Abstract
Circadian rhythms can be regulated by many environmental and endogenous factors. We show here a sensitivity of circadian clock function to oxidative stress that is revealed in flies lacking the foxo gene product. When exposed to oxidative stress, wild-type flies showed attenuated clock gene cycling in peripheral tissues, whereas foxo mutants also lost behavioral rhythms driven by the central clock. FOXO is expressed predominantly in the fat body, and transgenic expression in this tissue rescued the mutant behavioral phenotype, suggesting that foxo has non-cell-autonomous effects on central circadian clock function. Overexpression of signaling molecules that affect FOXO activity, such as the insulin receptor or Akt, in the fat body also increased susceptibility of the central clock to oxidative stress. Finally, foxo mutants showed a rapid decline in rest:activity rhythms with age, supporting the idea that the increase of oxidative stress contributes to age-associated degeneration of behavioral rhythms and indicating the importance of FOXO in mitigating this deterioration. Together these data demonstrate that metabolism affects central clock function and provide a link among insulin signaling, oxidative stress, aging, and circadian rhythms.
Keywords: circadian rhythms
Molecular circadian clocks consist of feedback loops in which clock proteins regulate the expression of their own mRNAs. In Drosophila melanogaster, expression of the period (per) and timeless (tim) genes is activated during the day by a heterodimer comprised of the CLOCK (CLK) and CYCLE (CYC) transcription factors through binding to E-box sequences in the per and tim promoters (1). As PER and TIM proteins accumulate during the night, they translocate into the nucleus to inhibit their own transcription (2). The phase of this intrinsic cycle can be reset to different light:dark cycles through light-dependent degradation of TIM, a process initiated by the circadian photoreceptor cryptochrome (3).
Cryptochrome activity and its response to light are regulated by a mechanism that involves redox activity (4, 5). In addition, light induces transcription of heme oxygenase 2 and cytochrome P450 oxidoreductase, which may in turn regulate cellular redox state by reducing the NADPH/NADP ratio (6). Interestingly, redox state may also affect clock activity; in vitro studies showed that the NADH/NAD ratio affects the DNA binding efficiency of CLK/BMAL1 and NPAS2/BMAL1 heterodimers (7). BMAL1 is the mammalian homolog of the CYC protein mentioned above, and NPAS2 is an analog of CLK that functions as a circadian clock component in some tissues (8). Because cellular redox state is closely coupled to metabolism (9, 10), this effect of redox state on the circadian photoreceptor or on the transcriptional activity of clock proteins indicates that metabolic activity may affect the phase, and perhaps even the functioning, of the circadian clock. Consistent with this idea, oxidative stress, which can be caused by high metabolic activity, suppresses the expression of melatonin and the overt rhythm of bioluminescence in the dinoflagellate Lingulodini (11). However, in general, the effects of oxidative stress on circadian rhythms are poorly understood.
Here we show that the circadian clock in Drosophila is sensitive to oxidative stress, particularly in flies that lack the FOXO protein. foxo is a homolog of the Caenorhabditis elegans daf-16 gene, and it has been associated with diverse cellular functions such as insulin signaling, metabolism, growth, oxidative stress response, and senescence (12–18). We found that in foxo mutants molecular and behavioral rhythms are diminished in response to the redox cycling agent paraquat (PQ). Overexpression of components of the insulin pathway, which are thought to decrease FOXO activity, results in phenotypes similar to that of the foxo mutation. Behavioral rhythms decline rapidly with age in foxo mutants, presumably because of the increased oxidative stress in these flies.
Results
foxo Mutants Lose Behavioral and Molecular Rhythms in Response to Oxidative Stress.
In the course of an EP (enhancer and promoter) screen for genes that produce aberrant rest:activity rhythms when overexpressed, we identified a circadian phenotype caused by overexpression of the foxo gene in clock cells [supporting information (SI) Fig. 7; see Materials and Methods for details]. To determine the physiological role of foxo in circadian rhythms we tested loss-of-function mutants (14). Mutant flies had normal rest:activity rhythms in constant darkness, indicating intact clock function in central clock cells (Fig. 1A and Table 1).
Fig. 1.
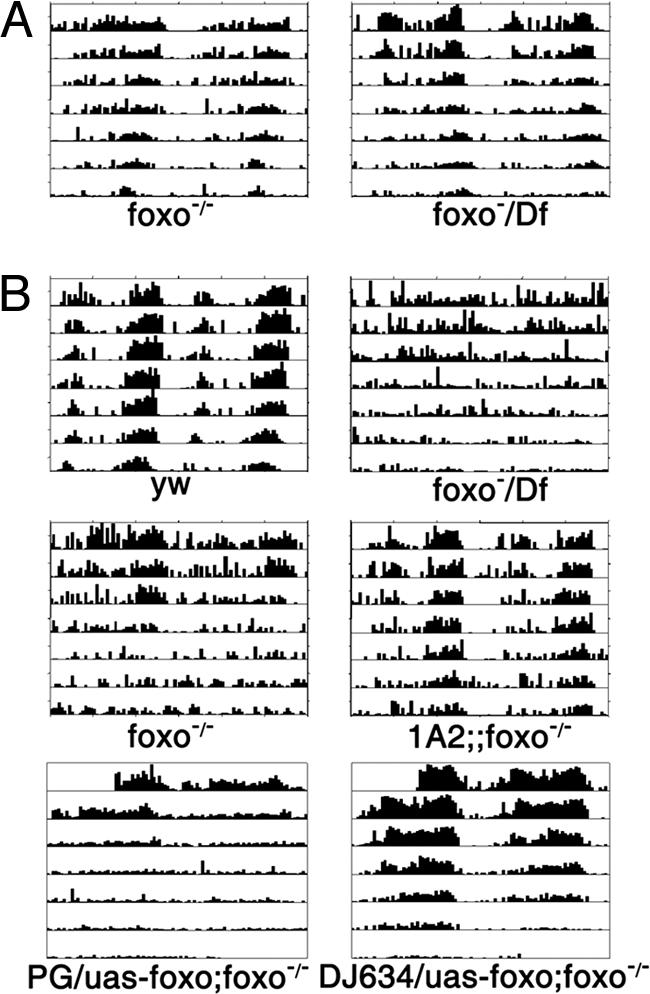
Circadian behavioral rhythms of foxo mutants are sensitive to oxidative stress. (A) In the absence of PQ, foxo mutants have normal rest:activity rhythms. Df, Df(3R)red31, which removes the foxo locus. (B) In the presence of 0.5 mM PQ, behavioral rhythms persist in control y w flies but degenerate rapidly in foxo mutants. Different allelic combinations of foxo, including the foxo21, foxo25, or Df(3R)red31 alleles, had the same phenotype. The rhythm defect was rescued by a transgene (1A2) carrying a wild-type foxo cDNA driven by its own promoter. This defect was also rescued by expression of foxo in the fat body, driven by DJ634, but not by expression of foxo in the lateral neurons (driven by Pdf-Gal4, PG). Representative actograms are shown. See SI Fig. 11 for expression patterns of the DJ634 and Pdf-Gal4 drivers.
Table 1.
Summary of circadian behavioral rhythm assays
| Genotypes | Control |
Treated |
|||
|---|---|---|---|---|---|
| n | Rhythmicity,* % | PQ, mM | n | Rhythmicity,* % | |
| y w | 31 | 100 | 0.5 | 32 | 91 |
| foxo−/− | 27 | 92 | 0.5 | 23 | 26 |
| foxo−/Df | 27 | 78 | 0.5 | 23 | 17 |
| 1A2;;foxo−/− | 0.5 | 31 | 81 | ||
| pdf-Gal4/UAS-foxo;foxo−/− | 0.5 | 31 | 1 | ||
| DJ634/UAS-foxo;foxo−/− | 0.5 | 24 | 83 | ||
| TUG/+ | 32 | 100 | 0.1 | 30 | 93 |
| UAS-InR/+ | 30 | 93 | 0.1 | 30 | 80 |
| TUG/UAS-InR | 20 | 75 | 0.1 | 27 | 33 |
| TUG/UAS-myr-akt | 15 | 87 | 1.0 | 16 | 37 |
| DJ634/+ | 32 | 100 | 1.0 | 32 | 94 |
| UAS-myr-akt/+ | 31 | 94 | 1.0 | 32 | 97 |
| DJ634/UAS-myr-akt | 24 | 80 | 1.0 | 27 | 11 |
| S106/+ † | 31 | 96 | 1.0 | 31 | 74 |
| UAS-InR/+ | 30 | 83 | 1.0 | 29 | 79 |
| S106/UAS-InR† | 32 | 91 | 1.0 | 31 | 38 |
| y w‡ | 29 | 83 | |||
| foxo−/−‡ | 51 | 20 | |||
| foxo−/Df‡ | 22 | 5 | |||
| Tp;; foxo−/−‡ | 27 | 74 | |||
| 1A2;;foxo−/−‡ | 29 | 93 | |||
Mutant phenotypes are indicated in boldface.
*Percentage rhythmicity by χ2 analysis of periodogram with 95% confidence interval.
†RU486 treatment of adults for 20 days increased sensitivity to 0.5 mM PQ in S106/UAS-InR flies (10% rhythmicity; n = 30), whereas sibling control S106/+ flies had 94% rhythmicity (n = 32).
‡One-month-old flies. Tp, Tp(3:Y) transposition carrying a WT allele of foxo.
Because of FOXO's known role in metabolism and the oxidative stress response, we considered the possibility that its effect on circadian rhythms would manifest under increased stress conditions. foxo mutants are particularly sensitive to oxidative stress, such that they show reduced survival in response to a high dose (20 mM) of PQ (14). We assessed circadian behavioral rhythms in wild-type flies and foxo mutants in the presence of low doses of PQ (0.5–1 mM). Under these conditions, wild-type flies retained their rhythms for a few weeks but foxo mutants rapidly became arrhythmic. This defect was rescued by a wild-type foxo transgene and also by a transposition or a duplication of the wild-type locus (Fig. 1B, Table 1, and data not shown). Interestingly, a general behavior like phototaxis was not affected by PQ treatment (SI Fig. 8).
Rest:activity rhythms are driven by a cluster of neurons in the central brain called lateral neurons. Time-of-day signals are generated by the molecular clock in these neurons and transmitted to other cells to produce overt rhythmic behavior. To determine whether the central clock was affected in the foxo mutants, we examined the rhythms of PER protein expression in lateral neurons. Cycling of PER was normal in foxo mutants maintained in normal conditions; however, in the presence of PQ, the amplitude of the cycling was reduced, largely because of a decrease in PER expression (Fig. 2). In addition, expression of PDF (pigment-dispersing factor), a clock-controlled factor secreted by lateral neurons, was reduced in specific projections from these neurons, although not in cell bodies (data not shown). In wild-type flies PER oscillations in lateral neurons were generally unaffected by PQ, although in some cases the expression was reduced (Fig. 2 and data not shown). There was no effect of PQ on PDF expression in wild-type flies. These data suggest that the molecular clock in the foxo mutants has increased susceptibility to oxidative stress.
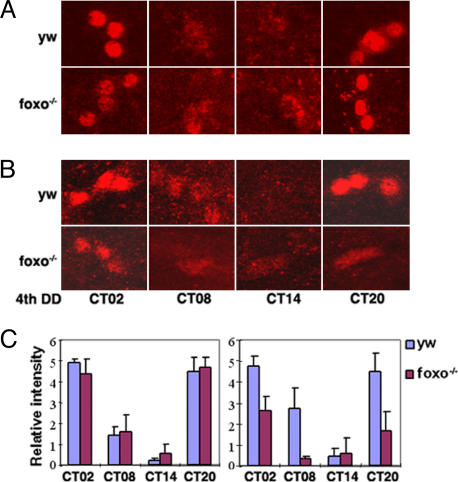
Fig. 2.
The central clock is susceptible to oxidative stress in foxo mutants. Flies were entrained to three light:dark cycles and transferred into DD with or without 1 mM PQ. On the fourth day of DD, brains were dissected at the indicated circadian times (CT) and immunostained with PER (red) and PDF (not shown) antibodies. At least six samples were examined for each time point, and representative confocal images of small ventral lateral neurons are shown. (A) In the absence of PQ, PER cycles normally in foxo mutants and y w controls. (B) In the presence of PQ, PER expression is reduced in foxo mutants. (C) Relative intensity of PER was blind-scored (mean ± SD). (Left) Control samples. (Right) PQ samples.
Peripheral Clocks Are Hypersensitive to Oxidative Stress.
As noted above, behavioral rhythms and central clock cells in wild-type flies are relatively resistant to the effects of PQ. However, circadian clocks are found in many cells other than those required to regulate rest:activity rhythms. To determine whether the clocks in other cells are sensitive to oxidative stress, we first assayed oscillations of a per-luciferase reporter (BG-luc) in the presence of PQ. The cyclic luciferase activity of this reporter reflects oscillations in peripheral tissues and can be measured in adult living flies by monitoring bioluminescence activity. As shown in Fig. 3A, in the presence of PQ, the amplitude of the cycling was dramatically reduced in wild-type flies and was abolished in the foxo mutants. BG-luc cycling was dampened in foxo mutants even in the absence of PQ (Fig. 3B), suggesting the presence of increased oxidative stress in these flies. The cycling of a CRE-luciferase reporter, which reflects clock-controlled CREB activity, was also dampened in foxo flies (data not shown).
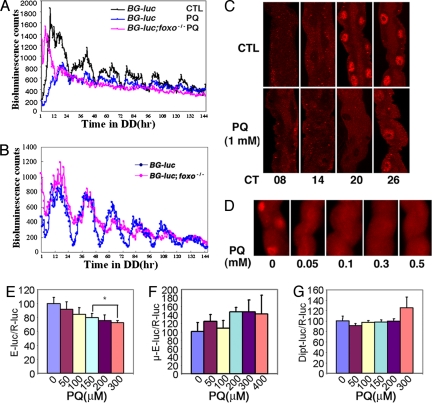
Fig. 3.
The molecular clock in peripheral tissues is hypersensitive to oxidative stress. (A) In response to PQ, BG-luc cycling is reduced in wild-type flies (n = 42) and abolished in foxo mutants (n = 27). Flies were treated with 1 mM PQ for 3 days in light:dark cycles and then monitored in the continued presence of PQ in DD. Control flies (CTL) did not receive any PQ treatment (n = 37). (B) BG-luc oscillations are dampened in foxo mutants. Flies were monitored in DD without PQ. CTL, n = 14; foxo, n = 24. (C) PER oscillations are diminished in MTs treated with PQ. PQ treatment was as described in A. MTs were dissected at the indicated circadian times on the first (circadian time 08–20) and second (circadian time 26) days of DD. PER expression was dramatically reduced at circadian time 20. (D) PER expression is reduced in MTs treated with low doses of PQ. Flies were entrained to three light:dark cycles with or without (0) PQ, and MTs were dissected at Zeitgeber time (ZT) 20. PER expression was diminished even with a dose of 0.05 mM PQ. At least five flies were examined for each data point, and representative images are shown in C and D. (E) CLK-mediated transactivation of a per-E-box-luc reporter is repressed by PQ. In contrast, PQ did not repress the expression of a reporter driven by a heat-shock basal promoter with a mutated (μ) E-box (F). dorsal-activated dipt-luc activity was also not repressed (G). Data from three experiments (E and F) and four experiments (G) were averaged. Asterisks indicate a significant difference between control (no PQ) and PQ-treated samples (P < 0.01) by ANOVA and post hoc Tukey analysis.
We also examined PER protein cycling in a peripheral tissue, the Malpighian tubule (MT). PER expression was reduced with PQ concentrations as low as 0.05 mM, suggesting that this organ is extremely vulnerable to PQ treatment (Fig. 3 C and D).
Based on a study showing that the NADH/NAD ratio affects the DNA binding efficiency of CLK/BMAL1 (7), we reasoned that redox state might affect CLK-mediated transcription of per. To test this idea, we assayed the effects of PQ on CLK activity in cultured cells. As expected, PQ altered cellular redox status (SI Fig. 9). We found that PQ repressed CLK activation of a per E box-luciferase reporter but did not affect transcription driven by a basal heat-shock promoter with a mutated E-box sequence. In addition, dorsal-activated transcription of a diptericin luciferase reporter (19) was not affected (Fig. 3 E–G). These data indicate that CLK activity is affected by oxidative stress, and this probably accounts for the effect of PQ on period expression in flies.
FOXO Is Expressed Predominantly in the Fat Body.
To understand how FOXO protects clock cells from oxidative stress, we examined its expression pattern in the whole fly. Interestingly, FOXO expression is largely restricted to fat body tissue, with no detectable expression in the circadian-relevant lateral and dorsal neurons of the brain (Fig. 4). The Drosophila fat body plays an important role in nutrient sensing and metabolism (20–22). Expression of FOXO in this tissue is consistent with its role in these processes. We did not observe FOXO expression in the lateral neurons even when flies were exposed to treatments that might induce its nuclear expression, e.g., under starvation conditions or in response to PQ. In addition, a UAS-GFP reporter driven by a foxo promoter-GAL4 was not detected in lateral neurons (SI Fig. 10), although the same foxo promoter sequence fused to a foxo cDNA rescued the phenotype of the foxo mutant (Fig. 1 and Table 1). Furthermore, expression of foxo in the fat body, using a fat body Gal4 driver DJ634 (23), rescued the PQ-sensitive phenotype of the foxo mutant whereas a lateral neuron Gal4 driver failed to rescue (Fig. 1B and SI Fig. 11). These data suggest that FOXO protects the lateral neuron clock from oxidative stress via a non-cell-autonomous mechanism.
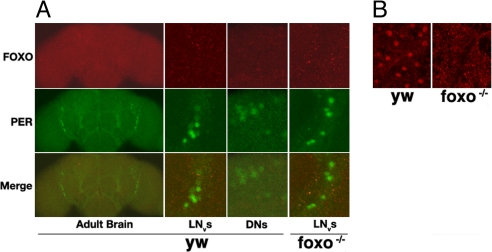
Fig. 4.
FOXO is expressed predominantly in the fat body. Adult tissues were dissected and immunostained with FOXO and PER antibodies. (A) FOXO is not detectable in the central clock cells of wild-type flies. Ventrolateral neurons (LNvs) and dorsal neurons (DNs) were positive for PER but not for FOXO. (B) FOXO is expressed abundantly in the fat body (both head and abdominal). At least five samples were examined, and representative images are shown.
Elevated Insulin Signaling Increases Sensitivity to Oxidative Stress.
FOXO is deactivated by insulin signaling through AKT-mediated phosphorylation (24). To determine whether elevated insulin signaling increases sensitivity of the circadian system to oxidative stress, we overexpressed components of the insulin pathway. Expression of the insulin receptor (UAS-InR) (25) under the control of the tim promoter (tim-UAS-Gal4 or TUG) (26), dramatically increased sensitivity to oxidative stress. These flies died within 2 days of being challenged with 1 mM PQ. When treated with 0.05–0.1 mM PQ they survived up to 6 days, and the majority showed arrhythmic locomotor behavior (Fig. 5A and Table 1). Similar but weaker effects were obtained when a constitutively active AKT (UAS-myr-Akt, kindly provided by M. Birnbaum, University of Pennsylvania) was overexpressed under the control of the same driver, consistent with the weaker signaling strength of myr-AKT as compared with insulin receptor (INR) (25, 27).
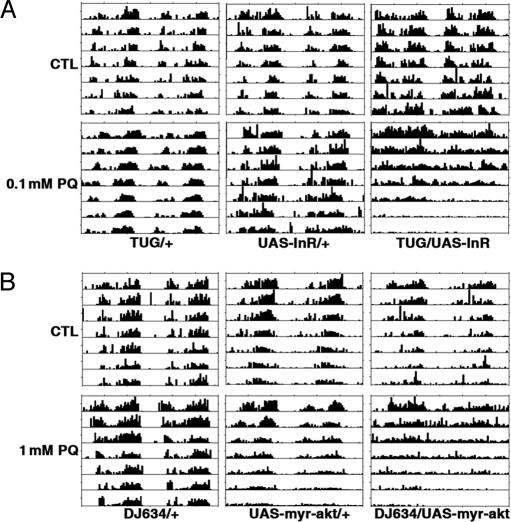
Fig. 5.
Overexpression of INR and AKT increases sensitivity of circadian behavioral rhythms to oxidative stress. (A) TUG-driven overexpression of INR results in arrhythmia in response to a low dose of PQ. (B) Overexpression of constitutively active AKT in the fat body increases sensitivity to PQ. CTL, control.
TUG is expressed in the fat body as well as the central clock neurons. To express INR in the fat body we used a variety of constitutive fat body drivers, but all were lethal in combination with the UAS-InR transgene. However, expression of myr-AKT under the control of the fat body driver DJ634 increased sensitivity of behavioral rhythms to PQ (Fig. 5B and Table 1). A similar phenotype was obtained when INR was overexpressed in adult fat bodies using an inducible fat body driver, S106 (12, 28) (SI Fig. 12 and Table 1). Together these results indicate that elevated insulin signaling increases the susceptibility of the circadian clock to oxidative stress, as does the foxo mutation. Importantly, increased insulin signaling, or loss of foxo, in the fat body tissue is sufficient to affect the sensitivity of the central circadian clock to oxidative stress.
Behavioral Rhythms Degenerate Rapidly in Aging foxo Mutants.
The insulin pathway regulates aging (12, 29), a process of cellular senescence long suspected to result from oxidative stress (30, 31). In addition, we showed previously that rest:activity rhythms break down with age and suggested that this occurs because of an increase of oxidative stress (32). Based on the increased sensitivity of foxo mutants to oxidative stress, we hypothesized that they would show accelerated breakdown of rest:activity rhythms. As shown in Fig. 6, 30-day-old wild-type flies had robust circadian rhythms, but foxo mutants showed weak rhythms similar to those seen in response to PQ in young flies, indicating a premature breakdown of circadian clock function in the foxo mutants. This defect was rescued by a foxo transgene (Fig. 6 and Table 1).
Fig. 6.
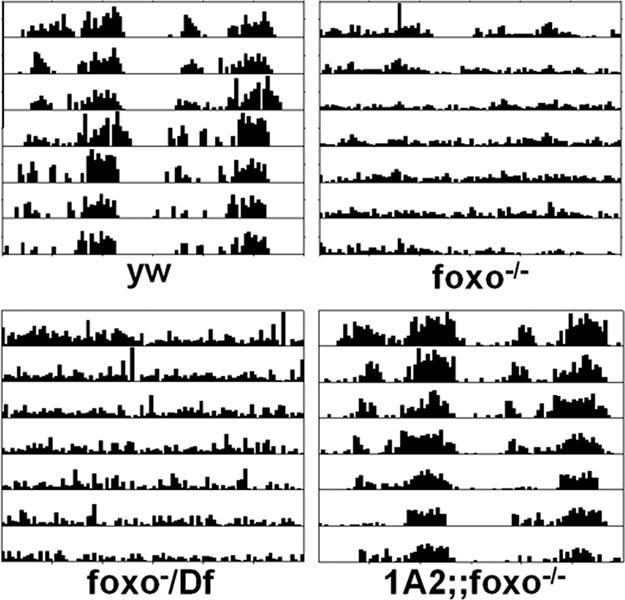
Aging foxo mutants have weak behavioral rhythms. Whereas 1-month-old wild-type flies have robust behavioral rhythms, foxo mutants show very weak behavioral rhythms. This defect was rescued by a transgene 1A2 carrying a wild-type foxo cDNA driven by its own promoter.
Discussion
We demonstrate here a link between oxidative stress and circadian rhythms in Drosophila. In particular, we found that the circadian clock is sensitive to oxidative stress and that this sensitivity is easily detected in the foxo mutants. When treated with a redox cycling agent, PQ, or even normally with aging, these mutants show a breakdown of rest:activity rhythms, which are driven by the molecular clock in the central brain. Cell culture transcription assays show that PQ treatment represses CLK-mediated activation of an E-box-luc reporter, consistent with the findings that PQ treatment reduces the amplitude of BG-luc cycling in the living fly, and decreases PER expression in the lateral neurons of foxo mutants. Although we did not detect an acute effect of PQ on rest:activity rhythms of wild-type flies, we did see a profound effect on clock gene expression in peripheral clocks, indicating that peripheral clocks are hypersensitive to oxidative stress. Molecular oscillations in MTs are diminished considerably even with very low doses of PQ. This could be because these cells are more susceptible to oxidative stress or because they are more accessible to ingested PQ. Based on the function of this tissue in excretion (33, 34), we suspect that PQ is filtered through the MTs, thus resulting in a relatively high dose in this tissue.
Increased signaling of the insulin pathway in the fat body is sufficient to increase sensitivity of the circadian clock to PQ. This effect may result from the down-regulation of FOXO activity because insulin signaling is thought to lead to AKT-dependent phosphorylation and exclusion of FOXO from the nucleus (17). FOXO in other organisms reportedly regulates the expression of several enzymes important for the cellular defense against oxidative stress: superoxide dismutase and catalase (35, 36). We compared mRNA levels of these enzymes in adult heads and found that they were not altered in foxo mutants (data not shown), but it is possible that they are affected in specific cells.
On the other hand, FOXO also regulates insulin activity through feedback mechanisms. In C. elegans, the insulin-like peptide ins-7 is up-regulated in daf-16 (foxo) mutants (37). Studies in Drosophila also support a non-cell-autonomous effect of FOXO on insulin signaling. Overexpression of FOXO in the fat body reduces expression of Drosophila insulin-like peptide 2 (dilp2) in brain neurons and extends lifespan (12). We found that dilp2 expression varies considerably among different backgrounds (data not shown), but mRNA levels of the Drosophila homolog of the mammalian IGF binding protein ALS (acid labile subunit) (20) are consistently higher in foxo mutants (SI Fig. 13). Because ALS is thought to promote insulin action (20), an increase in its levels may be indicative of increased insulin signaling, which could also contribute to the hypersensitivity to oxidative stress in foxo mutants.
In general, data showing an effect of metabolism on circadian rhythms are limited. Streptozotocin treatment attenuates mPER2 expression in the mouse liver although not in the suprachiasmatic nucleus or the cerebral cortex. Interestingly, daily injections of insulin restore mPer2 and mPER2 rhythms in the liver of streptozotocin-treated mice (38). As noted above, in vitro studies showed that the ratio of NADH/NAD affects the activity of CLK/BMAL1, with higher ratios resulting in increased activity (7). Consistent with this, we found that PQ treatment shifted the cellular redox environment to a more oxidized state (SI Fig. 9) and repressed CLK-mediated activation of per E-box reporter in cell culture assays. Reduced expression of period in the living fly also suggests that CLK activity is affected by redox state.
Our data indicate that the effects of FOXO on circadian rhythms are non-cell-autonomous, because FOXO is not detectable in lateral neurons, the cells that generate the rest:activity rhythm. Importantly, expression of UAS-foxo in the fat body rescues the foxo mutant phenotype, whereas expression of UAS-foxo in the lateral neurons does not (Fig. 1B and SI Fig. 11). The fat body in Drosophila is the primary site of lipid oxidative damage as measured by 4-hydroxy-2-nonenal accumulation (39). Because 4-hydroxy-2-nonenal is diffusible (40), it could also affect distant tissues such as the CNS. It is also worth noting that, although PQ did not affect phototaxis of foxo mutants, they were less phototactic to begin with, suggesting that they may have other defects.
Increasing evidence supports the oxidative stress theory of aging (30, 31). Consistent with the finding that foxo mutants are more susceptible to oxidative stress, their behavioral rhythms break down prematurely during aging. This breakdown of rhythms also occurs in wild-type flies, but at an older age (32). We speculate that even in the wild-type flies it is caused by oxidative stress [in fact, extended treatment with PQ also causes loss of rhythms in wild-type flies (32)] but takes longer to manifest. In line with our data showing that elevated insulin signaling results in increased sensitivity to oxidative stress, the insulin pathway is implicated in the aging process in multiple species. Decreased insulin signaling in worms, flies, and mammals, systemically or only in the adipose tissue, extends lifespan, although the mechanisms involved are not known (29, 41–45). Thus, the studies presented here have implications for understanding the connection among metabolism, oxidative stress, aging, and circadian rhythms.
Materials and Methods
EP Screening.
We conducted a screen for genes that produce aberrant rest:activity rhythms when overexpressed, by mobilizing an EP insertion located on the X chromosome. New insertions of the EP were expressed in clock cells by using a tim-Gal4 (TG) driver. Of 3,662 lines screened, 33 showed rhythm deficits that ranged from period changes to arrhythmia. Of these, three lines contained independent insertions in the first exon of the foxo gene. Because it was isolated multiple times and because of its obvious role in metabolism, we became interested in foxo.
Fly Stocks and Behavioral Assays.
All fly stocks were raised on standard molasses–yeast–corn meal. foxo21 and foxo25 (14) were maintained over a balancer, TM6B. Transheterozygotes of foxo21/foxo25 were used for experiments unless noted otherwise. Males of foxo21 and foxo25 were also out-crossed to y w, and F1 females were backcrossed to y w for six generations, allowing recombination. F6 males were then crossed into double balancer y w; CyO; TM6B to establish stocks. UAS-foxo was kindly provided by Ernst Hafen (University of Zurich, Zurich, Switzerland). Deficiency Df(3R)red31, transposition Tp(3:Y)ry506-85C, and duplication Df(3R)ry85;DP(3:2)ry[+], DJ634 (Gal4 insertion in the gene Lk6) were obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN). All lines reported here were genotyped by a combination of PCR, sequencing, Western blotting, immunohistochemistry, and morphology.
For behavioral rhythm assays, 3- to 5-day-old adult males were collected and entrained to a 12-h:12-h light:dark cycle at 25°C for 3 days. Individual flies were loaded into locomotor tubes containing control food (no PQ) or food with PQ (Sigma, St. Louis, MO) and monitored under constant dark conditions (DD) in parallel for 10 days. The PQ dosage was generally adjusted within a range of 0.5–1 mM to elicit the strongest possible circadian behavioral phenotype. As noted in Results, it was reduced to 0.1 mM for flies overexpressing the INR. Activity records were analyzed by using Clocklab software (Actimetrics). Circadian periodicity was evaluated by using χ2 periodogram analysis. In the aging experiment, 2- to 3-day-old adult males were collected and maintained on standard corn meal for 30 days at room temperature (≈23°C), then entrained to a 12-h:12-h light:dark cycle at 25°C for 3 days and analyzed as described above.
In the RU486 (Sigma) experiments, 2- to 3-day-old adult males were collected and raised on standard food with or without 0.5 mM RU486 for 6–8 days in a 12-h:12-h light:dark cycle at 25°C, then loaded into locomotor tubes in the continued presence or absence of RU486 with or without PQ.
P-Element-Mediated Germ-Line Transformation.
A 4.6-kb genomic fragment containing the promoter region and first two exons of foxo was fused to a full-length foxo cDNA, followed by an in-frame V5 tag sequence, and cloned into the pUAST vector. Ten transgenic lines were obtained by standard P-element-mediated germ-line transformation. Two of them, 1A2 and 2A1, were crossed into the foxo mutant background. The same 4.6-kb fragment was also cloned into the pPTGal4 transformation vector to obtain transgenic flies.
Whole-Fly per-Luciferase Reporter Assay.
Whole-fly luciferase activity monitoring was performed as reported (46), with some modifications: 3- to 5-day-old adult males were collected and entrained to a 12-h:12-h light:dark cycle at 25°C for 3 days on normal food with (experimental) or without (control) 1 mM PQ, then loaded into Optiplate-96F plates (PerkinElmer) containing 100 μM firefly luciferin (Biosynth) with or without 1 mM PQ. At least 5 days of data were collected in a Topcount NXT luminometer (Packard); each well was read twice, for 6 seconds each time, in a 30-min interval.
Cell Culture and CLK-Mediated Transcription Assay.
Serum-free medium (SFM) adapted D.mel2 cells (Invitrogen) were cultured in Express Five SFM (Invitrogen). Plasmids [10 ng of pAct-Clk, 50 ng of per-E-box-luc, 50 ng of per-μ-luc (mutated E-box), 20 ng of pAct-dl, 100 ng of dipt-luc, and 50 ng of actin-Rluc] were transfected into D.mel2 cells in 96-well plates using Effectene according to the manufacturer's protocol (Qiagen, Valencia, CA). Twelve hours after transfection, cells were treated with PQ or vehicle, and 1 μg/ml insulin was added for improved cell viability (note that insulin might down-regulate endogenous FOXO activity). Seventy-two hours after treatment, cells were harvested and luciferase activity was measured in a Victor 3 plate reader (PerkinElmer) by using Stop & Glow reagents (Promega, Madison, WI). Luc/R-luc readings were normalized to the non-PQ-treated sample (set as 100).
Quantitative Real-Time PCR.
Three- to 5-day-old adult males were collected and entrained to a 12-h:12-h light:dark cycle at 25°C for 3 days and collected on dry ice at indicated time points. Total RNA was isolated by using an Ultraspec RNA isolation system (Biotecx), and cDNAs were synthesized by using a high-capacity cDNA Archive kit (Applied Biosystems). Quantitative real-time PCR was performed in an ABI prism 7100 using a SYBR Green kit (Applied Biosystems). We essentially followed protocols from the manufacturer for primer design, PCR, and data analysis.
Immunohistochemistry and Microscopy.
Tissues were dissected out in 4% PFA, washed for 1 h in PBS buffer, and incubated with primary antibody (in PBS buffer with 3% normal donkey serum and 0.3% Triton X-100) overnight at 4°C. Tissues were extensively washed with PBS buffer and incubated with Cy3 donkey anti-rabbit (or rat) and FITC donkey anti-rat (or rabbit) secondary antibodies (Jackson ImmunoResearch Laboratories) for 1.5 h at room temperature, followed by extensive washes in PBS. Fluorescent images were taken with a FluoView confocal microscope (FV1000, ver.1.3c; Olympus) and analyzed with Openlab 4.0.1 (Improvision). Primary antibody dilutions were as follows: FOXO (24), 1:1,000; PDF, 1:1,000; PER, 1:1,500 (47). Secondary antibody dilutions were 1:1,000.
Supplementary Material
Acknowledgments
We thank Ernst Hafen, Morris Birnbaum, Jeffrey Hall (Brandeis University, Waltham, MA), Justin Blau (New York University, New York, NY), Michael Rosbash (Brandeis University), and Ron Davis (Baylor College of Medicine, Houston, TX) for fly stocks; Robert Tjian (University of California, Berkeley, CA) for the FOXO antibody; and Steve Kay (Scripps Research Institute, La Jolla, CA), Julie Williams (Robert Wood Johnson Medical School, Piscataway, NJ), and Toshinori Hoshi (University of Pennsylvania) for plasmids. We appreciate technical assistance from Amanda Hansen for quantitative RT-PCR and Hajime Takano (of the Imaging Center at the University of Pennsylvania, which is funded by the National Institute of Neurological Disorders and Stroke) for confocal microscopy. We also thank Jeff Field (University of Pennsylvania) and Morris Birnbaum for critical reading of the manuscript and members of the A.S. laboratory for helpful discussions. A.S. is an Investigator of the Howard Hughes Medical Institute. This work was funded in part by National Institutes of Health Grant NS048471.
Abbreviations
- MT
Malpighian tubule
- PQ
paraquat
- CT
circadian time
- DD
constant dark conditions
- INR
insulin receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701599104/DC1.
References
- 1.Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD, Weitz CJ, Takahashi JS, Kay SA. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 2.Hardin PE. Curr Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Ashmore LJ, Sehgal A. J Biol Rhythms. 2003;18:206–216. doi: 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- 4.Lin F-J, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Mol Cell Biol. 2001;21:7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froy O, Chang DC, Reppert SM. Curr Biol. 2002;12:147–152. doi: 10.1016/s0960-9822(01)00656-x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shlomo R, Akhtar R, Collins B, Judah D, Davies R, Kyriacou C. Chronobiol Int. 2005;22:455–471. doi: 10.1081/CBI-200062353. [DOI] [PubMed] [Google Scholar]
- 7.Rutter J, Reick M, Wu LC, McKnight SL. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 8.Reick M, Garcia JA, Dudley C, McKnight SL. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 9.Fernie AR, Carrari F, Sweetlove LJ. Curr Opin Plant Biol. 2004;7:254–261. doi: 10.1016/j.pbi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Harper M-E, Bevilacqua L, Hagopian K, Weindruch R, Ramsey JJ. Acta Physiol Scand. 2004;182:321–331. doi: 10.1111/j.1365-201X.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 11.Hardeland R, Coto-Montes A, Poeggeler B. Chronobiol Int. 2003;20:921–962. doi: 10.1081/cbi-120025245. [DOI] [PubMed] [Google Scholar]
- 12.Hwangbo DS, Gersham B, Tu M-P, Palmer M, Tatar M. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 13.Lin K, Dorman JB, Rodan A, Kenyon C. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 14.Junger M, Rintelen F, Stocker H, Wasserman J, Vegh M, Radimerski T, Greenberg M, Hafen E. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HK, Kim YK, Song I-H, Baek S-H, Lee S-R, Kim JH, Kim J-R. J Gerontol A Biol Sci Med Sci. 2005;60:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- 16.Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 17.Barthel A, Schmoll D, Unterman TG. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Morris BJ. J Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- 19.Gross I, Georgel P, Kappler C, Reichhart J, Hoffmann J. Nucleic Acids Res. 1996;24:1238–1245. doi: 10.1093/nar/24.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 21.Scott RC, Schuldiner O, Neufeld TP. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Häder T, Müller S, Aguilera M, Eulenberg KG, Steuernagel A, Ciossek T, Kühnlein RP, Lemaire L, Fritsch R, Dohrmann C, et al. EMBO Rep. 2003;4:511–516. doi: 10.1038/sj.embor.embor837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seroude L, Brummel T, Kapahi P, Benzer S. Aging Cell. 2002;1:47–56. doi: 10.1046/j.1474-9728.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 24.Puig O, Marr MT, Ruhf ML, Tjian R. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 26.Blau J, Young MW. Cell. 1999;99:661–671. doi: 10.1016/s0092-8674(00)81554-8. [DOI] [PubMed] [Google Scholar]
- 27.Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Science. 2002;295:2088–2091. doi: 10.1126/science.1068094. [DOI] [PubMed] [Google Scholar]
- 28.Roman G, Endo K, Zong L, Davis RL. Proc Natl Acad Sci USA. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 30.Finkel T, Holbrook NJ. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 31.Beckman KB, Ames BN. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 32.Koh K, Evans JM, Hendricks JC, Sehgal A. Proc Natl Acad Sci USA. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dow JAT, Davies SA. J Insect Physiol. 2006;52:365–378. doi: 10.1016/j.jinsphys.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Jung AC, Denholm B, Skaer H, Affolter M. J Am Soc Nephrol. 2005;16:322–328. doi: 10.1681/ASN.2004090729. [DOI] [PubMed] [Google Scholar]
- 35.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KWA, Coffer PJ, Huang T-T, Bos JL, Medema RH, Burgering BMT. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 36.McElwee J, Bubb K, Thomas JH. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 37.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 38.Kuriyama K, Sasahara K, Kudo T, Shibata S. FEBS Lett. 2004;572:206–210. doi: 10.1016/j.febslet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 39.Zheng J, Mutcherson R, Helfand SL. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 40.Barrera G, Di Mauro C, Muraca R, Ferrero D, Cavalli G, Fazio V, Paradisi L, Dianzani M. Exp Cell Res. 1991;197:148–152. doi: 10.1016/0014-4827(91)90416-r. [DOI] [PubMed] [Google Scholar]
- 41.Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloting N, Bluher M. Exp Gerontol. 2005;40:878–883. doi: 10.1016/j.exger.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 44.Tatar M. Exp Gerontol. 2004;39:1745–1750. doi: 10.1016/j.exger.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 45.van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RGJ. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 46.Stanewsky R, Jamison CF, Plautz JD, Kay SA, Hall JC. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers EM, Yu J, Sehgal A. Curr Biol. 2003;13:526–533. doi: 10.1016/s0960-9822(03)00167-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.