Abstract
Although psilocybin has been trained in the rat as a discriminative stimulus, little is known of the pharmacological receptors essential for stimulus control. In the present investigation rats were trained with psilocybin and tests were then conducted employing a series of other hallucinogens and presumed antagonists. An intermediate degree of antagonism of psilocybin was observed following treatment with the 5-HT2A receptor antagonist, M100907. In contrast, no significant antagonism was observed following treatment with the 5-HT1A/7 receptor antagonist, WAY-100635, or the DA D2 antagonist, remoxipride. Psilocybin generalized fully to DOM, LSD, psilocin, and, in the presence of WAY-100635, DMT while partial generalization was seen to 2C-T-7 and mescaline. LSD and MDMA partially generalized to psilocybin and these effects were completely blocked by M-100907; no generalization of PCP to psilocybin was seen. The present data suggest that psilocybin induces a compound stimulus in which activity at the 5-HT2A receptor plays a prominent but incomplete role. In addition, psilocybin differs from closely related hallucinogens such as 5-MeO-DMT in that agonism at 5-HT1A receptors appears to play no role in psilocybin-induced stimulus control.
Keywords: Psilocybin, Drug discrimination, Rat, LSD, M100907, WAY-100635, Ketanserin, Bufotenine, 2C-T-7, MDMA, PCP
1. Introduction
Archeological evidence suggests that hallucinogenic mushrooms have been in use for at least two millennia by the indigenous peoples of what are now Guatemala and Mexico. For example, a feature of Aztec civilization as encountered by Cortez in the 16th century was the use in religious ceremonies of mushrooms called teonanacatl (Schultes and Hofmann, 1980). Despite numerous first person accounts of teonanacatl intoxication (e.g., Wasson and Wasson, 1957), identification of its active principles awaited the cultivation of Psilocybe mexicana and the isolation of psilocybin and psilocin by Heim, Hofmann, and their colleagues (Hofmann et al., 1959; Hofmann and Troxler, 1959). Psilocybin [O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine] is rapidly dephosphorylated in vivo to form 4-hydroxy-N,N-dimethyltryptamine [psilocin]; the latter is the presumed active principle (Horita, 1963; Horita and Weber, 1961). In addition to anecdotal reports, a number of clinical studies were conducted during this period and detailed descriptions of the human experience were provided (Isbell, 1959; Hollister, 1961; Malitz et al., 1960; Rinkel et al., 1960). There followed a long hiatus in human research which ended in the late 1990s with the work of Vollenweider et al. (1998) and Gouzoulis-Mayfrank et al. (1999). [For brief reviews of human studies, see Nichols (2004) and Griffiths et al. (2006)] From a pharmacological perspective, perhaps the most significant advance since Hofmann’s isolation of psilocybin was the report by Vollenweider et al. (1998) of the antagonism of the effects of psilocybin in human subjects by the serotonergic antagonist, ketanserin, and by the serotonergic/dopaminergic antagonist, risperidone.
Dependent variables employed in the initial attempts to isolate active principles from P. mexicana Heim included general behavior in dogs and pupillary reaction time and piloerection in mice. The results were inconclusive; indeed, the very activity of cultivated mushrooms was called into question. The issue was settled by Hofmann’s self-administration of P. mexicana Heim resulting in an unequivocal demonstration of a psychotomimetic effect (Hofmann, 1968). Subsequent extraction of active principles culminating in the identification of psilocybin and psilocin employed human experimentation. Brimblecombe and Pinder (1975) would later write that psilocybin “demonstrates how difficult it is to obtain meaningful results in animals with a drug which produces effects chiefly on the psyche”. However, with the demonstration that mescaline and lysergic acid diethylamide [LSD] can function as discriminative stimuli in the rat (Hirschhorn and Winter, 1971), it was suggested that drug-induced stimulus control in nonverbal species might permit preclinical identification of such agents as well as provide insight into their possible mechanisms of action (Winter, 1974).
The first report of psilocybin-induced stimulus control was provided by Harris and Balster (1971) who observed, in two Sprague-Dawley rats, that stimulus control was “weak” relative to amphetamine and other psychoactive drugs. In addition, suggestive evidence was provided that psilocybin and LSD could not be distinguished by these rats. A similar conclusion was reached by Schechter and Rosecrans (1972) who employed a T-maze discrimination procedure. Subsequently it was reported that rats trained with psilocybin generalized fully to psilocin and to LSD but not to the phenethylamine [PEA] hallucinogen, mescaline (Koerner and Appel, 1982). However, the last-named observation appears not to be symmetrical, in that rats trained with mescaline (Callahan and Appel, 1988; Appel and Callahan, 1989) or [-]-DOM (Silverman and Ho, 1980), another PEA hallucinogen, generalize to psilocybin. In addition, psilocybin mimics LSD or quipazine in animals trained with the latter drugs (Appel et al., 1978; Jarbe, 1980; Callahan and Appel, 1988) while THC-trained rats do not generalize to psilocybin (Melges et al., 1970; Jarbe and Henriksson, 1974).
The goal of the present investigation was to characterize stimulus control by psilocybin in the rat more fully in terms of its generalization to other hallucinogens as well as to identify possible pharmacological antagonists of the stimulus effects of psilocybin. Previous studies of stimulus control by indoleamine and phenethylamine hallucinogens in the rat have strongly suggested a prominent role for agonism at serotonergic receptors especially those of the 5-HT1A, 5-HT2A, and 5-HT2C subtypes (Winter, 1978; Spencer et al., 1987; Glennon, 1990; Fiorella et al., 1995a) as well as the possibility of dopaminergic influences (Marona-Lewicka et al., 2005). For this reason, the interaction of selective antagonists at each of these receptors with psilocybin-induced stimulus control was examined. In an attempt to ascertain the symmetry of psilocybin generalizations, tests of generalization of the stimulus effects of LSD, phencyclidine [PCP], and methylenedioxymethamphetamine [MDMA, Ecstasy] to those of psilocybin were conducted.
2. Methods
2.1. Animals
All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Animal Care and Use Committee of the State University of New York at Buffalo. Male Fischer-344 rats obtained from Harlan Sprague-Dawley Inc. (Indianapolis, IN, USA) at an age of approximately 6 weeks were used in all discrimination experiments. Rats were housed in pairs with free access to water in a temperature-controlled room at the State University of New York at Buffalo under a constant 12-h light/dark cycle (all experiments were conducted during the light phase). Caloric intake was controlled to yield a mean body weight of approximately 300 g; supplemental feedings of standard rat chow were provided following experimental sessions.
2.2. Drug-induced stimulus control
Eight small animal test chambers (Med-Associates Model ENV-008), each equipped with a house light and an exhaust fan, and housed in larger lightproof Malaguard sound attenuating cubicles (Med-Associates Model ENV-022M) were used for these experiments. The chamber contained two levers mounted on opposite sides of one wall. Centered between the levers was a dipper that delivered 0.1 ml of sweetened condensed milk diluted 2:1 with tap water. A total of 50 rats were trained with psilocybin [0.5 mg/kg], LSD [0.1 mg/kg], MDMA [1.5 mg/kg], and PCP [3.0 mg/kg] in groups of 12, 12, 10, and 16, respectively. All injections were intraperitoneal [IP] with a pretreatment time of 15 min for all but PCP which was injected 30 min before training.
The general methods were as previously described (Winter et al., 2004). A non-resetting fixed ratio 10 (FR10) schedule of reinforcement was employed using the MED-PC version IV behavioral programming application. After learning to drink from the dipper, rats were trained to press first one and then the other of the two levers. The number of responses for each reinforcement was gradually increased from 1 to 10. During this time, the reinforced lever was alternated on a random basis. All subsequent training and testing sessions used a fixed ratio 10 (FR10) schedule of reinforcement. Discrimination training was then begun. Following the administration of drug, every tenth response on the drug-appropriate lever was reinforced. Similarly, responses on the vehicle-appropriate lever were reinforced on a FR10 schedule following the injection of vehicle. For half of the subjects, the left lever was designated as the drug-appropriate lever. During discrimination training, drug and vehicle were alternated on a daily basis. Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, 83% or more of all responses prior to the delivery of the first reinforcer were on the appropriate lever, i.e., no more than 2 incorrect responses prior to completion of the FR10 on the correct lever. After stimulus control was established with the training agents, tests of generalization were conducted once per week in each animal so long as performance did not fall below the criterion level of 83% correct responding in any one of the previous three training sessions. Values were calculated independently for each animal and mean percentages for the group are presented in all figures. Half of the test sessions were conducted the day after saline training sessions with the remainder following drug training sessions. During test sessions, no responses were reinforced and the session was terminated after the emission of ten responses on either lever. The distribution of responses between the two levers was expressed as a percentage of total responses emitted on the drug-appropriate lever. Response rate was calculated for each session by dividing the total number of responses emitted on both levers by the elapsed time prior to 10 responses on either lever.
Complete generalization of a training drug to a test drug is said to be present when (a) a mean of 80% or more of all test responses occurs on the drug-appropriate lever; (b) there is no statistically significant difference between the response distributions of the training drug and the test drug; and (c) there is a statistically significant difference between the response distributions of the test drug and saline control sessions. An intermediate degree of generalization is defined as being present when response distributions after a test drug are between 20 and 80% drug-appropriate and are significantly different from both training conditions. Finally, when the response distribution after a test drug is not statistically significantly different from that in saline control sessions, an absence of generalization of the training drug to the test drug is assumed. Similar criteria are applied to the definitions of full, partial, and no antagonism. Thus, full antagonism is assumed to be present when (a) less than 20% of all test responses are on the training drug-appropriate lever; (b) there is no significant difference between the response distributions in the test of antagonism and the saline control, and (c) there is a statistically significant difference between the response distributions of the test drug alone and in combination with the antagonist. Pretreatment times for antagonists are as follows. Ketanserin, pirenperone, M100907: 60 min; remoxipride: 40 min; SB-242084: 20 min. All antagonists were administered IP with the exception of WAY-100635 which was injected SC.
2.3. Data analysis
Drug discrimination data are expressed as percent drug-appropriate responding, which is the number of responses emitted on the drug-appropriate lever as a percentage of the total number of responses emitted. Data for any subjects failing to emit 10 responses within the constraints of the 10-min test session were not considered in the calculation of the percent drug-appropriate responding but were included in the analysis of response rates.
Two-sample comparisons were by means of Student’s t-test for paired values. In the event that the data failed tests of either normality or equal variance, the signed ranks test was employed. For multiple comparisons, data were submitted to repeated measures analysis of variance [ANOVA] with subsequent pair-wise comparisons using the Holm-Sidak method. In the event that the data failed tests of either normality or equal variance, Friedman repeated measures ANOVA on ranks was applied with subsequent pair-wise comparisons by the Tukey test. All differences were considered to be statistically significant if the probability of their having arisen by chance was <0.05. All statistical analyses were conducted using SigmaStat 3.1 for WindowsÔ (Jandel Scientific Software, San Rafael, CA). Control data were repeated for each comparison and statistical analyses were applied using the appropriate control sessions. However, for purposes of clarity, mean values for control data are shown in all figures.
2.4. Drugs
Psilocybin, psilocin, LSD [+-lysergic acid diethylamide (+)-tartrate 2:1], [+/-]-MDMA [methylenedioxymethamphetamine HCL], bufotenine [5-hydroxy-N,N-dimethyltryptamine], [-]-DOM [2,5-dimethoxy-4-methylamphetamine HCl], mescaline [3,4,5-trimethoxyphenylethylamine HCL], and DMT [N,N-dimethyltryptamine fumarate] were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC). 2C-T-7 [2,5-dimethoxy-4-propylthiophenethylamine HCl] was provided by Dr. Andrew Coop of the Drug Evaluation Committee of the College on Problems of Drug Dependence. M100907 was synthesized at the Laboratory of Medicinal Chemistry at the National Institutes of Diabetes, Digestive and Kidney Disorders at the National Institutes of Health (Bethesda, MD). SB-242084 was generously provided by GlaxoSmithKline, United Kingdom. Pirenperone and WAY-100635 were purchased from Sigma-Aldrich [St. Louis, MO]. DPAT [(+/-)-8-hydroxy-2-dipropylaminotetralin hydrobromide], remoxipride hydrochloride, and ketanserin tartrate were purchased from Tocris Bioscience [Ellisville, MO]. All drugs were dissolved in 0.9% sodium chloride solution. Doses of LSD, MDMA, DOM, DMT, and 2C-T-7 are expressed in terms of their respective salts. All injections were administered ip at a volume of 1.0 ml/kg.
3. Results
3.1. Tests of antagonism
Fig. 1 illustrates the effects on psilocybin-induced stimulus control of pirenperone, a 5-HT2A/2C antagonist, M100907, an antagonist selective for the 5-HT2A receptor, and WAY-100635, a selective antagonist at 5-HT1A/7 receptors. The doses of pirenperone and M100907 selected for use have earlier been shown to fully antagonize stimulus control by LSD (Winter et al., 2004). Our criteria for intermediate antagonism were met for both doses of pirenperone and of M100907 [pirenperone 0.08 mg/kg: F(2,10)=90.15; P<0.001; 0.16 mg/kg: F(2,10)=55.51; P<0.001; M100907 0.1 mg/kg: F(2,11)=45.28; P<0.001; 0.2 mg/kg: F(2,11)=39.55; P<0.001]. In light of the absence of antagonism of the stimulus effects of psilocybin by any of the doses of WAY-100635 tested, it should be noted that the same doses have previously been shown to block completely the stimulus effects of the selective 5-HT1A/7 agonist, DPAT (Reissig et al., 2005). Furthermore, it is seen in Fig. 1 that, while DPAT does not significantly mimic psilocybin, responding is diminished at a dose of 0.1 mg/kg and completely suppressed at a dose of 0.2 mg/kg of DPAT. However, when the subjects were pretreated with a dose of WAY-100635 of 0.3 mg/kg, the rate suppressant effects of DPAT were substantially antagonized [paired t-tests: 0.1 mg/kg: P<0.005; 0.2 mg/kg: P<0.001]. Thus, the failure of WAY-100635 to antagonize psilocybin cannot be attributed to inadequate occupation of 5-HT1A receptors by the antagonist. With respect to response rates, it should be noted as well that at both doses of pirenperone and M100907 and at the two higher doses of WAY-10035, response rates were significantly reduced compared with vehicle control rates. The influence which rate change per se may have had on diminishing psilocybin-appropriate responding is unknown.
Fig. 1.
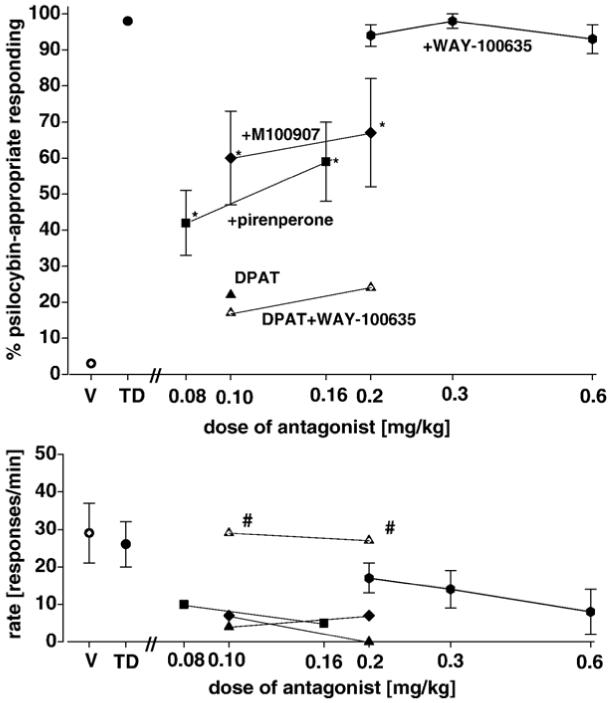
Effects of a range of doses of M100907 [diamonds], pirenperone [squares], and WAY-100635 [hexagons] on stimulus control by the training dose of psilocybin [0.5 mg/kg]. For M100907 and WAY-100635, each point represents the mean of one determination in each of 12 rats. For pirenperone, each point represents the mean of 2 determinations in each of 11 rats. Standard errors of the mean are indicated. Also shown is the generalization of psilocybin to DPAT alone [closed triangles] and in the presence of a dose of WAY-100635 of 0.3 mg/kg [open triangles]. The points at V and TD on the abscissa are for saline and psilocybin training sessions, respectively. *Significantly different from both training conditions. #Significantly different from DPAT alone. &Rate significantly different from psilocybin alone. Ordinate: upper panel: percent psilocybin-appropriate responding; lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
The results of further exploration of possible antagonists of psilocybin are shown in Fig. 2. A range of doses of the 5-HT2A/2C antagonist, ketanserin [squares], the 5-HT2C-selective antagonist, SB-242084 [diamonds], and the dopamine D2 antagonist, remoxipride [hexagons], was administered in combination with the training dose of psilocybin. We have previously shown that ketanserin at a dose of 1.0 mg/kg blocks the stimulus effects of LSD by approximately 75% (Fiorella et al., 1995a) and that SB-242084 at a dose of 2.0 mg/kg blocks the stimulus effects of the non-selective 5-HT2C agonist, meta-chlorophenylpiperazine (Eckler et al., 2004). Our criteria for intermediate antagonism were met for the two higher doses of ketanserin [2.0 mg/kg: F(2,8)=80.52; P<0.001; 4.0 mg/kg: F(2,8)=56.02; P<0.001]. Neither dose of SB242084 met the criteria for intermediate antagonism but for both doses, psilocybin-appropriate responding was significantly diminished in the presence of SB242084 [paired t-tests; 1.0 mg/kg: P<0.008; 3.0 mg/kg: P<0.023]. None of the doses of remoxipride resulted in significant antagonism of psilocybin.
Fig. 2.
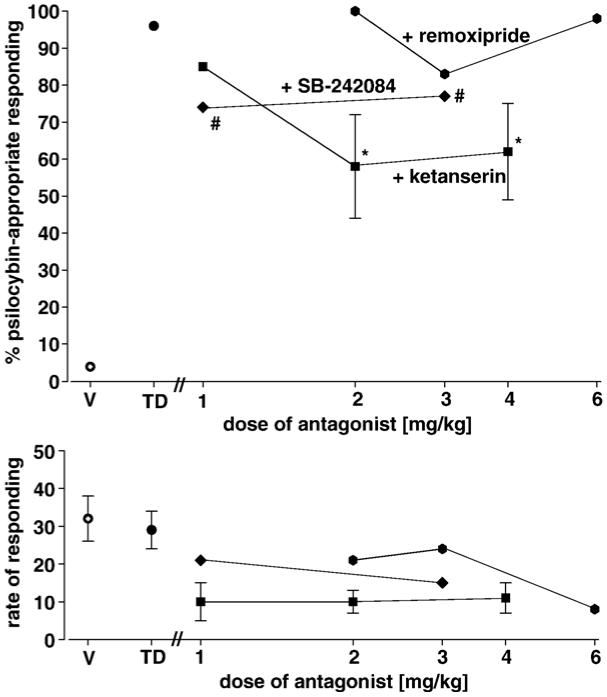
Effects of a range of doses of ketanserin [squares], SB-242084 [diamonds], and remoxipride [hexagons] on stimulus control by the training dose of psilocybin [0.5 mg/kg]. Each point represents the mean of one determination in each of 10 rats. *Significantly different from both training conditions. #Significantly different from psilocybin alone. Other details are as in Fig. 1.
3.2. Tests of generalization of psilocybin to other hallucinogens
In Fig. 3 are seen the percentages of psilocybin-appropriate responding following treatment with LSD [squares], psilocin [triangles], DMT [inverted triangles], and bufotenine [diamonds] in rats trained with psilocybin [0.5 mg/kg] as a discriminative stimulus. The results for LSD and psilocin fulfill our criteria for complete generalization. In contrast, bufotenine produced effects not significantly different from saline controls. Although there was no significant difference between the percentage of psilocybin-appropriate responding between the training dose of psilocybin and DMT at a dose of 2.5 mg/kg, the results did not fully meet our criteria for complete generalization in that only 73% of responding was psilocybin-appropriate following DMT. An interesting interaction between antagonism of 5-HT1A receptors by WAY-100635 and DMT is seen as well. In the presence of WAY-100635, the degree of substitution by DMT for psilocybin was slightly higher [82% versus 73%; open hexagon] thus fulfilling our criteria for full generalization while the rate of responding was significantly increased [11 versus 4 responses per minute; signed ranks test: P<0.004], and all subjects completed the tests. Also shown in Fig. 3 are the effects of pretreatment with M100907 on generalization to LSD and to DMT. While stimulus generalization of psilocybin to LSD was completely antagonized by M100907 [open square], no block of DMT was observed [open inverted triangle], response rates were suppressed, and only 6 of 12 animals completed the test session. The latter observation indicates a suppressive effect resulting from the antagonism of 5-HT2A receptors in the presence of DMT.
Fig. 3.
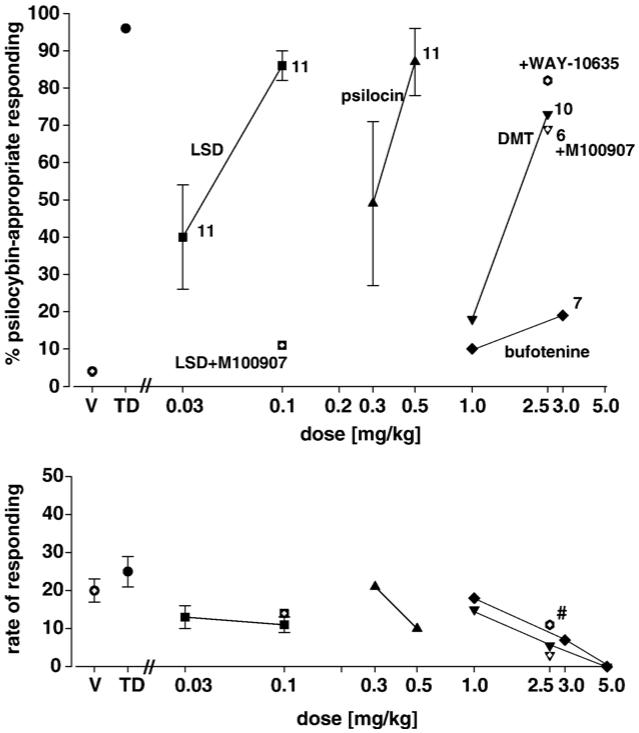
Effects of LSD [squares], psilocin [triangles], DMT [inverted triangles], and bufotenine [diamonds] in rats trained with psilocybin [0.5 mg/kg] as a discriminative stimulus and antagonism of the effects of LSD with M100907 [open square]. Interactions of WAY-100635 and M100907 with DMT are indicated by the open hexagon and open inverted triangle, respectively. Each point represents the mean of one determination in each of 12 rats. A number next to a data point indicates the number of subjects completing the session if less than 12. #Significantly different from DMT alone. Other details are as in Fig. 1.
The results of tests of generalization of psilocybin to the phenethylamine hallucinogens, [-]-DOM [squares], 2C-T-7 [circles], and mescaline [hexagons] in rats trained with psilocybin [0.5 mg/kg] as a discriminative stimulus are shown in Fig. 4. While psilocybin generalized completely to [-]-DOM, substitution of 2C-T-7 [1.0 mg/kg] and mescaline [3.0 mg/kg] for psilocybin reached maxima of 44% and 76% psilocybin-appropriate responding, respectively. Both values met our criteria for intermediate generalization [F(2,8)=31.53; P<0.001; F(2,11)=158.35; P<0.01, respectively. Also in Fig. 4 are shown the interactions between M100907 and [-]-DOM, 2C-T-7, and mescaline. It is seen that the complete substitution of [-]-DOM and the partial substitution 2C-T-7 and mescaline for psilocybin were completely antagonized by M100907 thus indicating a 5-HT2A receptor-mediated mechanism.
Fig. 4.
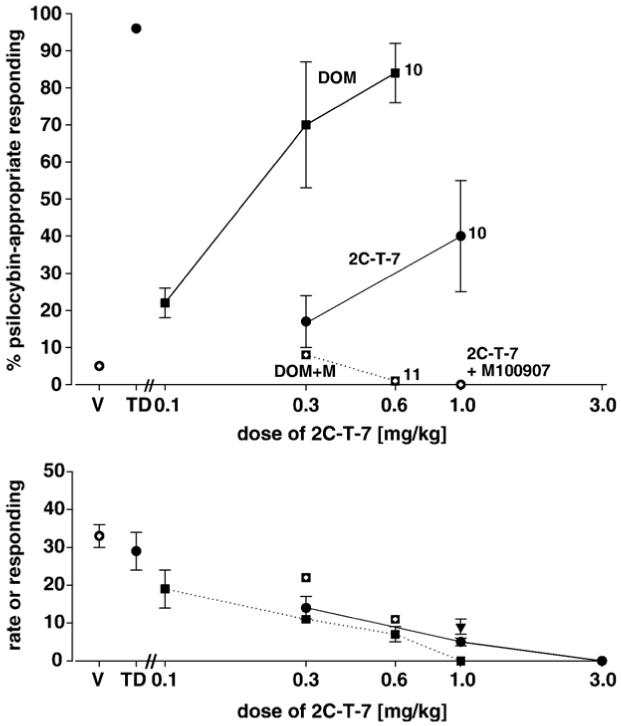
Effects of [-]-DOM [squares], 2C-T-7 [circles], and mescaline [hexagons] in rats trained with psilocybin [0.5 mg/kg] as a discriminative stimulus and antagonism of the effects of [-]-DOM and 2C-T-7 with M100907 [open squares, circle, and hexagons, respectively]. Each point represents the mean of one determination in each of 12 rats. A number next to a data point indicates the number of subjects completing the session if less than 12. Other details are as in Fig. 1.
3.3. Tests of generalization of LSD, MDMA, and PCP to psilocybin
Separate groups of rats were trained with LSD, MDMA, and PCP, respectively, and the generalization of these drugs to psilocybin was examined. The results for LSD-trained subjects are shown in Fig. 5. Although the pattern of responding following psilocybin [diamonds] appears to be intermediate in nature, the points at doses of psilocybin of 0.8 and 1.0 mg/kg did not fulfill our statistical criteria for intermediate responding as a consequence of the diminished number of rats completing the tests. However, when subjects were treated with 1.0 mg/kg psilocybin following pretreatment with 0.3 mg/kg WAY-100635 [SC], 10 of 12 subjects completed the test giving a mean of 43% LSD-appropriate responding; this value satisfied our criteria for intermediate responding [F(9,2)=152.9; P<0.001]. In contrast with the effects of WAY-100635, pretreatment with M100907 completely antagonized the LSD-like effects of psilocybin, again indicating a 5-HT2A receptor-mediated mechanism.
Fig. 5.
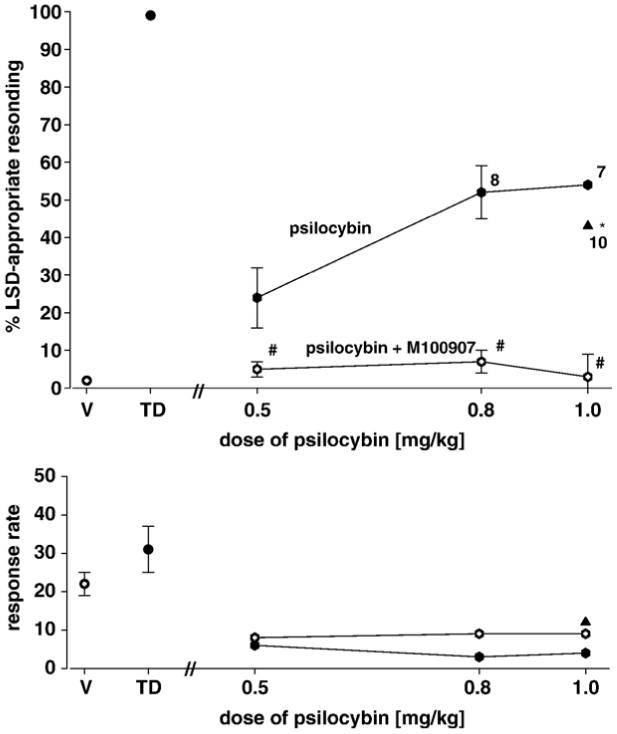
Effects of psilocybin alone [closed hexagons] and in combination with M100907 [open hexagons] or WAY-100635 [triangle] in rats trained with LSD [0.1 mg/kg] as a discriminative stimulus. Each point represents the mean of one determination in each of 12 rats. A number next to a data point indicates the number of subjects completing the session if less than 12. *Significantly different from both training conditions. #Significantly different from psilocybin alone. Ordinate: upper panel: percent LSD-appropriate responding. Other details are as in Figs. 1, 3, and 4.
Fig. 6 shows the results of tests of generalization of MDMA to psilocybin. An intermediate degree of generalization of MDMA to psilocybin was observed at a dose of psilocybin of 0.5 mg/kg [F(11,2)=37.9; P<0.001] and this effect was completely antagonized by pretreatment with M100907 [diamond; paired t-test, P=0.015]. In PCP-trained rats, no PCP-appropriate responding was observed over a range of doses of psilocybin of 0.3-1.0 mg/kg [data not shown]. At the highest dose of PCP responding was completely suppressed.
Fig. 6.
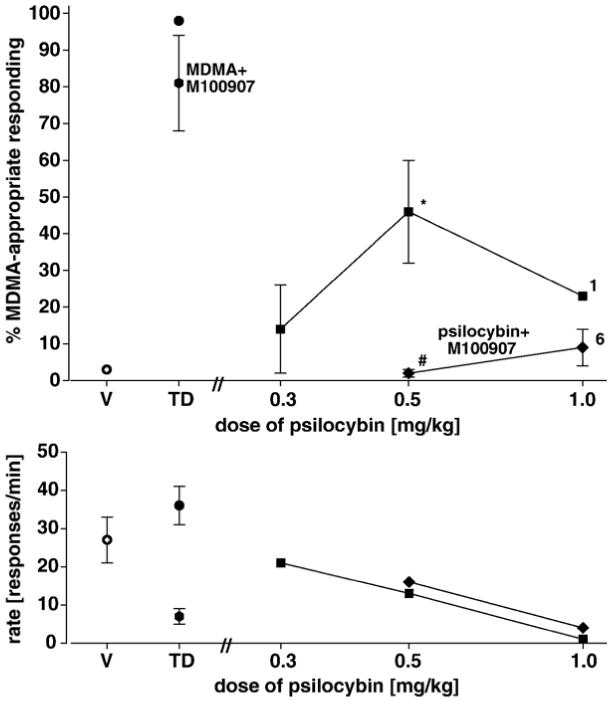
Effects of psilocybin alone [closed hexagons] and in combination with M100907 [open hexagons] in rats trained with MDMA [1.5 mg/kg] as a discriminative stimulus. Each point represents the mean of one determination in each of 10 rats. A number next to a data point indicates the number of subjects completing the session if less than 10. The data points at TD on the dose axis are for the training dose of MDMA alone [closed circle] and in combination with M100907 [hexagon]. *Significantly different from both training conditions.#Significantly different from psilocybin alone. Ordinate: upper panel: percent LSD-appropriate responding. Other details are as in Figs. 1 and 4.
4. Discussion
As was noted above, previous studies of psilocybin-induced stimulus control in animals have been limited in number and have not included the use of pharmacological antagonists. The results of interactions of psilocybin with the 5-HT2A/2C antagonist, pirenperone, and the selective 5-HT2A antagonist, M100907, shown in Fig. 1 suggest that stimulus control by psilocybin is partially mediated by 5-HT2A receptors. This conclusion is further supported by the data of Fig. 5 which indicate that the partial substitution of psilocybin for LSD is completely antagonized by M100907. That psilocybin should induce stimulus control in part via 5-HT2A receptors is not surprising in that there is abundant evidence from the drug discrimination literature that these receptors play a prominent role in the actions of other hallucinogens including LSD as well as those of phenethylamines such as mescaline and [-]-DOM (Fiorella et al., 1995a,b,c; Glennon, 1990; Winter, 1978). In addition, as was noted above, the serotonergic antagonist, ketanserin, blocks the psychotomimetic effects of psilocybin in human subjects (Vollenweider et al., 1998) and the data of Fig. 2 indicate a partial antagonism by ketanserin of stimulus control in rats. In contrast, the 5-HT1A antagonist, WAY-100635, had no effects whatsoever on stimulus control by psilocybin [Fig. 1]. Though long regarded as a selective 5-HT1A antagonist, WAY-100635 has recently been shown to possess significant affinity for 5-HT7 receptors (Bard et al., 1993) and as an agonist at dopamine D4 receptors as well (Chemel et al., 2006). These findings do not alter the conclusion drawn from Fig. 1 that there is no functionally significant, 5-HT1A receptor-mediated element in stimulus control by psilocybin in the rat.
The results with WAY-100635 shown in Fig. 1 are of particular interest for at least two reasons. First we anticipated that psilocybin via its metabolite, psilocin, would have prominent actions at 5-HT1A receptors. This hypothesis was based on previous work which indicated that stimulus control in the rat by the related indoleamine hallucinogen, 5-methoxy-N,N-dimethyltryptamine [MDMT], is mediated by 5-HT1A receptors (Spencer et al., 1987; Winter et al., 2000). Second, Carter et al. (2005) observed in human subjects that psilocybin-induced deficits in attentional performance are not antagonized by ketanserin and concluded that activity at 5-HT1A receptors was thus responsible for this aspect of the actions of psilocybin. Binding data for psilocybin and psilocin are limited and not directly comparable (McKenna et al., 1990; Blair et al., 2000) but are compatible with actions of psilocybin mediated by 5-HT1A, 5-HT2A, 5-HT2c receptors. We have previously provided evidence that actions at 5-HT1A and 5-HT2c receptors may modulate activity of hallucinogens at 5-HT2A receptors (Fiorella et al., 1995a,b,c; Reissig et al., 2005).
Of the three antagonists for which data are shown in Fig. 2, only ketanserin met our criteria for intermediate antagonism. This is in contrast with the complete antagonism of the psychotomimetic effects of psilocybin reported by Vollenweider et al. (1998). In addition, those authors argue for the primacy of effects mediated by 5-HT2A receptors over those of 5-HT2C receptors. The data of Fig. 2 do not disagree with that conclusion but it must be noted that Vollenweider et al. (1998) make their argument on the basis of the affinity of ketanserin being 100-fold greater at 5-HT2A receptors as compared with 5-HT2C receptors. Our calculations suggest that at a concentration of 10 times the KD, approximately 90% of 5-HT2A receptors would be occupied as compared with approximately 26% of 5-HT2C receptors. Thus, while ketanserin is relatively selective for 5-HT2A as compared with 5-HT2C receptors, an effect of the latter receptor cannot be ruled out. On the other hand, the quite modest effects of the selective 5-HT2C receptor antagonist, SB-242084, shown in Fig. 2 argue against a major 5-HT2C receptor-mediated element in stimulus control by psilocybin. However, it should be noted that this conclusion is weakened by the unavailability of a selective agonist at 5-HT2C receptors. Remoxipride, a dopamine D2 receptor-selective antagonist (Mohell et al., 1993), was tested on the basis of numerous suggestions that the hallucinogenic effects of LSD might arise in part via activity at dopamine receptors (Watts et al., 1995). In addition, Marona-Lewicka et al. (2005) have recently provided evidence that stimulus control by LSD may be mediated by both dopaminergic and serotonergic effects. However, the data of Fig. 2 provide no support for the hypothesis that dopamine D2 receptors mediate stimulus control by psilocybin. Furthermore, on the basis of the relative antagonism by ketanserin, risperidone, and haloperidol of the effects of psilocybin in human subjects, Vollenweider et al. (1998) concluded that dopamine D2 receptor-mediated factors play at most a minor role.
The complete generalization of psilocybin to LSD and to psilocin shown in Fig. 3 was not unexpected in light of previous reports noted above (Harris and Balster, 1971; Schechter and Rosecrans, 1972; Koerner and Appel, 1982). However it is noteworthy that M100907 fully antagonized the effects of LSD while only partially blocking psilocybin itself [Fig. 1]. A plausible explanation of these differential effects of M100907 is that, while LSD mimics psilocybin via a 5-HT2A-mediated mechanism, psilocybin induces a compound stimulus in which multiple elements are present. Unfortunately, the antagonism data of Figs. 1 and 2 do not fully identify what those elements might be. Relevant to this question are the data in Fig. 3 indicating a substantial substitution for psilocybin by the simple tryptamine hallucinogen, DMT, and the interactions of DMT with antagonists of at 5-HT2A and 5-HT1A receptors, respectively. Thus the combination of DMT and WAY-100635 increased psilocybin appropriate responding to greater than 80% and significantly increased the rate of responding. We have recently observed a similar interaction in psilocybin-trained rats between WAY-100635 and the closely related hallucinogen, N,N-dipropyltryptamine (Fantegrossi et al., 2005). In contrast, M100907 had no effect on psilocybin-appropriate responding but decreased the number of animals completing the test sessions. The results with WAY-100635 suggest a rate suppressant effect of DMT which is mediated by 5-HT1A receptors while the results with M100907 provide further evidence of a rate decreasing interaction between tryptamine hallucinogens and 5-HT2 receptor antagonists (Winter and Rabin, 1988). The failure of bufotenine to mimic psilocybin is in keeping with previous reports in rats in which bufotenine did not substitute for 5-MeO-DMT (Spencer et al., 1987), for LSD (Helsley et al., 1998), or for TVX Q 7821, a 5-HT1A receptor agonist (Spencer and Traber, 1987). The hallucinogenicity of bufotenine has been a matter of controversy for some time (Shulgin and Shulgin, 1997, pages 473-478; Torres and Repke, 2006). Nonetheless, the absence of activity with respect to stimulus activity in the rat as exemplified in Fig. 3 is plausibly explained on the basis of low lipid solubility and an associated inability to cross the blood-brain barrier. While psilocin has a chloroform/water coefficient of 5.52, that of bufotenine is only 0.06 (Gessner et al., 1968). In keeping with a primarily peripheral mechanism in the rat for bufotenine is the observation that the hypothermic effects of bufotenine are antagonized by xylamidine, a peripherally acting 5-HT antagonist (Winter, 1972).
In reporting the full generalization of psilocybin to psilocin and LSD, Koerner and Appel (1982) noted an absence of generalization to mescaline [3,4,5-trimethoxy-phenylethylamine]. Those results, taken in light of the fact that the human experiences with psilocybin and mescaline share many features (Hollister, 1968) led the authors to conclude that the “failure of a known hallucinogen to transfer to another hallucinogen provides evidence of the inability of existing drug discrimination procedures to detect ‘hallucinogenicity’ in drugs”. However, as is noted above, subsequent studies found that rats trained with mescaline (Callahan and Appel, 1988; Appel and Callahan, 1989) or [+/-]-DOM (Silverman and Ho, 1980), generalize to psilocybin. These latter results, coupled with the data shown in Fig. 4 for the generalization of psilocybin to [-]-DOM, 2C-T-7, and mescaline, rather than arguing for a rejection of the rat drug-discrimination model of hallucinogenicity, suggest that the overlap between the discriminative stimulus effects of the phenethylamine hallucinogens and psilocybin is variable depending upon the structural details of specific drugs. For example, one may speculate based on the data of Fig. 4 that the presence of the alpha-methyl group present in [-]-DOM may account for its differential effects relative to the phenethylamines, 2C-T-7 and mescaline, in mimicking psilocybin. Also seen in Fig. 4 is complete antagonism by M100907 of psilocybin-appropriate responding following the administration of [-]-DOM, 2C-T-7, and mescaline. This stands in contrast with only partial antagonism by M100907 of stimulus control by psilocybin itself [Fig. 1]. A plausible explanation of results such as those seen in Figs. 1 and 4 is provided by the hypothesis that a drug-induced stimulus may be comprised of multiple elements with the salience of each element being determined by training and testing conditions (Ator and Griffiths, 1989; Winter, 1984; Winter and Rabin, 1988; Winter et al., 2000).
When psilocybin was tested in rats trained with LSD, MDMA, and PCP, respectively, the results were mixed. No generalization of PCP to any dose of psilocybin was observed whereas intermediate degrees of generalization of LSD [Fig. 5] and MDMA [Fig. 6] to psilocybin were seen. The results in LSD trained rats are in general agreement with previous reports though these earlier studies observed complete generalization (Appel et al., 1978; Jarbe, 1980; Callahan and Appel, 1988) and attempts were not made to antagonize psilocybin. We are unaware of any previous tests of psilocybin in rats trained with either MDMA or PCP as discriminative stimuli. Although binding studies indicate that psilocybin has significant affinity for 5-HT1A, 5-HT2A, 5-HT2c receptors (McKenna et al., 1990; Blair et al., 2000), complete antagonism by M100907 of the intermediate effects of psilocybin in rats trained with LSD [Fig. 5] and with MDMA [Fig. 6] is compatible with a 5-HT2A receptor-mediated mechanism for these partial generalizations. In contrast, it is seen in Fig. 6 that M100907 does not significantly antagonize the training dose of MDMA suggesting that whatever 5-HT2-mediated element might be present, it is not essential for MDMA-induced stimulus control. Previous studies have reported mixed results when various serotonergic antagonists have been employed in attempts to antagonize MDMA in rats trained with the drug (Baker et al., 1995; Glennon et al., 1992; Goodwin et al., 2003; Schechter, 1988; Young et al., 2005).
In summary, the present data indicate that the stimulus properties of psilocybin in the rat are broadly compatible with those of other ergoline, indoleamine, and phenethylamine hallucinogens. However, significant differences are apparent as well. Thus, the full generalization of psilocybin to LSD [Fig. 3] and to DOM [Fig. 4] is completed blocked by the selective 5-HT2A receptor antagonist, M100907, but stimulus control by psilocybin is only partially antagonized by M100907 [Fig. 1]. In addition, while it is known that a significant 5-HT1A receptor-mediated element is induced by 5-MeO-DMT (Spencer et al., 1987; Winter et al., 2000), stimulus control by psilocybin is influenced not at all by the selective 5-HT1A receptor antagonist, WAY-100635 [Fig. 1].
Acknowledgement
Supported in part by the National Institute on Drug Abuse, grant DA 03385 [JCW, RAR, DJA].
References
- Appel JB, Callahan Involvement of 5-HT receptor subtypes in the discriminative stimulus properties of mescaline. Eur J Pharmacol. 1989;159:41–6. doi: 10.1016/0014-2999(89)90041-1. [DOI] [PubMed] [Google Scholar]
- Appel JB, White FJ, Kuhn DM. The use of drugs as discriminative stimuli in behavioral pharmacodynamics. In: Colpaert FC, Rosecrans JA, editors. Stimulus properties of drugs: ten years of progress. Elsevier/ North Holland; Amsterdam: 1978. pp. 7–29. [Google Scholar]
- Ator NA, Griffiths RR. Asymmetrical cross-generalization in drug discrimination with lorazepam and pentobarbital training conditions. Drug Dev Res. 1989;16:355–64. [Google Scholar]
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, et al. Assessment of the discriminative stimulus effects of the optical isomers of ecstasy [MDMA] Behav Pharmacol. 1995;6:263–75. [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor [5-HT7] positively linked to adenylate cyclase. J Biol Chem. 1993;268:422–6. [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, et al. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–10. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Brimblecombe RW, Pinder RM. Hallucinogenic drugs. Wright-Scientechnica; Bristol: 1975. p. 16. [Google Scholar]
- Callahan PM, Appel JB. Differences in the stimulus properties of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in animals trained to discriminate hallucinogens from saline. J Pharmacol Exp Ther. 1988;246:866–70. [PubMed] [Google Scholar]
- Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci. 2005;17:1497–508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 agonist. Psychopharmacology. 2006;188:244–51. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT2C receptor-mediated interaction between 2,5-dimethoxy-4-methylamphetamine [-]-DOM] and citalopram in the rat. Pharmacol Biochem Behav. 2004;79:25–30. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Rabin RA, Winter JC, Coop A, et al. Hallucinogen-like actions of 2C-T-7 in mice and rats. Psychopharmacology. 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs I: Antagonist correlation analysis. Psychopharmacology. 1995a;121:347–56. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs II: Reassessment of LSD false positives. Psychopharmacology. 1995b;121:357–63. doi: 10.1007/BF02246075. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Helsley SE, Lorrain DS, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs III: The mechanistic basis for supersensitivity to the LSD stimulus following serotonin depletion. Psychopharmacology. 1995c;121:364–72. doi: 10.1007/BF02246076. [DOI] [PubMed] [Google Scholar]
- Gessner PK, Godse DD, Krull AH, McMullan JM. Structure-activity relationships among 5-methoxy-N,N-dimehtyltryptamine, 4-hydroxy-N,N-dimethyltryptamine [psilocin], and other substituted tryptamines. Life Sci. 1968;7:267–77. doi: 10.1016/0024-3205(68)90200-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Do hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacology. 1990;3:509–17. [PubMed] [Google Scholar]
- Glennon RA, Higgs R, Young R, Issa H. Further studies on N-methyl-1[3,4-methylenedioxyphenyl]-2-aminopropane as a discriminative stimulus: antagonism by 5-HT3 antagonists. Pharmacol Biochem Behav. 1992;43:1099–106. doi: 10.1016/0091-3057(92)90488-2. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE. Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a 3-choice discrimination. Pharmacol Biochem Behav. 2003;74:987–95. doi: 10.1016/s0091-3057(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Thelen B, Habermeyer E, Kunert HJ, Kovar KA, Lindenblatt H, et al. Psychophathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine [MDE], psilocybin, and D-methamphetamine in healthy volunteers. Results of an experimental double-blind placebo-controlled study. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–83. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Harris RT, Balster RL. An analysis of the function of drugs in the stimulus control of operant behavior. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. Appleton-Century Crofts; New York: 1971. pp. 111–32. [Google Scholar]
- Helsley S, Fiorella D, Rabin RA, Winter JC. Behavioral and biochemical evidence for a nonessential 5-HT2A component of the ibogaine-induced discriminative stimulus. Pharmacol Biochem Behav. 1998;59:419–25. doi: 10.1016/s0091-3057(97)00451-6. [DOI] [PubMed] [Google Scholar]
- Hirschhorn I, Winter JC. Mescaline and LSD as discriminative stimuli. Psychopharmacologia. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Hofmann A. Psychotomimetic agents. In: Burger A, editor. Drugs affecting the central nervous system. Edward Arnold; London: 1968. pp. 169–235. [Google Scholar]
- Hofmann A, Troxler F. Identifzierung von psilocin. Experientia. 1959;15:101–2. doi: 10.1007/BF02166696. [DOI] [PubMed] [Google Scholar]
- Hofmann A, Heim R, Brack A, Kobel Psilcybin, ein psychotroper Wirkstuff aus dem mexikanischen Rauchpilz Psilocybe Mexicana Heim. Experientia. 1959;15:107–11. doi: 10.1007/BF02159243. [DOI] [PubMed] [Google Scholar]
- Hollister LE. Clinical, biological, and psychological effects of psilocybin. Arch int Pharmacodyn Ther. 1961;130:42–52. [PubMed] [Google Scholar]
- Hollister LE. Charles C. Thomas; Springfield, IL: 1968. Chemical psychoses; pp. 32–40. [Google Scholar]
- Horita A. Some biochemical studies on psilocybin and psilocin. J Neuropsychiatry. 1963;4:270–3. [PubMed] [Google Scholar]
- Horita A, Weber LJ. Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Proc Soc Exp Biol Med. 1961;106:32–4. doi: 10.3181/00379727-106-26228. [DOI] [PubMed] [Google Scholar]
- Isbell H. Comparison of the reactions induced by psilocybin and LSD-25 in man. Psychopharmacologia. 1959;1:29–38. doi: 10.1007/BF00408109. [DOI] [PubMed] [Google Scholar]
- Jarbe TU. LSD-25 as a discriminative stimulus for response selection by pigeons. Pharmacol Biochem Behav. 1980;13:549–54. doi: 10.1016/0091-3057(80)90279-8. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (delta 8-THC and delta 9-THC), and other drugs. Psychopharmacologia. 1974;40:1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- Koerner KA, Appel JB. Neuropharmacological reassessment of the discriminative stimulus properties of lisuride. Psychopharmacology. 1982;91:67–73. doi: 10.1007/BF00690929. [DOI] [PubMed] [Google Scholar]
- Malitz S, Esecover H, Wilkens B, Hoch PH. Some observation on psilocybin, a new hallucinogen, in human subjects. Compr Psychiatry. 1960;1:8–17. doi: 10.1016/s0010-440x(60)80045-4. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Thisted RA, Nichols DE. Distinct temporal phases in the behavioral pharmacology of LSD: dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology. 2005;180:427–35. doi: 10.1007/s00213-005-2183-9. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ. Differential interactions of indolealkylamines with 5-HT receptor subtypes. Neuropharmacology. 1990;29:193–8. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Melges FT, Tinlenberg JR, Hollister LE, Gillespie HE. Marihuana and temporal disintegration. Science. 1970;168:1118–20. doi: 10.1126/science.168.3935.1118. [DOI] [PubMed] [Google Scholar]
- Mohell N, Sallemark M, Rosqvist S, Malmberg A, Hogberg T, Jackson DM. Binding characteristics of remoxipride and its metabolites to dopamine D2 and D3 receptors. Eur J Pharmacol. 1993;238:121–5. doi: 10.1016/0014-2999(93)90515-j. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology. 2005;182:197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkel M, Atwell CR, Dimascio A, Brown J. Experimental psychiatry. V. Psilocybine, a new psychotogenic drug. N Engl J Med. 1960;262:295–7. doi: 10.1056/NEJM196002112620606. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine [MDMA, “ecstasy”] Pharmacol Biochem Behav. 1988;31:817–24. doi: 10.1016/0091-3057(88)90390-5. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA. Lysergic acid diethylamide (LSD) as a discriminative cue: drugs with similar stimulus properties. Psychopharmacologia. 1972;26:313–6. doi: 10.1007/BF00422707. [DOI] [PubMed] [Google Scholar]
- Schultes RE, Hofmann A. The botany and chemistry of hallucinogens. 2nd edition Charles C. Thomas; Springfield, IL: 1980. [Google Scholar]
- Shulgin A, Shulgin A. TiHKAL, the continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- Silverman PB, Ho BT. The discriminative stimulus properties of 2,5-dimethoxy-4-methylamphetamine (DOM): differentiation from amphetamine. Psychopharmacology. 1980;68:209–15. doi: 10.1007/BF00428105. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Traber J. The interoceptive discriminative stimuli induced by the novel putative anxiolytic TVX Q 7821: behavioral evidence for the specific involvement of serotonin 5-HT1A receptors. Psychopharmacology. 1987;91:25–9. doi: 10.1007/BF00690921. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Glaser T, Traber J. Serotonin receptor subtypes mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology. 1987;93:158–66. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Torres M, Repke D. Anadenanthera: visionary plant of ancient South America. The Haworth Herbal Press; New York: 2006. pp. 143–86. [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Lawler CP, Fox DR, Neve KA, Nichols DE, Mailman RB. LSD and structural analogs: pharmacological evaluation at D1 dopamine receptors. Psychopharmacology. 1995;118:401–9. doi: 10.1007/BF02245940. [DOI] [PubMed] [Google Scholar]
- Wasson VP, Wasson RG. Mushrooms, Russian and History. Pantheon; New York: 1957. [Google Scholar]
- Winter JC. Xylamidine tosylate: differential antagonism of the hypothermic effects of N,N-dimethyltryptamine, bufotenine, and 5-methoxytryptamine. Arch int Pharmacodyn Ther. 1972;198:61–6. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli. Fed Proc. 1974;33:1825–32. [PubMed] [Google Scholar]
- Winter JC. Stimulus properties of phenethylamine hallucinogens and lysergic acid diethylamide: the role of 5-hydroxytryptamine. J Pharmacol Exp Ther. 1978;204:416–23. [PubMed] [Google Scholar]
- Winter JC. The stimulus properties of p-methoxyamphetamine: a non-essential serotonergic component. Pharmacol Biochem Behav. 1984;20:201–3. doi: 10.1016/0091-3057(84)90242-9. [DOI] [PubMed] [Google Scholar]
- Winter JC, Rabin RA. Interactions between serotonergic agonists and antagonists in rats trained with LSD as a discriminative stimulus. Pharmacol Biochem Behav. 1988;30:617–24. doi: 10.1016/0091-3057(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Winter JC, Eckler JR, Rabin RA. Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with phencyclidine and LSD as discriminative stimuli. Psychopharmacology. 2004;172:233–40. doi: 10.1007/s00213-003-1636-2. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RF, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: a hallucinogen which induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Young R, Khorana N, Bondareva T, Glennon RA. Pizotyline effectively attenuates the stimulus effects of MDMA. Pharmacol Biochem Behav. 2005;82:404–10. doi: 10.1016/j.pbb.2005.09.010. [DOI] [PubMed] [Google Scholar]


