. [PubMed] [Google Scholar]
- Voelkel NF. Lung endothelial cell biology. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 565–571. [Google Scholar]
- Vogel J, Hopf C, Eysel P, et al. Application of extracorporeal shock-waves in the treatment of pseudarthrosis of the lower extremity. Preliminary results. Arch Orthop Trauma Surg. 1997;116:480. doi: 10.1007/BF00387581. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, et al. Behavior teratologic effects of prenatal exposure to continuous-wave ultrasound in unanesthetized rats. Teratology. 1994;50:238. doi: 10.1002/tera.1420500309. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Weisenburger WP, et al. A teratologic evaluation of continuous-wave, daily ultrasound exposure in unanesthetized rats. Teratology. 1991;44:667. doi: 10.1002/tera.1420440609. [DOI] [PubMed] [Google Scholar]
- Wang S-J, Lewallen DG, Bolander ME, et al. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994;12:40. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- Ward B, Baker AC, Humphrey VF. Nonlinear propagation applied to the improvement of resolution in diagnostic medical ultrasound. J Acoust Soc Am. 1997;101:143. doi: 10.1121/1.417977. [DOI] [PubMed] [Google Scholar]
- Watson JW. Elastic, resistive, and inertial properties of the lung. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton, FL: CRC Press; 1992. pp. 175–216. [Google Scholar]
- Weaver LK, Morris A. Venous and arterial gas embolism associated with positive pressure ventilation. Chest. 1998;113:1132. doi: 10.1378/chest.113.4.1132. [DOI] [PubMed] [Google Scholar]
- Weber C, Moran ME, Braun EJ, et al. Injury of rat renal vessels following extracorporeal shock wave treatment. J Urol. 1992;147:476. doi: 10.1016/s0022-5347(17)37283-x. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Dimensions of the tracheobronchial tree and alveoli. In: Altman PL, Dittmer DS, editors. Biological Handbooks: Respiration arid Circulation. Bethesda, MD: Federation of American Societies for Experimental Biology; 1971. p. 105. [Google Scholar]
- West JB. Respiratory Physiology—The Essentials. 4. Baltimore, MD: Williams and Wilkins; 1990. p. 1. [Google Scholar]
- WFUMB (World Federation of Ultrasound in Medicine and Biology). WFUMB Symposium on Safety and Standardization in Medical Ultrasound. Conclusions and Recommendations Regarding Nonthermal Mechanisms for Biological Effects of Ultrasound. Ultrasound Med Biol. 1998;24(Suppl 1) [PubMed] [Google Scholar]
- Williams AR. Absence of meaningful thresholds for bioeffect studies on cell suspensions in vitro. Br J Cancer. 1982;45:192. [PMC free article] [PubMed] [Google Scholar]
- Williams AR. Ultrasound: Biological Effects and Potential Hazards. New York, NY: Academic Press; 1983. [Google Scholar]
- Williams AR, Delius M, Miller DL, et al. Investigation of cavitation in flowing media by lithotripter shock waves both in vitro and in vivo. Ultrasound Med Biol. 1989;15:53. doi: 10.1016/0301-5629(89)90132-4. [DOI] [PubMed] [Google Scholar]
- Williams AR, Kubowicz G, Cramer E, et al. The effects of the microbubble suspension SH U 454 (Echovist®) on ultrasonically induced cell lysis in a rotating tube exposure system. Echocardiography. 1991;8:423. doi: 10.1111/j.1540-8175.1991.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Williams AR, Miller DL. The role of non-acoustic factors in the induction and proliferation of cavitational activity in vitro. Phys Med Biol. 1989;34:1561. doi: 10.1088/0031-9155/34/11/005. [DOI] [PubMed] [Google Scholar]
- Wiison WL, Wiercinski FL, Nyborg WL, et al. Deformation and motion produced in isolated living cells by localized ultrasonic vibration. J Acoust Soc Am. 1966;40:1363. doi: 10.1121/1.1910235. [DOI] [PubMed] [Google Scholar]
- Winkelmann JW, Kenner MD, Dave R, et al. Contrast echocardiography. Ultrasound Med Biol. 1994;20:507. doi: 10.1016/0301-5629(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Wray RA, Zoghbi WA, Quinones MA, et al. Contrast echocardiography: Relation of acoustic power and time gain compensation to contrast intensity duration. J Am Soc Echocardiog. 1992;4:286. [Google Scholar]
- Wu J, Tong J. Experimental study of stability of contrast agents in an ultrasound field. Ultrasound Med Biol. 1998a;24:257. doi: 10.1016/s0301-5629(97)00245-7. [DOI] [PubMed] [Google Scholar]
- Wu J, Tong J. Measurements of nonlinearity parameter B/A of contrast agents. Ultrasound Med Biol. 1998b;24:153. doi: 10.1016/s0301-5629(97)00207-x. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhu Z, Du G. Nonlinear behavior of a liquid containing uniform bubbles: Comparison between theory arid experiments. Ultrasound Med Biol. 1995;21:545. doi: 10.1016/0301-5629(94)00134-y. [DOI] [PubMed] [Google Scholar]
- Xavier CAM, Duarte LR. Treatment of nonunions by ultrasound stimulation: First clinical applications. San Francisco, CA. Annual Meeting of the American Academy of Orthopaedic Surgeons; January 25, 1987. [Google Scholar]
- Yang KH, Parvizi J, Wang SJ, et al. Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthopaed Res. 1996;14:802. doi: 10.1002/jor.1100140518. [DOI] [PubMed] [Google Scholar]
- Yarali H, Gurgan T, Erden A, et al. Color Doppler hys terosalpingosonography: A single and potentially useful method to evaluate fallopian tubal patency. Hum Reprod. 1994;9:64. doi: 10.1093/oxfordjournals.humrep.a138321. [DOI] [PubMed] [Google Scholar]
- Yount DE. On the evolution, generation, and regeneration of gas cavitation nuclei. J Acoust Soc Am. 1982;71:1473. [Google Scholar]
- Yumita N, Nishigaki R, Umemura K, et al. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Nishigaki R, Umemura K, et al. Synergistic effect of ultrasound and hematoporphyrin on Sarcoma 180. Jpn J Cancer Res. 1990;81:304. doi: 10.1111/j.1349-7006.1990.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumita N, Sasaki K, Umemura S, et al. Sono-dynamically induced antitumor effect of gallium-porphyrin complex by focused ultrasound on experimental kidney tumor. Cancer Lett. 1997;112:79. doi: 10.1016/s0304-3835(96)04548-x. [DOI] [PubMed] [Google Scholar]
- Zachary JF, O’Brien WD., Jr Lung lesion induced by continuous- and pulsed-wave (diagnostic) ultrasound in mice, rabbits, and pigs. Vet Pathol. 1995;32:43. doi: 10.1177/030098589503200106. [DOI] [PubMed] [Google Scholar]
- Zagzebski JA. Essentials of Ultrasound Physics. St Louis, MO: CV Mosby; 1996. [Google Scholar]
- Zhong P. Effects of tissue constraining on shock wave-induced bubble oscillation in vivo. J Acoust Soc Am. 1998;103:3038. doi: 10.1121/1.423905. [DOI] [PubMed] [Google Scholar]


