Abstract
The indeterminate shoot apical meristem of plants is characterized by the expression of the Class 1 KNOTTED1-LIKE HOMEOBOX (KNOX1) genes. KNOX1 genes have been implicated in the acquisition and/or maintenance of meristematic fate. One of the earliest indicators of a switch in fate from indeterminate meristem to determinate leaf primordium is the down-regulation of KNOX1 genes orthologous to SHOOT MERISTEMLESS (STM) in Arabidopsis (hereafter called STM genes) in the initiating primordia. In simple leafed plants, this down-regulation persists during leaf formation. In compound leafed plants, however, KNOX1 gene expression is reestablished later in the developing primordia, creating an indeterminate environment for leaflet formation. Despite this knowledge, most aspects of how STM gene expression is regulated remain largely unknown. Here, we identify two evolutionarily conserved noncoding sequences within the 5′ upstream region of STM genes in both simple and compound leafed species across monocots and dicots. We show that one of these elements is involved in the regulation of the persistent repression and/or the reestablishment of STM expression in the developing leaves but is not involved in the initial down-regulation in the initiating primordia. We also show evidence that this regulation is developmentally significant for leaf formation in the pathway involving ASYMMETRIC LEAVES1/2 (AS1/2) gene expression; these genes are known to function in leaf development. Together, these findings reveal a regulatory point of leaf development mediated through a conserved, noncoding sequence in STM genes.
Keywords: evolution, KNOX1, shoot apical meristem
The shoot apical meristem (SAM) of plants is an indeterminate structure and the source of stem cells from which all aerial organs are derived. Indeterminacy during development is regulated by a suite of genes that function in the SAM. The process of organ initiation begins when cells in the incipient organ primordium change identity from indeterminate to determinate. The indeterminate SAM is characterized by the expression of the Class 1 KNOTTED1-LIKE HOMEOBOX (KNOX1) genes. KNOX1 genes have been implicated in the acquisition and/or maintenance of meristematic fate (1, 2). One of the earliest indicators of a switch in fate from indeterminate meristem to determinate leaf primordium is the down-regulation of KNOX1 genes orthologous to SHOOT MERISTEMLESS (STM) in Arabidopsis (hereafter called STM genes) in the incipient primordia (1).
The SAM can give rise to two different leaf forms, simple or compound (3), and KNOX1 genes are down-regulated in the incipient primordia in both compound and simple leafed species. In simple leafed plants, this down-regulation persists during leaf formation (4–7), and overexpression of these genes is sufficient to cause leaf lobing and ectopic meristem formation (8–11). However, KNOX1 expression is reestablished later in the developing primordia of compound leafed plants (12–15). Additionally, overexpression of KNOX1 genes in transgenic tomato plants or spontaneous tomato mutants results in leaves with increased numbers of leaflets (13, 14, 16). Therefore, it has been concluded that KNOX1 genes are involved in compound leaf formation by establishing an indeterminate environment within developing primordia.
In Arabidopsis thaliana, loss-of-function of the STM gene results in loss of the SAM in agreement with its role in meristem acquisition/maintenance (1). STM represses ASYMMETRIC LEAVES1 (AS1) (17–20), and AS1 in turn represses KNAT1 (also known as BREVIPEDICELLUS), another Arabidopsis KNOX1 gene (17, 19–26). Some upstream factors are reported to regulate KNAT1 expression (27–30), whereas upstream regulation of STM genes is still largely obscure. Thus, two questions still remain relating to how the expression of STM genes might be regulated: (i) how their expression is initially excluded from the incipient primordia and (ii) how their expression is repressed persistently in developing leaves of simple leafed species and reestablished in compound leafed species.
Control of gene expression requires cis-acting regulatory nucleotide sequences. Conserved noncoding sequences (CNSs) that have been maintained across orthologs in distantly related taxa can be candidates for such regulatory elements. Because CNS function is implied by evolutionary conservation, CNSs have attracted a lot of attention in various organisms (31–35) and have also been identified in plants (36–38). Some examples of CNSs include sequences present in introns where they function to repress ectopic expression of the maize knotted1 (kn1) gene (36). In this report, we identify two CNSs within the 5′ upstream region of STM genes in monocots and dicots. We show that one of the elements has a significant role in leaf development, representing a regulatory point of leaf formation processes.
Results and Discussion
Identification of CNSs in 5′ Upstream Regions of STM Orthologous Genes.
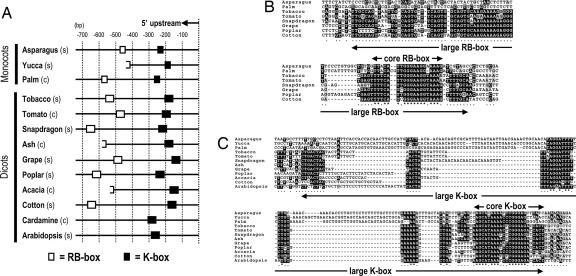
We identified two CNSs in the 5′ upstream regions of STM genes by comparing gene sequences from various species, including both simple (asparagus, yucca, grape, poplar, cotton, arabidopsis, tobacco, and snapdragon) and compound (palm, acacia, tomato, and ash) leafed species across monocots and dicots. These identified CNSs were named the RB-box and the K-box. Their positions relative to the translation initiation codon are shown in Fig. 1A. The core RB-box (≈23 nucleotides) is conserved in both monocots and dicots, whereas the large RB-box (≈80 nucleotides) is conserved only in dicots (Fig. 1B). The positions of the RB-box vary between 415 and 630 nucleotides upstream from the translation initiation codon (Fig. 1A). Among all of the species that we checked, the RB-box was absent only in Arabidopsis and the recently reported STM gene of Cardamine hirsuta (39). It remains to be seen if the RB-box is absent only in STM genes of the clade including Arabidopsis and Cardamine or in the entire Brassicaceae family. The core K-box (≈23 nucleotides) is conserved well among both monocots and dicots, whereas the large K-box (≈90 nucleotides) is conserved especially in dicots (Fig. 1C). The K-box is located in the region between 105 and 271 nucleotides upstream from the translation initiation codon (Fig. 1A).
Fig. 1.
Two CNSs in the 5′ upstream regions of STM orthologous genes. (A) The positions of RB-boxes (white boxes) and K-boxes (black boxes) in the 5′ upstream region from the translation start codon are illustrated. s and c indicate simple and compound leafed species, respectively. Halved white boxes for some RB-boxes show that sequences only downstream of the core RB box are available because the 5′ degenerate primer for the core RB-box was used to obtain upstream sequences. (B and C) The alignment of sequences of the RB-box and K-box are illustrated, respectively.
In the Poaceae, including rice and maize, the kn1 ortholog is known to participate in the maintenance of the SAM (2). Two recently published papers regarding monocot STM genes (40, 41) and also our unpublished analysis strongly suggest that the STM ortholog was lost in the Poaceae. The Poaceae kn1 gene is related to KNAT1. The STM function appears to be taken over by the kn1 in the Poaceae. Here, we report on our functional analysis of the tobacco (Solanaceae) K-box and RB-box and the Arabidopsis (Brassicaceae) K-box.
K-Box Represses NTH15 Expression Outside SAM in Tobacco.
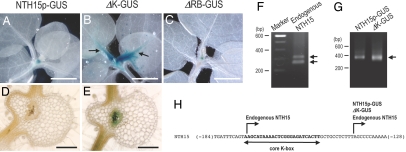
To investigate how the CNSs are involved in the regulation of the tobacco NTH15 gene (tobacco ortholog of Arabidopsis STM), we carried out β-glucuronidase (GUS) reporter fusion experiments. When the GUS reporter gene was fused to the 5′ upstream region from the translation start codon of the tobacco NTH15 gene and transformed into tobacco plants (NTH15p-GUS), GUS expression was detected only in the SAM (Fig. 2A). When the large K-box (81 bp) or the large RB-Box (68 bp) was removed from the reporter construct (ΔK-GUS and ΔRB-GUS, respectively), we observed a dramatic expansion of GUS expression in the ΔK-GUS plants but not in the ΔRB-GUS plants (Fig. 2 B and C). GUS expression was detected not only in the SAM but also in the basal region of the ΔK-GUS leaves (Fig. 2B), and transverse sections of leaves showed GUS expression in the midvein (Fig. 2E). These results suggest that the K-box has a role in the repression of STM gene expression outside of the SAM. Thus, in this study we focused on elucidating the function of the K-box.
Fig. 2.
GUS expression from the NTH15 upstream region in tobacco and the determination of the transcription initiation sites. (A–C) GUS expression in transgenic tobacco seedlings harboring NTH15p–GUS, ΔK-GUS, and ΔRB-GUS transgenes were detected, respectively. Arrows indicate ectopic expression of GUS proteins in leaves. (D and E) GUS expression in transverse sections of leaves of transgenic tobacco harboring NTH15p–GUS and ΔK-GUS transgenes are shown, respectively. (F and G) Agarose gel electrophoresis of nested PCR products from the RLM-RACE procedure for endogenous NTH15 gene and GUS reporter transgenes, respectively. Molecular size markers (base pairs) are shown on the left. Arrows on the right mark the major PCR products. (H) Sequence around the core K-box of the NTH15 gene is illustrated, and arrows above the sequence indicate the identified transcription start sites. Horizontal arrow below the sequence indicates the core K-box. (Scale bars: A–C, 2 mm; D and E, 0.4 mm.)
K-Box Functions as a DNA Element.
To determine whether the K-box is an element of the promoter or a part of the 5′ UTR of the mRNA, we analyzed the transcription initiation sites of the endogenous NTH15 gene by 5′ RNA ligase-mediated RACE (RLM-RACE), which is 5′ RACE-specific for capped mRNA. We detected two major PCR products in this experiment (Fig. 2F). Sequence analysis showed that one product started at the 5′ end of the core K-box and that the other started downstream of the K-box (Fig. 2H). To confirm this, we also performed 5′ RLM-RACE to determine the transcription initiation sites of the GUS reporter transgenes in the NTH15p-GUS and ΔK-GUS plants. We detected one major amplification product from both transgenes (Fig. 2G), and sequence analysis indicated that transcription started at the same position downstream of the K-box in both cases. This position was identical to the shorter endogenous NTH15 mRNA lacking a K-box (Fig. 2H). NTH15p-GUS and ΔK-GUS plants showed different GUS expression patterns despite the same transcription initiation site downstream of the K-box (Fig. 2 A and B). These results indicate that the presence of the K-box in the 5′ UTR of the mRNA is not required for SAM-specific expression of the NTH15 gene. Therefore, we conclude that the K-box likely functions as a DNA element in the promoter region.
K-Box Represses STM Expression in Developing Leaves but Not in Initiating Leaf Primordia of Arabidopsis.
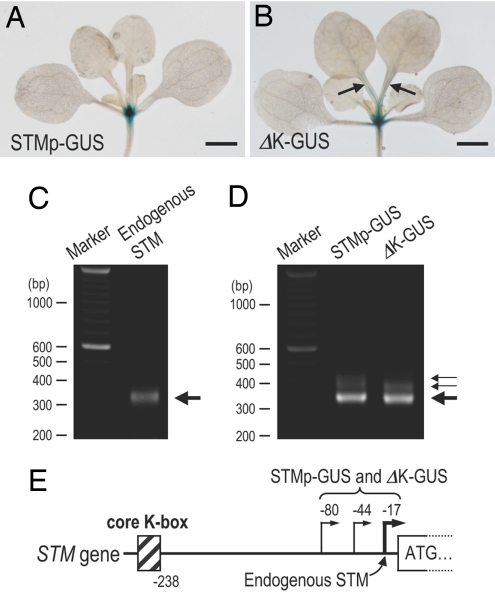
We next carried out GUS reporter experiments in Arabidopsis to confirm whether K-box function is conserved in this species. When the GUS reporter was fused to the 5′ region of the Arabidopsis STM gene upstream of the translation start codon and then transformed into Arabidopsis (STMp–GUS), GUS expression was detected only in the SAM (Fig. 3A). However, when the core K-box (25 bp) was removed from the reporter construct (ΔK-GUS), GUS expression was detected not only in the SAM but also in the midvein of the leaf petioles (Fig. 3B). We also determined the transcriptional start sites of the endogenous STM gene and GUS reporter transgenes and confirmed that the K-box is upstream of the identified transcriptional start sites (Fig. 3 C–E). Thus, the K-box appears to function as a DNA element in the promoter of both Arabidopsis and tobacco to restrict STM expression to the SAM.
Fig. 3.
GUS expression from the STM upstream region in Arabidopsis and the determination of the transcription initiation sites. (A and B) GUS expression in transgenic Arabidopsis seedlings harboring STMp–GUS and ΔK-GUS transgenes were detected, respectively. Arrows indicate ectopic expression of GUS proteins in leaf petioles. (C and D) Agarose gel electrophoresis of nested PCR products from the RLM-RACE procedure for endogenous STM gene and GUS reporter transgenes, respectively. Molecular size markers (base pairs) are indicated on the left. Thick arrows and thin arrows on the right mark the major and minor PCR products, respectively. (E) The identified transcription start sites are illustrated. (Scale bars: 1.5 mm.)
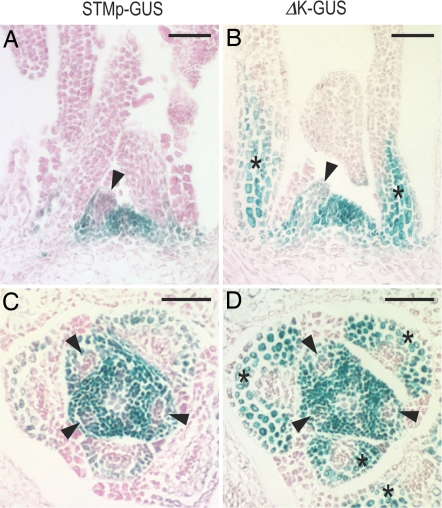
KNOX1 genes are known to be expressed in the SAM but not in initiating leaf primordia. This repression in leaf primordia is persistent during leaf development in simple leafed species such as Arabidopsis. To observe STM expression regulation in the shoot apex, we analyzed the GUS expression in STMp–GUS and ΔK-GUS Arabidopsis plants by using thin paraffin sections. STMp–GUS plants showed strong GUS expression in the SAM, but this expression was remarkably eliminated from the new leaf primordia (Fig. 4 A and C, arrowheads). We also noticed a weak trace of GUS expression in the base of leaf primordia of STMp–GUS plants (Fig. 4C) compared with the expression patterns of the endogenous STM gene reported in ref. 42. Similar observations were reported when a STMp–GUS reporter transgene was used (43). It is likely that this is a characteristic of the STM promoter fragment used. Thus, the STM upstream region used in these experiments had the elements necessary for down-regulation of STM expression in new primordia. This down-regulation was also observed in the ΔK-GUS plants (Fig. 4 B and D, arrowheads). However, GUS expression was reestablished in older developing leaves (Fig. 4 B and D, asterisks). These results indicate that the K-box mediates the persistent repression of STM expression in developing leaves but not the initial down-regulation in leaf primordia.
Fig. 4.
Paraffin sections of GUS-stained Arabidopsis SAM tissues. GUS expression in longitudinal (A and B) and transverse (C and D) sections of SAM of transgenic Arabidopsis seedlings harboring STMp–GUS (A and C) and ΔK-GUS (B and D) transgenes. Arrowheads indicate down-regulation of GUS expression in young leaf primordia. Asterisks indicate the reestablishment of GUS expression in the developing leaf primordia. (Scale bars: 50 μm.)
K-box-Mediated STM Expression Regulates Leaf Development.
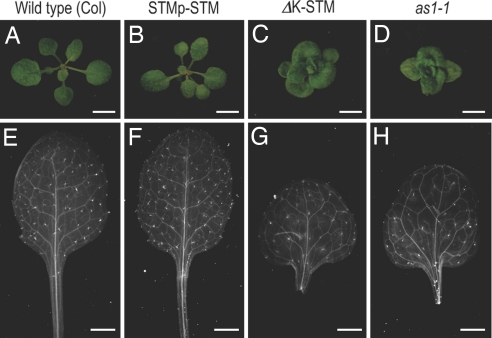
To analyze the developmental role of the K-box, a translational fusion between GFP and STM driven by the 5′ upstream region of the STM gene with or without the K-box (STMp–STM or ΔK-STM, respectively) was introduced into Arabidopsis. The STMp–STM transgene rescued the shoot meristem defect of stm mutant seedlings [see supporting information (SI) Fig. 7 and SI Table 1] and did not show an ostensible phenotypic alteration in the wild-type background (Fig. 5A, B, E, and F). ΔK-STM plants, on the other hand, showed an altered plant phenotype with rounded leaves and short petioles, resembling as1 mutant phenotypes (Fig. 5 C, D, G, and H). ΔK-STM plants also harbor leaf-lobing phenotypes. The degree of leaf lobing varies among individuals (for example, compare Figs. 5G and 6D) as is also the case in as1-1 mutants grown under controlled conditions. This transgene also rescued the phenotype of stm mutant seedlings (SI Fig. 7 and SI Table 1), and the rescued seedlings showed as1 mutant-like phenotypes (SI Fig. 7I). These results indicate that the K-box might have a role in the control of leaf formation in the pathway related to AS1, a known participant in leaf development (17), and that the K-box is not required for the acquisition and/or maintenance of SAM fate by the STM gene.
Fig. 5.
Transgenic Arabidopsis plants expressing the STM protein from the STM upstream region with or without the K-box. Vegetative rosettes (A–D) and cleared rosette leaves (E–H) of wild-type (Col) (A and E), STMp–STM (B and F), and ΔK-STM (C and G) and as1-1 (D and H) Arabidopsis plants are shown. (Scale bars: A–D, 5 mm; E–H, 2 mm.)
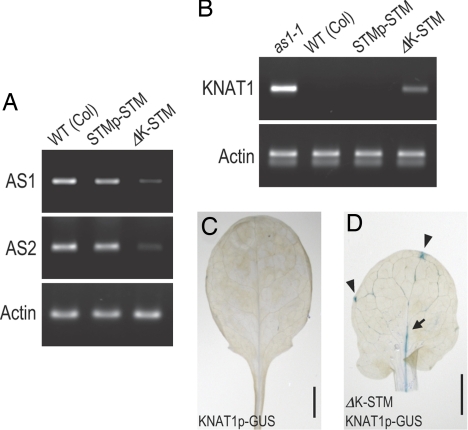
Fig. 6.
Involvement of STM regulation by K-box in the AS1 pathway during leaf development. (A and B) Products by RT-PCR for AS1, AS2, and ACT2 mRNA in tissues containing the SAM and leaf primordia of Arabidopsis plants (A) and KNAT1 and ACT2 mRNA in leaves of Arabidopsis plants (B) are shown. (C and D) GUS expression in transgenic Arabidopsis seedlings harboring KNAT1p–GUS with or without ΔK-STM, respectively. Arrow and arrowheads indicate GUS expression in the leaf midvein and leaf hydathodes, respectively. (Scale bars: 2 mm.)
AS1 is known to function downstream of the STM gene together with AS2 (17–21, 24). RT-PCR experiments in the ΔK-STM plants showed a severe reduction of the expression of AS1 and AS2 mRNA compared with wild-type and STMp–STM plants (Fig. 6A). To further confirm the involvement of the K-box in the AS1 pathway, we also analyzed the expression of KNAT1 mRNA in leaves of STMp–STM and ΔK-STM plants because KNAT1 is reported to be down-regulated by AS1/2 (17, 18, 22, 24). We did not detect KNAT1 expression in wild-type leaves, but the expression was present in as1 leaves (Fig. 6B). Similarly, KNAT1 expression was not detected in STMp–STM leaves but was seen in ΔK-STM leaves (Fig. 6B). Also, when we compared the GUS expression patterns in leaves of KNAT1p–GUS plants with or without the ΔK-STM transgene (Fig. 6 C and D), as1-mutant-like leaves induced by the ΔK-STM transgene showed ectopic GUS expression in leaf midveins and hydathodes. These observations are consistent with the previous reports showing GUS expression patterns from the KNAT1p–GUS reporter gene in as1 and as2 mutant plants (22, 25, 27, 28). Thus, the control of STM expression via the K-box may be significant for leaf development in the pathway involving AS1/2 gene expression.
Function of K-box in STM Genes Suggests Regulatory Points in Leaf Development.
The regulation of leaf development via STM orthologous KNOX1 genes can be partitioned into two steps. The first step is the initial down-regulation of STM expression in the incipient primordia, and the second step is either the persistent repression of expression in the developing leaves of simple leafed species or the reestablishment of expression in compound leafed species. In this study, we identify two CNSs within the 5′ upstream region of STM genes and show that one of them, K-box, plays a significant role in regulation of STM expression. The K-box functions in the persistent repression of STM expression in developing leaves of simple leafed species (Figs. 2 and 3). On the other hand, the initial down-regulation of STM expression in the incipient primordia is likely controlled in a K-box-independent manner. Current studies suggest that auxin is a key factor responsible for the initial down-regulation of KNOX1 genes (44–47). Our results show the initial down-regulation in both STMp–GUS and ΔK-GUS plants (Fig. 4), indicating that the signaling pathway triggered by auxin is eventually connected to the STM upstream region but outside of the K-box. Future investigation is required to reveal the relationship between auxin and unidentified elements of the STM upstream region responsible for the initial down-regulation of STM expression.
Simple leafed plants such as Arabidopsis show persistent repression of KNOX1 expression after the initial down-regulation of the expression, whereas in compound leafed species, KNOX1 expression is reestablished later in developing leaf primordia. Our results show that, in developing leaves of ΔK-GUS plants, STM promoter activity is reestablished (Figs. 2–4). K-box sequences are conserved among both simple and compound leafed species (Fig. 1). This conservation suggests that understanding upstream regulation of the K-box might be crucial to our understanding of the difference in STM expression between both types of species. This regulation may involve transacting factors that work through the K-box, although database searches have not yielded any recognizable binding sites of transcription factors in the K-box. Furthermore, a recent report (39) showed that changes of unknown cis-elements in the STM promoter are responsible for altered STM expression patterns between compound leafed Cardamine and simple leafed Arabidopsis, but our sequence analysis showed that the K-box is conserved between these two species. It is possible that the K-box cooperates with other unknown cis-elements of the STM promoter to control STM expression differently between simple and compound leafed species.
Our findings indicate that the regulation of STM gene expression is divided into two steps controlled by distinct mechanisms: a K-box-independent mechanism in the SAM and a K-box-dependent one in the developing leaves. This regulation of STM suggests a regulatory point in leaf development that may have been involved in elaborating a variety of leaf shapes in nature.
Materials and Methods
Sequences of STM Orthologous KNOX1 Upstream Region from Various Plant Species.
Upstream sequences of STM genes of grape (Vitis vinifera), cotton (Gossypium raimondii), snapdragon (Antirrhinum majus), and cardamine (Cardamine hirsuta) were obtained from the public database (GenBank accession nos. AM458687, CO081267, AY072735.1, and DQ526380, respectively). The genomic sequences of STM genes of Arabidopsis and poplar were found in the sequenced genome information. The upstream sequence of the tobacco NTH15 gene was provided by M. Matsuoka (Nagoya University, Chikusa, Japan) (48). The genomic sequence of the tomato LeT6 gene was reported in ref. 13. After the identification of the RB-boxes among the above sequences, degenerate primer sets corresponding to the core RB-box and protein-coding sequences were used to amplify genomic fragments of asparagus, yucca, acacia, and ash. Further 5′ extension of the obtained sequences was carried out by thermal asymmetric interlaced PCR. The GenBank database accession numbers of the sequences of palm (Washingtonia robusta), asparagus (Asparagus officinalis), yucca (Yucca aloifolia), acacia (Gleditsia triacanthos), and ash (Fraxinus angustifolia) are EF612706, EF494254, EF494255, EF494256, and EF494257, respectively.
Plasmid Construction and Plant Transformation.
The partial deletion (68 bp, from −516 to −584, relative to the translation initiation codon for the large RB-box and 81 bp, from −164 to −245, for the large K-box) of the 2.5-kb NTH15 promoter (48) was carried out by PCR. Then, the original fragment and the deleted fragments were cloned into the pCR8/GW/TOPO vector (Invitrogen, Carlsbad, CA). These constructs served as the entry vectors to transfer promoter fragments into the pKGWFS7 vector containing a GFP–GUS reporter gene (49). This construct was made via the GATEWAY system by using the LR reaction (Invitrogen). The 3.3-kb STM promoter region of the binary vector harboring the GUS reporter gene (50) was replaced with the deleted version (deletion of the 25-bp core K-box, from −238 to −263, relative to the translation initiation codon) after modification by PCR. STM-coding sequences, GFP6-coding sequences, and cauliflower mosaic virus 35S transcriptional terminator sequences were amplified by PCR by using an Arabidopsis cDNA library reverse-transcribed from the total RNA, pK2GW7 vector (49) and the pMDC204 vector (51), respectively. They were cloned into the pCR8/GW/TOPO vector together with the 3.3-kb STM promoter region or with the modified version. These expression cassettes were transferred to the pMDC123 binary vector (51) using the LR reaction. The constructs described above were introduced into Agrobacterium GV3101 for Arabidopsis transformation or EHA105 for tobacco transformation by electroporation. Arabidopsis (Col) and Nicotiana tabacum (cv. Samsun NN) were transformed by the floral dip method (52) and the tissue culture method, respectively.
Genotyping.
Detection of the stm-1 allele was performed as described in ref. 53 with some modifications. The primer set used for the dCAPS PCR marker was TTTATTAGATTAAATGATAACATTTAAGTCGATATGAACAATGAATTTGTAGATGCA and GTATAAGGGAAGAGAGTTACCGAAG. The 248-bp PCR product was digested into 198-bp and 50-bp fragments with NsiI when amplified from the stm-1 allele but not when amplified from the wild-type allele (SI Fig. 7 B and C). One primer spanned an intron–exon boundary to prevent the interference by the STM cDNA contained in the transgene used in this study.
Determination of Transcription Initiation Site.
Total RNA was isolated from the shoot apex of plants with an RNeasy Plant Mini kit (Qiagen, Valencia, CA). Determination of the transcription initiation sites was performed by RNA ligase-mediated 5′ rapid amplification of cDNA ends (5′ RLM-RACE) by using a GeneRacer kit (Invitrogen). Briefly, total RNA was dephosphorylated by using calf intestinal phosphatase to eliminate the 5′ phosphates from the truncated (noncapped) mRNA without affecting the 5′ capped mRNA, and the RNA was then treated with tobacco acid pyrophosphatase to remove the 5′ cap structure. GeneRacer RNA Oligo was ligated to the 5′ end of the decapped mRNA by a T4 RNA ligase. cDNA synthesis was performed with SuperScript III reverse transcriptase and with the gene-specific primers for the coding regions (AGGAAGTAAGAGTGAGAGC for the endogenous NTH15, AAGTCGTGCTGCTTCATG for the NTH15p(ΔK)–GUS transgene, TGAGGATGAGCCATGATC for the endogenous STM gene, and CAAACGGTGATACGTACAC for the STMp(ΔK)–GUS transgene). PCR was performed using the primer sets of the GeneRacer 5′ Primer and the gene-specific primers (GACAAGAGACGAGGGTAGTGAGGATGA for the endogenous NTH15, CTGAAGCACTGCACGCCGTAGGT for the NTH15p(ΔK)–GUS transgene, GATTCCTCCTGCAACGATTTCGTTATG for the endogenous STM gene, and CTGATGCTCCATCACTTCCTGATTATTGAC for the STMp(ΔK)–GUS transgene). Nested PCR was carried out using the primer sets of the GeneRacer 5′ Nested Primer and the gene-specific primers [GAGGACATAGGGCTGCATTTCCACT for the endogenous NTH15, TTCAGGGTCAGCTTGCCGTAGGT for the NTH15p(ΔK)–GUS transgene, CATTAGAGAATAGGCAGGAGCACAAG for the endogenous STM gene, and CGTAATGAGTGACCGCATCGAAAC for the STMp(ΔK)–GUS transgene]. The amplified products were gel-purified with the Qiaquick Gel Extraction kit (Qiagen), were cloned into the pCR4Blunt–TOPO vector (Invitrogen), and then were sequenced.
GUS Assay and Histology.
Detection of GUS expression was performed with 5-bromo-4-chloro-3-indolyl-β-d-glucuronidase (X-Gluc; Rose Scientific, Edmonton, AB, Canada). Tissues were vacuumed in 50 mM sodium phosphate buffer, pH 7.0 (1% Triton X-100/1% DMSO/10 mM EDTA/0.5 mM potassium ferrocyanide/0.5 mM potassium ferricyanide/1 mM X-Gluc) and then incubated at 37°C. The stained samples were washed twice with 70% ethanol, fixed in 50% ethanol/5% acetic acid/3.7% formaldehyde, and cleared with 70% ethanol. Tissues were mounted in 50% glycerol, and microscopy was conducted. When necessary, tissues were sectioned with a vibratome (Leica, Deerfield, IL) before observation. Histological analysis followed by paraffin sectioning was performed as described in ref. 54 with some modifications. Briefly, tissues were gently fixed in 90% acetone, washed with water, vacuumed in 50 mM sodium phosphate buffer, pH 7.0 (0.2% Triton X-100Z/10 mM EDTA/10 mM potassium ferrocyanide/10 mM potassium ferricyanide/1 mM X-Gluc), and incubated at 37°C. The stained tissues were fixed in 50% ethanol/5% acetic acid/3.7% formaldehyde, treated with Eosin-Y, and then embedded in Paraplast Plus (McCormick Scientific, St. Louis, MO). Paraffin sections (7 μm) were microtomed (Leica) and were observed after deparaffinization. Observation of leaf vein patterns was performed after incubation in the clearing agent chloral hydrate.
Primer Sets for RT-PCR Analysis.
The following primer sets were used for RT-PCR: ACT2, TGCTGGATTCTGGTGATG and TTCCAGCAGCTTCCATTC; AS1, GGAAGTTGCTCTTGAGTTTG and CAGCAATCTTCTTCCACTTG; AS2, CAAAGAAAGGCTTCTTTAATTTACTC and CTGACGAAGCTGATGTTG; and KNAT1, GAAGATCGGGAACTCAAG and TCCATCACCCATGTAATGAC. All targeted regions contain exon–exon junctions to avoid the amplification derived from contamination of genomic DNA.
Supplementary Material
Acknowledgments
We thank Sarah Hake (Plant Gene Expression Center, Albany, CA) for the KNAT1p–GUS Arabidopsis seeds, Makoto Matsuoka (Nagoya University, Chikusa, Japan) for NTH15 promoter sequences, and Kathryn Barton (Carnegie Institution, Stanford, CA) for the STMp–GUS plasmid. We also thank John Harada and the members of our laboratory for critical reading of the manuscript. Tobacco transformation was performed by the Ralph M. Parsons Foundation Plant Transformation Facility (University of California, Davis, CA), and we thank David Tricoli and Kim Carney for conducting the transformations. The as1-1 (CS3374) and stm-1 seeds (CS8154) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). This work was funded by National Science Foundation Grants IBN 0316877, 0344743, and 0641696 (to N.S.). N.U. is a Department of Energy–Energy Biosciences Research Fellow of the Life Sciences Research Foundation. N.U. was also supported by the Uehara Memorial Foundation and by the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Abbreviations
- SAM
shoot apical meristem
- CNSs
conserved noncoding sequences
- GUS
β-glucuronidase
- RLM
RNA ligase-mediated.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. EF494254 (Asparagus officinali STM), EF494255 (Yucca aloifolia STM), EF494256 (Gleditsia triacanthos STM), EF494257 (Fraxinus angustifolia STM), and EF612706 (Washingtonia robusta STM)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0707577104/DC1.
References
- 1.Long JA, Moan EI, Medford JI, Barton MK. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 2.Vollbrecht E, Reiser L, Hake S. Development (Cambridge, UK) 2000;127:3161–172. doi: 10.1242/dev.127.14.3161. [DOI] [PubMed] [Google Scholar]
- 3.Champagne C, Sinha N. Development (Cambridge, UK) 2004;131:4401–4412. doi: 10.1242/dev.01338. [DOI] [PubMed] [Google Scholar]
- 4.Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura A, Tamaoki M, Sato Y, Matsuoka M. Plant J. 1999;18:337–347. doi: 10.1046/j.1365-313x.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith LG, Greene B, Veit B, Hake S. Development (Cambridge, UK) 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Waites R, Selvadurai HR, Oliver IR, Hudson A. Cell. 1998;93:779–789. doi: 10.1016/s0092-8674(00)81439-7. [DOI] [PubMed] [Google Scholar]
- 8.Chuck G, Lincoln C, Hake S. Plant Cell. 1996;8:1277–1289. doi: 10.1105/tpc.8.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muehlbauer GJ, Fowler JE, Girard L, Tyers R, Harper L, Freeling M. Plant Physiol. 1999;119:651–662. doi: 10.1104/pp.119.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneeberger RG, Becraft PW, Hake S, Freeling M. Genes Dev. 1995;9:2292–2304. doi: 10.1101/gad.9.18.2292. [DOI] [PubMed] [Google Scholar]
- 11.Sinha NR, Williams RE, Hake S. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- 12.Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR. Science. 2002;296:1858–1860. doi: 10.1126/science.1070343. [DOI] [PubMed] [Google Scholar]
- 13.Chen JJ, Janssen BJ, Williams A, Sinha N. Plant Cell. 1997;9:1289–1304. doi: 10.1105/tpc.9.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. Cell. 1996;84:735–744. doi: 10.1016/s0092-8674(00)81051-x. [DOI] [PubMed] [Google Scholar]
- 15.Janssen BJ, Lund L, Sinha N. Plant Physiol. 1998;117:771–786. doi: 10.1104/pp.117.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E. Plant Cell. 1997;9:2143–2158. doi: 10.1105/tpc.9.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- 18.Byrne ME, Simorowski J, Martienssen RA. Development (Cambridge, UK) 2002;129:1957–1965. doi: 10.1242/dev.129.8.1957. [DOI] [PubMed] [Google Scholar]
- 19.Timmermans MC, Hudson A, Becraft PW, Nelson T. Science. 1999;284:151–153. doi: 10.1126/science.284.5411.151. [DOI] [PubMed] [Google Scholar]
- 20.Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA. Science. 1999;284:154–156. doi: 10.1126/science.284.5411.154. [DOI] [PubMed] [Google Scholar]
- 21.Lin WC, Shuai B, Springer PS. Plant Cell. 2003;15:2241–2252. doi: 10.1105/tpc.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ori N, Eshed Y, Chuck G, Bowman JL, Hake S. Development (Cambridge, UK) 2000;127:5523–5532. doi: 10.1242/dev.127.24.5523. [DOI] [PubMed] [Google Scholar]
- 23.Schneeberger R, Tsiantis M, Freeling M, Langdale JA. Development (Cambridge, UK) 1998;125:2857–2865. doi: 10.1242/dev.125.15.2857. [DOI] [PubMed] [Google Scholar]
- 24.Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, Machida Y. Development (Cambridge, UK) 2001;128:1771–1783. doi: 10.1242/dev.128.10.1771. [DOI] [PubMed] [Google Scholar]
- 25.Theodoris G, Inada N, Freeling M. Proc Natl Acad Sci USA. 2003;100:6837–6842. doi: 10.1073/pnas.1132113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Xu Y, Dong A, Sun Y, Pi L, Xu Y, Huang H. Development (Cambridge, UK) 2003;130:4097–4107. doi: 10.1242/dev.00622. [DOI] [PubMed] [Google Scholar]
- 27.Hay A, Barkoulas M, Tsiantis M. Development (Cambridge, UK) 2006;133:3955–6391. doi: 10.1242/dev.02545. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Xu L, Wang H, Yuan Z, Cao X, Yang Z, Zhang D, Xu Y, Huang H. Plant Cell. 2005;17:2157–2171. doi: 10.1105/tpc.105.033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelps-Durr TL, Thomas J, Vahab P, Timmermans MC. Plant Cell. 2005;17:2886–2898. doi: 10.1105/tpc.105.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Huang W, Wang H, Cai R, Xu Y, Huang H. Plant Mol Biol. 2006;61:63–78. doi: 10.1007/s11103-005-5992-7. [DOI] [PubMed] [Google Scholar]
- 31.Bird CP, Stranger BE, Dermitzakis ET. Curr Opin Genet Dev. 2006;16:559–564. doi: 10.1016/j.gde.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Dermitzakis ET, Reymond A, Lyle R, Scamuffa N, Ucla C, Deutsch S, Stevenson BJ, Flegel V, Bucher P, Jongeneel CV, et al. Nature. 2002;420:578–582. doi: 10.1038/nature01251. [DOI] [PubMed] [Google Scholar]
- 33.Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, et al. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas JW, Touchman JW, Blakesley RW, Bouffard GG, Beckstrom-Sternberg SM, Margulies EH, Blanchette M, Siepel AC, Thomas PJ, McDowell JC, et al. Nature. 2003;424:788–793. doi: 10.1038/nature01858. [DOI] [PubMed] [Google Scholar]
- 35.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, et al. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 36.Inada DC, Bashir A, Lee C, Thomas BC, Ko C, Goff SA, Freeling M. Genome Res. 2003;13:2030–2041. doi: 10.1101/gr.1280703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplinsky NJ, Braun DM, Penterman J, Goff SA, Freeling M. Proc Natl Acad Sci USA. 2002;99:6147–6151. doi: 10.1073/pnas.052139599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas BC, Rapaka L, Lyons E, Pedersen B, Freeling M. Proc Natl Acad Sci USA. 2007;104:3348–3353. doi: 10.1073/pnas.0611574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hay A, Tsiantis M. Nat Genet. 2006;38:942–947. doi: 10.1038/ng1835. [DOI] [PubMed] [Google Scholar]
- 40.Hirayama Y, Yamada T, Oya Y, Ito M, Kato M, Imaichi R. Dev Genes Evol. 2007;217:363–372. doi: 10.1007/s00427-007-0149-0. [DOI] [PubMed] [Google Scholar]
- 41.Jouannic S, Collin M, Vidal B, Verdeil JL, Tregear JW. New Phytol. 2007;174:551–568. doi: 10.1111/j.1469-8137.2007.02020.x. [DOI] [PubMed] [Google Scholar]
- 42.Long J, Barton MK. Dev Biol. 2000;218:341–353. doi: 10.1006/dbio.1999.9572. [DOI] [PubMed] [Google Scholar]
- 43.Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. Curr Biol. 2002;12:1557–1565. doi: 10.1016/s0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 44.Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M. Development (Cambridge, UK) 2004;131:5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- 45.Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 46.Treml BS, Winderl S, Radykewicz R, Herz M, Schweizer G, Hutzler P, Glawischnig E, Ruiz RA. Development (Cambridge, UK) 2005;132:4063–4074. doi: 10.1242/dev.01969. [DOI] [PubMed] [Google Scholar]
- 47.Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. Development (Cambridge, UK) 2000;127:5157–5165. doi: 10.1242/dev.127.23.5157. [DOI] [PubMed] [Google Scholar]
- 48.Tamaoki M, Sato Y, Matsuoka M. Genes Genet Syst. 1997;72:1–8. [Google Scholar]
- 49.Karimi M, Inze D, Depicker A. Trends Plants Sci. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 50.McConnell JR, Barton MK. Development (Cambridge, UK) 1998;125:2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- 51.Curtis MD, Grossniklaus U. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clough SJ, Bent AF. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 53.Gallois JL, Woodward C, Reddy GV, Sablowski R. Development (Cambridge, UK) 2002;129:3207–3217. doi: 10.1242/dev.129.13.3207. [DOI] [PubMed] [Google Scholar]
- 54.Sessions A, Weigel D, Yanofsky MF. Plant J. 1999;20:259–263. doi: 10.1046/j.1365-313x.1999.00594.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.