Abstract
NF-κB is constitutively active in many solid tumors, including breast cancer. However, the role of NF-κB in breast carcinogenesis is unknown. IkkαAA/AA “knockin” mice in which activation of IκB kinase α (IKKα) is prevented by replacement of activation loop serines with alanines exhibit delayed mammary gland growth during pregnancy, because IKKα activity is required for cyclin D1 induction and proliferation of lobuloalveolar epithelial cells. Given the role of cyclin D1 in breast and mammary cancer, we examined involvement of IKKα in mammary carcinogenesis induced by oncogenes or a chemical carcinogen, 7,12-dimethylbenz[a]anthracene (DMBA). The IkkαAA mutation retarded tumor development in response to either 7,12-dimethylbenzaanthracene or the MMTV-c-neu (ErbB2/Her2) transgene but had no effect on MMTV-v-Ha-ras-induced cancer, although both oncogenes rely on cyclin D1. Strikingly, primary IkkαAA/AA/MMTV-c-neu carcinoma cells exhibited diminished self-renewal capacity, resulting in the inability to establish secondary tumors. IkkαAA/AA/MMTV-c-neu carcinoma cells underwent premature senescence when cultured under conditions used for propagation of mammary gland stem cells. Thus, IKKα is not only a regulator of mammary epithelial proliferation, but is also an important contributor to ErbB2-induced oncogenesis, providing signals that maintain mammary tumor-initiating cells. IKKα may represent a novel and specific target for treatment of ErbB2-positive breast cancer.
Keywords: breast cancer, NF-κB
Breast cancer is the most common malignancy in women, accounting for nearly one-third of all cancers. Among the many signaling molecules thought to be involved in breast carcinogenesis, the role of NF-κB has not been fully investigated. Whereas the role of NF-κB transcriptional factors as critical regulators of innate and adaptive immunity is well established (1), there is increasing evidence that constitutively elevated NF-κB activity is also a common feature of breast cancer and other solid malignancies (2–4). Indeed, recent work demonstrated that NF-κB plays a critical tumor promoting role in colon and liver cancers (5). NF-κB activation depends on the IκB kinase (IKK) complex, which contains two catalytic subunits IKKα and IKKβ (6). Although IKKβ is the critical subunit in most NF-κB activation responses, we found that IKKα was particularly important in mammary epithelial cells exposed to receptor activator of NF-κB (RANK) ligand (RANKL). Just like Rank-null and Rankl-null mice (7), Ikkα AA/AA mice, in which IKKα activation is prevented by replacement of its activation loop serines with alanines, showed retarded growth of the lobuloalveolar tree during pregnancy (8). Defective proliferation of mammary epithelial cells in Ikkα AA/AA mice is caused by impaired RANK-mediated induction of cyclin D1, the critical G1 cyclin of these cells, which is encoded by an NF-κB target gene (8). Ablation of cyclin D1 results in a mammary gland defect similar to that of Ikkα AA/AA mice (9), whereas a MMTV-cyclin D1 transgene rescues the mammary gland development defect of Ikkα AA/AA mice (8). Cyclin D1 is overexpressed in >50% of human mammary carcinomas and is amplified in up to 20% of human breast cancers (10). Transgenic cyclin D1 overexpression in the mouse mammary epithelium results in lobuloalveolar hyperplasia and mammary cancer (11). Conversely, cyclin D1 gene disruption protects mice against breast cancers induced by transgenic expression of ErbB2/Her2/neu and Ha-ras (12).
Here, we provide in vivo evidence for an important role of IKKα in mammary carcinogenesis in two different mouse models of breast cancer. One of these models is based on transgenic overexpression of ErbB2/neu from the mouse mammary tumor virus (MMTV) promoter, a validated mouse model for ErbB2-positive human breast cancer (13). The other model is mammary chemical carcinogenesis based on treatment with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) and the tumor promoter progesterone (14). Inactivation of IKKα reduces the incidence and multiplicity and prolongs the latency of mammary carcinomas in both models. Furthermore, cancer cells derived from Ikkα AA/AA/MMTV-c-neu tumors show lower cyclin D1 expression and a highly reduced ability to form secondary tumors because of the impaired self-renewal capacity of mammary tumor-initiating cells (TIC). These results strongly suggest that IKKα represents an attractive target for development of therapeutics for specific treatment of ErbB2-positive human breast cancer.
Results
The Ikkα AA Mutation Reduces Mammary Carcinogenesis by the ErbB2/neu Oncogene.
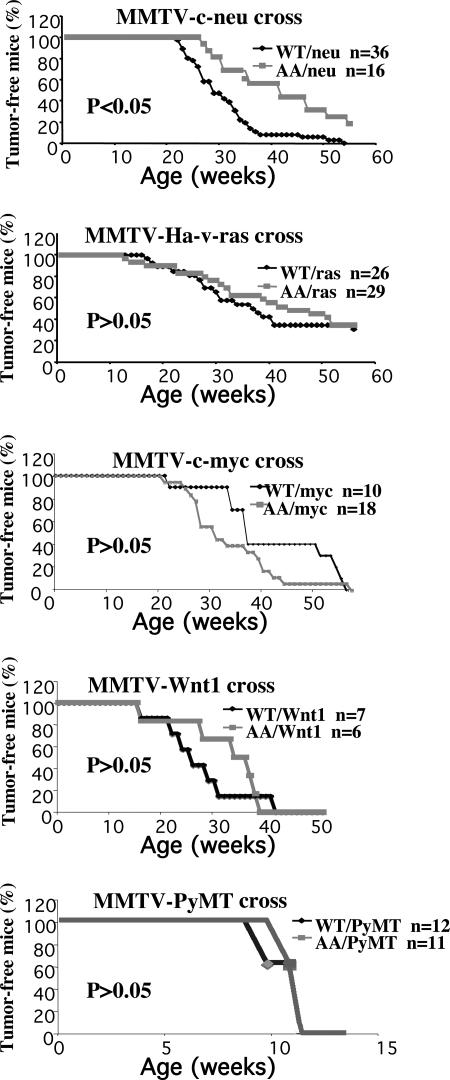
To examine the role of IKKα in mammary carcinogenesis, we crossed Ikkα AA/AA mice with different transgenic strains that express ErbB2/neu, Ha-ras, polyomavirus middle T antigen (PyMT), c-myc, and Wnt1 under control of the mammary epithelium-specific MMTV promoter (15–18) and monitored littermate females of all genotype combinations for tumor formation by biweekly palpation. Although homozygosity for the Ikkα AA mutation reduced the incidence and multiplicity and delayed the onset of mammary cancer caused by MMTV-c-neu (P < 0.05), it exerted no significant inhibitory effect on cancers induced by MMTV-v-Ha-ras, MMTV-PyMT, MMTV-c-myc, and MMTV-Wnt1 (Fig. 1). In fact, it slightly accelerated the appearance of c-myc-induced tumors. In >1 year, all (n = 36) Ikkα+/+/MMTV-c-neu females developed tumors, whereas 20% of Ikkα AA/AA/MMTV-c-neu females (n = 16) remained tumor free. It took 42 weeks for 50% of Ikkα AA/AA/MMTV-c-neu females to display tumors compared with 29 weeks for Ikkα+/+/MMTV-c-neu. Tumor numbers were also markedly decreased in the Ikkα AA/AA MMTV-c-neu group (Fig. 2B). Tumor growth rates were also decreased in the Ikkα AA/AA background. Histological analysis revealed no differences between Ikkα AA/AA/MMTV-c-neu and Ikkα+/+/MMTV-c-neu tumors (Fig. 2A). The Ikkα AA mutation also had no effect on the histology of MMTV-v-Ha-ras tumors.
Fig. 1.
IKKα kinase activation is required for full mammary tumorigenesis by MMTV-c-neu. Occurrence of mammary cancer in Ikkα +/+/MMTV-c-neu, Ikkα AA/AA/MMTV-c-neu, Ikkα+/+/MMTV-v-Ha-ras, Ikkα AA/AA/MMTV-v-Ha-ras, Ikkα+/+/MMTV-c-myc, Ikkα AA/AA/MMTV-c-myc, Ikkα+/+/MMTV-Wnt1, and Ikkα AA/AA/MMTV-Wnt1 mice that were followed for 1 year and in Ikkα+/+/MMTV-PyMT and Ikkα AA/AA/MMTV-PyMT mice that were followed for 4 months. WT, Ikkα+/+; AA, Ikkα AA/AA.
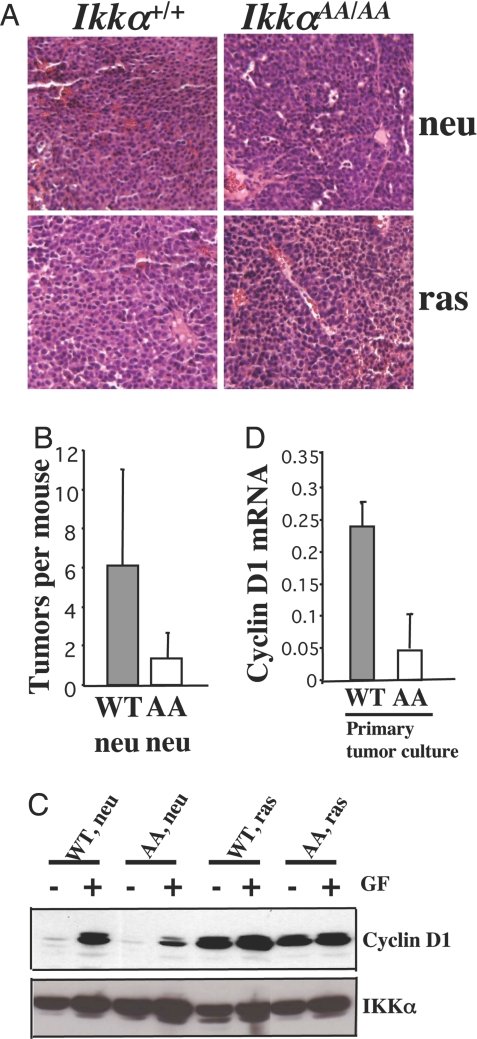
Fig. 2.
The Ikkα AA mutation reduces cyclin D1 expression in MMTV-c-neu mammary cancers. (A) Tumor histology. (Magnification: ×20.) (B) Average tumor numbers per mouse in Ikkα +/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu mice. Tumors were excised from mice of indicated genotypes, paraffin-embedded, sectioned, and H&E-stained. (C) Cyclin D1 expression in primary cancer cell cultures kept with (+) or without (−) 10% serum and 10 ng/ml EGF for 2 days before analyses. (D) Cyclin D1 mRNA levels in Ikkα+/+/MMTV-c-neu (WT) and Ikkα AA/AA/MMTV-c-neu (AA) tumors and primary tumor cell cultures.
The IkkαAA Mutation Reduces Cyclin D1 Expression in MMTV-c-neu Mammary Carcinomas.
Primary cancer cells were cultured for biochemical and physiological characterization. Ikkα AA/AA/MMTV-c-neu cells expressed less cyclin D1 upon growth factor (serum + EGF) addition relative to Ikkα+/+/MMTV-c-neu cells (Fig. 2C). Growth factors had little effect on constitutive cyclin D1 expression in Ha-ras-transformed cells of either genotype (Fig. 2C). Real-time (17) PCR analyses have confirmed the differences in cyclin D1 mRNA between Ikkα+/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu carcinoma cells (Fig. 2D).
The Ikkα AA Mutation Inhibits Preneoplastic Alterations in MMTV-c-neu Mice.
MMTV-induced mammary carcinoma is frequently preceded by preneoplastic alterations, such as hyperplastic alveolar nodules (19). Accordingly, we examined whole mounts of mammary glands from 5-month-old nulliparous Ikkα+/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu littermates without detectable tumors (Fig. 3A). Whereas Ikkα+/+/MMTV-c-neu mammary glands exhibited pronounced lobular hyperplasia with marked increase in terminal branch and alveolar numbers, resembling hormonally stimulated glands of pregnant females, the structure of the Ikkα AA/AA/MMTV-c-neu mammary gland was similar to those of oncogene-devoid WT mice (Fig. 3A). Immunohistochemistry staining showed that both WT and mutant epithelial cells express similar amounts of c-Neu/ErbB2 per cell (Fig. 3B). Pulse labeling of 5-month-old MMTV-c-neu females of either genotype with BrdU revealed that the Ikkα AA mutation reduced the proliferation of c-Neu/ErbB2-expressing cells (Fig. 3C).
Fig. 3.
Preneoplastic changes in MMTV-c-neu transgenic mice. (A) Mammary glands of 5-month-old Ikkα+/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu female mice were fixed for whole-mount staining (Upper) and H&E staining (Lower). (B) Immunohistochemical staining for neu/ErbB2 expression on paraffin sections. (C) Immunofluorescence staining for BrdU incorporation in the same samples as A and B. Mice were injected with BrdU 2 h before analysis. (Magnification: ×10.)
The Ikkα AA Mutation Reduces the Proliferative Capacity and Malignant Potential of ErbB2-Transformed Cancer Cells.
To determine whether homozygosity for the Ikkα AA mutation altered the malignant potential of mammary carcinoma cells, we performed transplantation experiments. Similar-sized tumors were isolated from Ikkα+/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu mice and cultured. After 1 week, 5 × 105 viable carcinoma cells of each genotype were injected into the right and left no. 4 mammary glands of nulliparous Rag1−/− females. Ikkα AA/AA/MMTV-c-neu cancer cells required much a longer time to generate secondary tumors compared with Ikkα+/+/MMTV-c-neu cancer cells transplanted into the same recipient (Fig. 4A). In two of the four repeats of this experiment with independently isolated cancer cells, the Ikkα AA/AA/MMTV-c-neu carcinoma cells did not form any secondary tumors at the time when secondary Ikkα+/+/MMTV-c-neu tumors were 2.5 cm in diameter. These results are consistent with those shown in Fig. 1, indicating that the Ikkα AA mutation dramatically prolongs the latency of MMTV-c-neu-induced tumors. Also consistent with the data in Fig. 1, the Ikkα AA mutation had no deleterious effect on induction of secondary tumors by cultured MMTV-v-Ha-ras cancer cells (Fig. 4A). Because each recipient mouse contains a uniform set of stromal and immune cells and provides a uniform hormonal environment, the difference in secondary tumor regeneration therefore ought to be intrinsic to the transplanted carcinoma cell compartment.
Fig. 4.
IKKα kinase activation is required for continuous proliferation and secondary tumor generation by MMTV-c-neu cancer cells. (A) Growth curves of secondary tumors formed by primary cancer cells transplanted into mammary glands of Rag1−/− mice. (B and C) Proliferation rates of cultured primary cancer cells after 1 week of culture were assessed by cell counting (B) and BrdU incorporation (C). WT, Ikkα+/+/MMTV-c-neu; AA, Ikkα AA/AA/MMTV-c-neu.
To measure cell proliferation, confluent primary cultures of cancer cells of either genotype were split after 1 week in culture, 105 cells were placed in new culture dishes, and cell number was measured every 4 days. Whereas Ikkα+/+/MMTV-c-neu cells continued to multiply, Ikkα AA/AA/MMTV-c-neu cells did not (Fig. 4B). Cell proliferation was also measured by BrdU incorporation, further demonstrating the proliferation defect of Ikkα AA/AA cancer cells (Fig. 4C). However, TUNEL assays failed to reveal higher rates of apoptosis in Ikkα AA/AA/MMTV-c-neu cultures relative to cultures of WT c-neu-transformed cells (data not shown). Collectively, these experiments suggest that homozygosity for the Ikkα AA mutation diminishes the proliferative potential of c-Neu/ErbB2-transformed cancer cells.
The Ikkα AA Mutation Impairs Self-Renewal of MMTV-c-neu TIC.
Recent studies have demonstrated that only a small number of primary breast cancer cells, referred to as cancer stem cells or TIC, can form secondary tumors (20, 21). When cultivated under specific conditions in the absence of serum, TIC-enriched cancer cells form nonadherent mammospheres, similar to normal mammary gland stem cells (22, 23). We applied this culture system to Ikkα AA/AA and WT c-Neu/ErbB2-transformed cells. When WT or IkkαAA/AA cancer cells were isolated from fresh primary tumors, they formed mammospheres of similar appearance (Fig. 5A). However, after dispersion into single cells (Fig. 5B) only WT c-Neu/ErbB2-transformed cells formed secondary mammospheres, whereas Ikkα AA/AA/MMTV-c-neu cells formed dead cell aggregates (Fig. 5C). WT secondary mammospheres could reform mammospheres at least one more time after dispersion (data not shown). This finding suggests that the Ikkα AA mutation severely impairs the self-renewal capacity of MMTV-c-neu TIC. When primary mammospheres were plated in serum-rich medium, both WT and Ikkα AA/AA cancer cells assumed typical epithelial appearance and continued to grow to some extent, but many of the IkkαAA/AA/MMTV-c-neu cells underwent senescence, as indicated by β-gal expression (Fig. 5D).
Fig. 5.
IKKα kinase activation is required for self-renewal of TIC. (A) Primary cultures of isolated cancer cells from Ikkα+/+/MMTV-c-neu and Ikkα AA/AA/MMTV-c-neu mice were grown as mammospheres. (B) Primary mammospheres were trypsinized into single cells. (C) A small portion of dispersed WT but not Ikkα AA/AA/MMTV-c-neu cells reformed mammospheres 5 days later. (D) Primary mammospheres differentiate into epithelial cells when grown in serum-rich medium, and cells expressing the senescence-associated marker β-gal appear. More senescent cells appear if the cultures were derived from Ikkα AA/AA/MMTV-c-neu carcinomas. (Magnification: ×10, A, B, and D; ×20, C.)
The IkkαAA Mutation Inhibits Development of Chemically Induced Mammary Cancer.
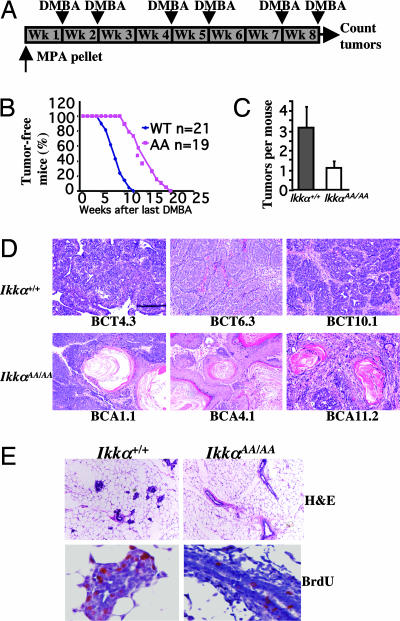
The chemical carcinogen DMBA induces mammary carcinomas in rodents, whose course resembles that of human breast cancer (14). To examine the role of IKKα in DMBA-induced mammary cancer, we introduced the mutant allele into the DMBA-sensitive BALB/c background (24, 25). Six-week-old WT and Ikkα AA/AA females were s.c.-implanted with a slow-release pellet of medroxyprogesterone acetate (MPA), which acts as a tumor promoter, followed by oral gavage with a DMBA-containing solution (Fig. 6A). Again, the Ikkα AA mutation delayed tumor onset and reduced tumor number (Fig. 6 B and C). In the Ikkα+/+ group, tumors appeared as early as 5 weeks after termination of DMBA administration and 50% of the mice had developed tumors by 7 weeks, whereas no tumors were detected before 10 weeks in the Ikkα AA/AA group, half of which displayed tumors 13.5 weeks after the last DMBA administration (Fig. 6B). Tumor numbers were also lower in the Ikkα AA/AA group (Fig. 6C). Thus IKKα signaling is also involved in chemically induced mammary carcinogenesis.
Fig. 6.
IKKα kinase activation is required for chemically induced mammary carcinogenesis. (A) Breast cancer induction scheme: virgin mice were implanted with an MPA pellet at 6 weeks of age and given DMBA by oral gavage as indicated. (B) Occurrence of mammary tumors in Ikkα+/+ and Ikkα AA/AA mice treated as in A. (C) Average tumor numbers in DMBA plus MPA-treated Ikkα+/+ and Ikkα AA/AA mice. (D) Representative histology of mammary tumors isolated from individual Ikkα+/+ and Ikkα AA/AA mice. Tumors were treated as described in Fig. 1C. (E) H&E staining and BrdU incorporation of mammary glands isolated 3 weeks after the last DMBA administration. (Magnification: ×20.)
The Ikkα AA Mutation Alters the Spectrum of DMBA-Induced Tumor Histotypes.
Unlike transgene-induced mammary tumors that are histologically homogenous, DMBA-induced tumors display a variety of different histotypes (14). The distribution of DMBA-induced tumor histotypes was altered by the Ikkα AA mutation. Whereas the majority of the DMBA-induced tumors in WT mice (18–20 of 30 examined) were typical adenocarcinomas at different stages of progression, 75% of the tumors in Ikkα AA/AA mice (9 of 12 examined) showed squamous metaplasia, with prominent keratin swirls (Fig. 6D). By contrast, keratin swirls characteristic of squamous cell carcinoma (SCC), were seen in only 13% of WT tumors. Because keratinization is characteristic of Wnt/β-catenin-induced tumors (26, 27), our results suggest an enrichment for tumors with activated β-catenin in Ikkα AA/AA mice, probably because the Ikkα AA mutation does not interfere with the oncogenic activity of the Wnt-β-catenin pathway (Fig. 1). Histology and cell proliferation were examined 3 weeks after the last DMBA administration, a time point at which no tumors were detected. Mammary glands of WT mice consistently exhibited a higher degree of preneoplastic hyperplasia compared with Ikkα AA/AA mice (Fig. 6E). Correspondingly, Ikkα AA/AA mammary glands exhibited a lower level of BrdU incorporation than WT mammary glands (Fig. 6E).
Discussion
The results described above indicate that, in addition to lobuloalveolar proliferation in response to pregnancy-related signals (4), IKKα kinase activation is also required for c-Neu/ErbB2- and DMBA-induced mammary carcinogenesis. Importantly, IKKα kinase activity is only required for optimal c-Neu/ErbB2-mediated oncogenic transformation, as primary malignant tumors do appear in Ikkα AA/AA/MMTV-c-neu mice, albeit at a slower rate. Interestingly, this dependence on IKKα is not seen when v-Ha-ras is the mammary-targeted oncogene and correlates with the requirement for IKKα activity for induction of cyclin D1 expression in c-Neu/ErbB2-transformed mammary carcinoma cells, but not in Ha-Ras-transformed cells. Notably, in transient transfection experiments we failed to observe significant activation of IKKα in response to Neu/ErbB2 overexpression (data not shown). By contrast, IKKα is readily activated in response to RANK engagement (8). Although we find higher NF-κB activity in MMTV-c-neu tumors relevant to normal mammary glands, it should be noted that the cell content is very different between the two, with many more epithelial cells in the tumor, which may contribute to some of the difference. We did not observe any difference in NF-κB activation in tumors derived from WT and Ikkα AA/AA mice expressing the MMTV-c-neu transgene. As IKKα is a signal-responsive protein kinase, we interpret our results to suggest that the self-renewal ability of c-Neu/ErbB2-transformed mammary TIC depends on extracellular signals that cannot be replaced by intrinsic c-Neu/ErbB2-generated signals, but are made dispensable upon overexpression of the “strong” Ha-Ras oncoprotein. Although Ras is activated downstream of c-Neu, the Ha-v-ras used in this study is constitutively active and therefore a more potent activator of the MAPK pathway than c-Neu. Western blot analysis of ERK phosphorylation supports this postulation (data not shown). Therefore, we believe that the difference between Ras and c-neu in respect to their dependence on IKKα could be a matter of signal strength. At this point, the origin of the signal that is transmitted by IKKα is not clear, but we suggest that it may be generated by RANK or a similar receptor whose engagement leads to IKKα-dependent cyclin D1 induction (8). Nevertheless, our results suggest that the self-renewal ability of TIC depends on a constant supply of signals that in the case of c-Neu/ErbB2 are not generated intrinsically. Given the requirement of IKKα for transmission of such signals and that many human breast cancers are Neu/ErbB2-dependent (10), we suggest that IKKα inhibition may prevent the reoccurrence of breast cancer in those patients that respond to a therapeutic regimen consisting of a Neu/ErbB2 inhibitor and cytotoxic drugs (28).
The dependence on IKKα kinase activity for efficient oncogenic transformation is also seen in mice treated with the clinical carcinogen DMBA. Curiously, DMBA exposure results in induction of two different types of tumors: one similar to ErbB2-induced adenocarcinoma and the other more similar to SCC. Only the adenocarcinoma seems to depend on IKKα activation such that its occurrence is reduced in Ikkα AA/AA tumors, resulting in an increased proportion of tumors with an SCC phenotype. Nonetheless, inactivation of IKKα significantly reduces the total burden of DMBA-induced mammary cancer. Thus, although inhibition of IKKα has no effect on SCC-like tumors in which Wnt-β-catenin is the dominant oncogenic pathway, it is capable of curtailing the formation of mammary adenocarcinomas in which ErbB2 is the dominant oncogene. At this point, however, we do not know how responsiveness to IKKα inhibition is modulated by the estrogen receptor status of the tumor. This and other questions need to be addressed along with development of IKKα-selective inhibitors that do not affect IKKβ activity before examining the utility of IKKα inhibition in human breast cancer patients.
Materials and Methods
Mice.
MMTV-c-neu, MMTV-v-Ha-ras, MMTV-c-myc, and MMTV-Wnt1 mice were purchased from Charles River Laboratories (Wilmington, MA). MMTV-PyMT mice were obtained from the National Cancer Institute, and each of the transgenic lines was crossed with Ikkα AA/AA mice generated in our laboratory. Female mice used in these experiments were on a mixed FVB/BL6/129 background and, except for the MMTV-c-myc mice, they were kept as virgins throughout the observation period. Tumor formation was monitored by palpation.
For DMBA carcinogenesis, Ikkα AA/AA mice were backcrossed for five generations with BALB/C mice. Six-week-old virgins were s.c.-embedded in the right flank with a slow-release pellet of 40 mg MPA, followed by oral gavage of 1 mg per mouse DMBA in cottonseed oil. DMBA was given six times with two consecutive weekly dosings followed by a 2-week drug-free interval. Appearance of mammary tumors was monitored as above after the last DMBA administration.
Rag1−/− mice were from The Jackson Laboratory (Bar Harbor, ME) and kept under sterile conditions. All mice were handled according to National Institutes of Health and University of California at San Diego guidelines.
Whole-Mount, Histology, and Immunofluorescence Analyses.
Whole-mount staining of mammary glands and histological and immunofluorescence analyses were as described (8). Anti-BrdU antibody was from BD Pharmingen (Franklin Lakes, NJ), and anti-ErbB2 antibody was from Upstate Biologicals (Charlottesville, VA). Signals were visualized by either cyanine 3 (PerkinElmer, Wellesley, MA) or chromogen diaminobenzidine reaction (BD Pharmingen). For BrdU labeling, mice were i.p.-injected with 100 μl/10 g body weight of a BrdU solution (Amersham, Piscataway, NJ) 2 h before death.
Protein and RNA Analyses.
Antibodies used for immunoblotting were anti-cyclin D1 (Upstate) and anti-IKKα (Imgenex, San Diego, CA). Tissue lysates were prepared as described (8). Total RNA was isolated from tumors or cultured cells with the RNeasy kit (Qiagen, Valenica, VA). Two-microgram samples were reverse-transcribed into cDNA with MMTV reverse transcriptase (Promega, Madison, WI). Cyclin D1 mRNA was quantified by real-time PCR using the SYBR GREEN Master Mix (Applied Biosystems, Foster City, CA) and ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Primers were: forward, 5′-CTGTGCGCCCTCCGTATCTTA and reverse, 5′-GGCGGCCAGGTTCCACTTGAG. The results were normalized to the level of cyclophilin mRNA.
Primary Tumor Cell Cultures and Transplantation.
Mammary carcinomas were cultured as described for mammary epithelial cells (29, 30), except that freshly dissected tumors averaging 1.5 cm in diameter were used as starting material. Briefly, tumors were mechanically minced and digested with 1 mg/ml collagenase (Sigma, St. Louis, MO) in RPMI medium 1640 supplemented with 10 mM Hepes and 2.5% BSA at 37°C for 4–5 h and cultured for ≈5 days before harvesting in F-12 medium supplemented with 10% FBS, 10 ng/ml EGF, 5 μg/ml insulin, and 1 μg/ml hydrocortisone. Epithelial cells were counted, and 5 × 105 cells suspended in 20 μl of PBS were injected into the no. 4 mammary glands of 2- to 5-month-old Rag1−/− virgin females. Tumor formation was examined by palpation twice a week. Tumor sizes were measured with a caliper, and the values represent averages for three different animals.
TIC were isolated essentially as described (22, 31). Secondary tumors isolated from transplanted Rag1−/− mice were used as starting materials and were mechanically and enzymatically disaggregated as described. Cells were cultured according to conditions as described (22).
Cell Proliferation and Senescence.
Proliferation of primary tumor cells was measured by BrdU labeling (Calbiochem, San Diego, CA). TIC grown as nonadherent mammospheres were collected by brief centrifugation and plated on Petri dishes in serum-rich medium. Cells were fixed after 2 days for senescence-associated β-gal staining with a Senescence Detection Kit (BioVision, Mountain View, CA).
Acknowledgments
This work was supported by grants from the National Institutes of Health and the California Breast Cancer Research Program. Y.C. was supported by the American Association for Cancer Research–Genentech BioOncology Career Development Award for Cancer Research on the HER family pathway and a grant from the California Breast Cancer Research Program. J.-l.L. was supported by the University of California at San Diego Pete Lopiccola Cancer Research Fellowship and a Life Science Fellowship. M.K. is an American Cancer Society research professor.
Abbreviations
- IKK
IκB kinase
- RANK
receptor activator of NF-κB
- MMTV
mouse mammary tumor virus
- DMBA
7,12-dimethylbenz[a]anthracene
- TIC
tumor-initiating cells
- MPA
medroxyprogesterone acetate
- SCC
squamous cell carcinoma.
Footnotes
The authors declare no conflict of interest.
References
- 1.Li Q, Verma IM. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Cao Y, Greten FR, Li ZW. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 3.Nakshatri H, Goulet RJ., Jr Curr Probl Cancer. 2002;26:282–309. doi: 10.1067/mcn.2002.129977. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Karin M. J Mammary Gland Biol Neoplasia. 2003;8:215–223. doi: 10.1023/a:1025905008934. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Karin M. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 7.Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, et al. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 9.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW, Horne CH. Oncogene. 1995;11:885–891. [PubMed] [Google Scholar]
- 11.Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- 12.Yu Q, Geng Y, Sicinski P. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 13.Cardiff RD. Microsc Res Tech. 2001;52:224–230. doi: 10.1002/1097-0029(20010115)52:2<224::AID-JEMT1007>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Escrich E. Int J Biol Markers. 1987;2:197–206. doi: 10.1177/172460088700200312. [DOI] [PubMed] [Google Scholar]
- 15.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinn E, Muller W, Pattengale P, Tepler I, Wallace R, Leder P. Cell. 1987;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 17.Stewart TA, Pattengale PK, Leder P. Cell. 1984;38:627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 19.Medina D. In: The Mouse in Biomedical Research. Foster HL, Small JD, Fox JG, editors. Vol IV. New York: Academic; 1982. pp. 373–396. [Google Scholar]
- 20.Al-Hajj M, Clarke MF. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 21.Ponti D, Zaffaroni N, Capelli C, Daidone MG. Eur J Cancer. 2006;42:1219–1224. doi: 10.1016/j.ejca.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 24.Pazos P, Lanari C, Meiss R, Charreau EH, Pasqualini CD. Breast Cancer Res Treat. 1992;20:133–138. doi: 10.1007/BF01834643. [DOI] [PubMed] [Google Scholar]
- 25.Pazos P, Lanari C, Elizalde P, Montecchia F, Charreau EH, Molinolo AA. Carcinogenesis. 1998;19:529–531. doi: 10.1093/carcin/19.3.529. [DOI] [PubMed] [Google Scholar]
- 26.Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Am J Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo MM, et al. Oncogene. 2002;21:5548–5556. doi: 10.1038/sj.onc.1205686. [DOI] [PubMed] [Google Scholar]
- 28.Emens LA. Am J Ther. 2005;12:243–253. [PubMed] [Google Scholar]
- 29.Pullan SE, Streuli CH. The Mammary Epithelial Cell. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 30.Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]