Abstract
Photosynthetic light reactions rely on the proper function of large protein complexes (including photosystems I and II) that reside in the thylakoid membrane. Although their composition, structure, and function are known, the repertoire of assembly and maintenance factors is still being determined. Here we show that an immunophilin of the cyclophilin type, CYP38, plays a critical role in the assembly and maintenance of photosystem II (PSII) supercomplexes (SCs) in Arabidopsis. Mutant plants with the CYP38 gene interrupted by T-DNA insertion showed stunted growth and were hypersensitive to high light. Leaf chlorophyll fluorescence analysis and thylakoid membrane composition indicated that cyp38 mutant plants had defects in PSII SCs. Sucrose supplementation enabled the rescue of the mutant phenotype under low-light conditions, but failed to mitigate hypersensitivity to high-light stress. Protein radiolabeling assays showed that, although individual thylakoid proteins were synthesized equally in mutant and wild type, the assembly of the PSII SC was impaired in the mutant. In addition, the D1 and D2 components of the mutant PSII had a short half-life under high-light stress. The results provide evidence that CYP38 is necessary for the assembly and stabilization of PSII.
Keywords: thylakoid lumen, immunophilin, photosynthesis, protein folding, chaperone
The light reactions and attendant evolution of oxygen in photosynthesis are carried out by four multisubunit protein complexes residing in the chloroplast thylakoid membranes: photosystems I (PSI) and II (PSII), cytochrome b6f complex, and CFO–CF1 complex (1–3). For a complete understanding of the photosynthetic process, it is essential to understand the biogenesis and maintenance of the participating complexes. Earlier studies on thylakoid protein supercomplex (SC) assembly, especially PSII, concentrated on the role of stromal factors, such as the translation and import machinery (4), because only a limited number of proteins were known to reside in the thylakoid lumen. However, recent proteomic findings suggest a population of 80–100 proteins in that compartment (5–7). The immunophilin family is one of the predominant groups identified.
Immunophilins were originally discovered in their capacity as cellular receptors for immunosuppressive drugs: cyclosporin A and FK506 (8, 9). The receptors for cyclosporin A and FK506, named cyclophilins (CYPs) and FK506-binding proteins (FKBPs), respectively, were collectively designated as immunophilins. A common feature of most immunophilins is the associated peptidyl-prolyl cis-trans isomerase activity that catalyzes the cis-trans conversion of X-Pro peptide bonds, a rate-limiting step in protein folding (8). These proteins are now known to occur widely in organisms ranging from bacteria and fungi to animals and plants. Studies in animal and plant systems have uncovered diverse functions of immunophilins, such as protein foldases, chaperones, and scaffolding facilitators. They also possibly have unknown catalytic capabilities (10, 11).
In Arabidopsis, genes encoding 52 putative immunophilins have been identified: 23 FKBPs and 29 CYPs (12). This group is by far the largest immunophilin family identified in any organism. A striking feature of Arabidopsis immunophilin family is that a large fraction is localized in the chloroplast, possibly explaining why the immunophilin family is more extensive in plants than animals. At least 16 immunophilins (11 FKBPs and 5 CYPs) were identified in the thylakoid lumen (5, 6, 12), with little known of their function. The presence of such a large number of immunophilins with protein-folding capability suggests that these enzymes play a central role in the assembly and maintenance of protein complexes, such as the two photosystems whose subunits reside in or at least contact the thylakoid lumen. This possibility has been strengthened by a recent study showing a requirement for one of the FKBPs, 20-2, for the maintenance of PSII (13). The present study shows that a member of the other immunophilin family in the thylakoid lumen, a CYP, is essential for assembling and stabilizing PSII. The results provide evidence for a function of a CYP in chloroplasts.
Results and Discussion
Identification of cyp38 Mutant Alleles.
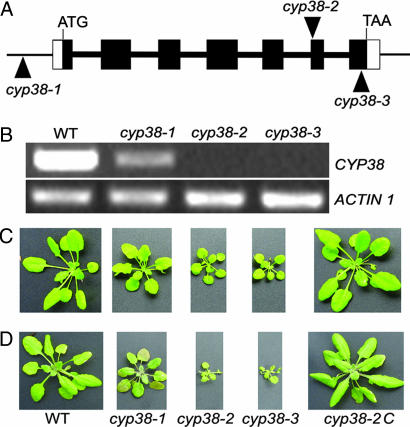
To identify their function, we isolated multiple Arabidopsis T-DNA insertion mutants for each immunophilin in the thylakoid lumen and screened them for potential defects in growth under greenhouse conditions (long-day cycle with light intensity ≈150 μmol·m−2·s−1). Three T-DNA insertion alleles for the gene encoding a 38-kDa CYP, CYP38 (At3g01480), were obtained. One allele, referred to as cyp38-1, contained a T-DNA insertion in the promoter region of the gene. The second allele, cyp38-2, had a T-DNA insertion in the sixth exon of the coding region (+2,236 bp), and a T-DNA insertion was found in the seventh exon (+2,506 bp) in the third allele (cyp38-3) (Fig. 1A).
Fig. 1.
Genetic characterization and phenotype of cyp38 mutant plants. (A) Localization of T-DNA insertion sites in CYP38 genomic DNA of three cyp38 alleles: cyp38-1, cyp38-2, and cyp38-3. The CYP38 genomic DNA includes seven exons (black boxes) and six introns (bold lines between black boxes). ATG, initiation codon; TAA, stop codon. (B) CYP38 mRNA levels in wild type (WT), cyp38-1, cyp38-2, and cyp38-3 as shown by RT-PCR. (C) Phenotype of wild type, cyp38-1, cyp38-2, cyp38-3, and cyp38-2 transformed with CYP38 genomic DNA (cyp38-2C) grown for 7 weeks under low light (25–35 μmol·m−2·s−1) with a long-day cycle (16–8 h). (D) The plants shown in C were exposed to illumination (300 μmol·m−2·s−1) for 2 days after 7 weeks of growth in low light (25–35 μmol·m−2·s−1).
RT-PCR analysis showed that the promoter insertion line (cyp38-1) had a significantly reduced level of the CYP38 transcript (Fig. 1B). The mRNA of CYP38 was not detectable by using RT-PCR in either cyp38-2 or cyp38-3, indicating that these two alleles are null mutants. All three mutant alleles showed reduced growth and pale green leaves compared with wild type when grown under low-light conditions (25–35 μmol·m−2·s−1), and the phenotype of the cyp38-2 and cyp38-3 null mutants was more severe than that of the cyp38-1, which was concluded to be a weak mutant (Fig. 1C). The chlorophyll content in the null mutants is ≈60% of the wild type based on fresh weight.
To demonstrate that the phenotype of cyp38-2 was attributable to the mutation of the CYP38 gene, we conducted complementation experiments with the cyp38-2 mutant as background by using a 6-kb genomic fragment containing a 2.5-kb promoter region, the 2.6-kb full-length gene sequence, and the 900-bp downstream sequence. The transgenic plants (cyp38-2C) displayed wild-type growth, thus confirming that growth defects in cyp38-2 resulted from mutation of the CYP38 gene (Fig. 1C). We also carried out a complementation test with a CYP38 cDNA and found that it rescued the mutant as well (data not shown).
During the isolation of homozygotes from the cyp38-2 and cyp38-3 mutants, we noticed that the mutants were sensitive to high-light stress and died at an early stage unless cultivated under low light. To investigate the light sensitivity of these mutants, wild-type and mutant plants were transferred to high light after being grown initially for 7 weeks under low light. Two days after exposure to 300 μmol·m−2·s−1 light, the mature leaves of the null mutants (cyp38-2 and cyp38-3) started to wilt, whereas the weak mutant (cyp38-1) showed a typical high-light stress phenotype, shrinking and becoming purplish. Wild-type and complemented plants also showed a slightly purplish phenotype because of the accumulation of anthocyanins (14), but they grew well nonetheless (Fig. 1D).
Previous proteomic data suggested that CYP38 is included in the chloroplast luminal proteome (5, 6). Its orthologue in spinach, TLP40, also was found to be localized in the chloroplast lumen and, further, was proposed to function in the phosporylation of thylakoid proteins (15–17). The CYP38 also shares homology with PAP-I, a protein purified from the pea and was hypothesized to be an essential factor for RNA poly(A) polymerase (18). Amino acid sequence analysis identified several related proteins from cyanobacteria and algae, but not from fungi or animals, suggesting conservation among photosynthetic organisms. The protein features a C-terminal CYP domain and a leucine zipper at the N-terminal half (12, 15).
Because the two null alleles showed a similar phenotype, which was more severe than that of the weak allele, we chose one of them, cyp38-2, for subsequent experiments to dissect the defects in the cyp38 mutants. Results from experiments characterizing the mutant (phenotype, 2D gels, and immunoblots) were consistently similar with cyp38-3 (data not shown).
Chlorophyll Fluorescence Analysis Reveals Defects in PSII Function in the cyp38 Mutants.
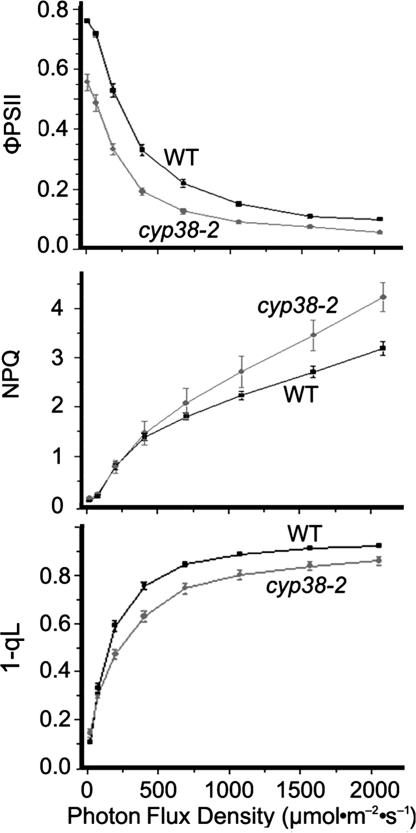
The mutants' stunted growth and sensitivity to high light suggested a possible defect in photosynthesis. To pursue this possibility, we measured chlorophyll fluorescence to assess photosynthetic electron transport in the leaves of whole plants. The Fv/Fm ratio, which represents the maximum efficiency of PSII photochemistry, was remarkably reduced in the mutant (0.65 in the mutant vs. 0.82 in the wild type), suggesting that the PSII is photodamaged or misassembled even under low-light growth conditions. ΦPSII, the quantum efficiency of PSII photochemistry at different photon flux densities (19), also was decreased in the mutant (Fig. 2Top), indicating a lower efficiency of photosynthesis and thus suppressed growth. Nonphotochemical quenching (NPQ), a reflection of the plant's ability to dissipate excess light energy as heat (20), was found to be similar in mutant and wild-type plants under low-light intensities (≤200 μmol·m−2·s−1) (Fig. 2 Middle). However, at light intensities of >400 μmol·m−2·s−1, the NPQ of the mutant was significantly higher than that of the wild type. Further analysis revealed that the additional NPQ was not rapidly reversible, indicating that the increased NPQ is due to a larger component of photoinhibitory quenching in the mutant. The energy-dependent quenching was similar in the mutant and wild type (data not shown). The parameter, 1-qL, which is considered to be a better parameter than 1-qP to reflect the redox state of the QA electron acceptor of PSII (21), was lower in the mutant (Fig. 2 Bottom). This result indicated a more oxidized plastoquinone pool in the mutant, a response likely due to a deficiency of PSII rather than downstream defects. Taken together, the chlorophyll fluorescence analyses suggested that the cyp38 mutation affected PSII function.
Fig. 2.
Chlorophyll fluorescence analyses of wild-type (WT) and cyp38-2 plants. Plants were grown under 25–35 μmol·m−2·s−1 for 7 weeks. Data represent means ± SE (n = 6). ΦPSII, efficiency of PSII photochemistry; 1-qL, parameter estimating the fraction of PSII in close states or excitation pressure of PSII based on a lake mode (21).
The PSII SC Is Compromised in cyp38 Mutants.
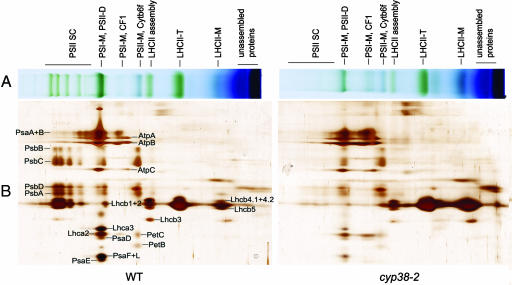
The defects in photosynthesis displayed by the cyp38 mutants were possibly caused by a reduced level or malfunction in protein complexes in the electron transport chain. To examine this possibility, we analyzed mutant and wild-type thylakoid membranes by using 2D gel electrophoresis involving blue native (BN)/PAGE followed by SDS/PAGE.
BN gels developed with mutant and wild-type preparations containing equal amounts of chlorophyll revealed significant differences in the accumulation of PSII SCs, which were obvious in the wild type but almost absent in the mutant (Fig. 3A), thus confirming defects in the PSII of mutant plants. By contrast, the LHCII monomer band was more abundant in the mutant, which is not surprising because equal chlorophyll amounts were loaded onto the gel. Furthermore, the band corresponding to the monomeric PSI and CFO–CF1 complex was more abundant in the mutant.
Fig. 3.
BN/PAGE and subsequent analysis of thylakoid membrane complexes in 2D SDS/PAGE. (A) Thylakoid membranes (5 μg of chlorophyll) were solubilized with 1% dodecyl-β-maltoside and separated by 5–13.5%. Native gel slices were horizontally laid on the top of 2D SDS/PAGE. PSI-M, PSI monomer; PSII-D, PSII dimer; LHCII, PSII light-harvesting complex; LHCII-T, PSII light-harvesting complex trimer; LHCII monomer, PSII light-harvesting complex monomer. (B) Thylakoid membrane complexes separated by BN/PAGE in A were further subjected to 12.5% SDS/PAGE and stained with silver. Protein identification was based on ref. 40.
The SDS/PAGE gel following the BN gel revealed details in individual protein components of the SC of the mutant and wild type that were not apparent in the BN gel. For instance, the mutant contained only trace amounts of PSII core proteins, whereas these components were abundant in the wild type (PSII SC in Fig. 3). In the right center area of the gel, mutant plants had more of the components corresponding to LHCII trimer and unassembled proteins than the wild type. Thus, LHCII proteins were distributed much less in the left, PSII SC (assembled) area and more in the low molecular weight (unassembled) area, especially in the LHCII monomer form.
To analyze protein differences between the mutant and wild type further, we applied immunoblotting to assess key components of each of the four major complexes of the chloroplast thylakoid membrane quantitatively: PSII, PSI, cytochrome b6f, and CFO–CF1. We prepared total protein and thylakoid membrane fractions from both mutant and wild type and probed them with antibodies against subunits of each complex: D2 and D1 for PSII, CF1α for CFO–CF1, PsaD and PsaF for PSI, and Cytf and Rieske (PetC) for Cytb6f complex.
The results summarized in Fig. 4 showed that the D2 and D1 proteins of PSII were reduced in both the total protein (T) and membrane (M) fractions of the mutant. However, although the level in the total protein fraction appeared not to change, the PSI subunits of the mutant, PsaD and PsaF, were significantly reduced in thylakoid membranes. The level of CF1α in the membrane fraction of the mutant also was decreased, although the level of total protein appeared not to change. The Cytb6f complex displayed similar patterns at the level of Cytf and Rieske subunits in the mutant and wild type. This result, which generally agrees with the 2D BN/SDS analysis, suggests that the PSII, PSI, and CF1 complexes in the mutant were compromised to various degrees, whereas the Cytb6 f complex was unaffected. Consistent with the in vivo chlorophyll fluorescence analyses, the results suggest that the cyp38 mutants had primary defects in PSII, whereas the altered levels of PSI and CF1 subunits were likely due to secondary effects of PSII deficiencies as described in other PSII mutants (22, 23).
Fig. 4.
Immunodetection of thylakoid membrane proteins. Protein samples (1 μg of chlorophyll) were resolved by 12.5% SDS/PAGE, transferred to a nitrocellulose membrane, and probed with antibodies against the indicated thylakoid membrane proteins. WTT, total protein sample from wild-type plants; 38-2T, total protein sample from cyp38-2; WTM, thylakoid membrane sample from wild-type plants; 38-2M, thylakoid membrane sample from cyp38-2.
cyp38 Mutants Display Defects in the Assembly of PSII SCs.
The reduced level of PSII SCs in the mutants could be attributed to (i) reduced synthesis or assembly or both, or (ii) increased degradation. We followed several approaches to distinguish these possibilities.
Clearly, cyp38 mutants showed both severe defects in components of their thylakoid membranes and lower photosynthetic activity. Thus, the stunted phenotype of the mutant as well as its hypersensitivity to high light could be due to secondary effects of the mutation resulting from insufficient energy needed for normal nourishment. To distinguish between primary defects due to lack of CYP38 and secondary defects caused by a deficiency in the products of photosynthesis, we supplied wild-type and mutant plants with 2% sucrose as a source of carbon to support normal growth and development.
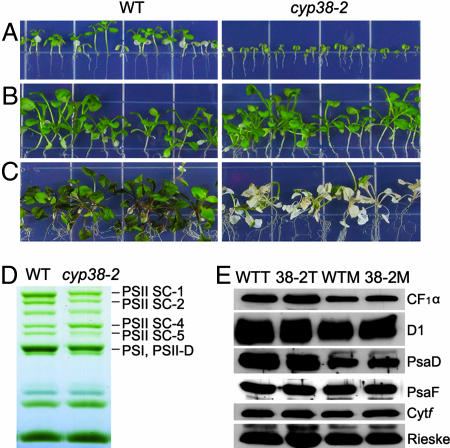
Fig. 5A shows that, when grown on half-strength Murashige-Skoog (MS) medium for 4 weeks under low light without sucrose, cyp38-2 plants grew much less than their wild-type counterparts (Fig. 5A). By contrast, in the presence of 2% sucrose, both plant types grew normally (Fig. 5B). However, when light intensity was increased, the mutant plants shown in Fig. 5B became bleached (Fig. 5C). Thus, while rescuing the stunted growth phenotype of the mutant, the addition of sucrose failed to mitigate its sensitivity to high-light stress, suggesting that this defect is caused primarily by a lack of the CYP38 protein.
Fig. 5.
Effect of sucrose treatment on wild-type and cyp38-2 plants. (A) Wild-type and cyp38-2 plants were grown vertically on half-strength MS medium without sucrose for 4 weeks under low-light conditions (25–35 μmol·m−2·s−1). (B) Wild-type and cyp38-2 plants were grown vertically on half-strength MS medium supplemented with 2% sucrose under low-light conditions (25–35 μmol·m−2·s−1) for 4 weeks. (C) Plants grown as described in B were exposed to high light (300 μmol·m−2·s−1) for 5 days. (D) BN/PAGE of thylakoid membrane complexes from wild-type (WT) and cyp38-2 plants after 4 weeks of growth on 2% sucrose half-width MS medium. PSI-M, PSI monomer; PSII-D, PSII dimer. (E) Immunoanalysis of thylakoid proteins (1 μg of chlorophyll) from wild-type total protein (WTT), cyp38-2 total protein (38-2T), wild-type membrane protein (WTM), and cyp38-2 membrane protein (38-2M). Plants were grown on 2% sucrose medium for 4 weeks.
To identify the PSII defects, we conducted BN/PAGE analysis of the thylakoid membranes from the cyp38 and the wild-type plants grown with 2% sucrose under low light. The gels revealed that differences still existed (Fig. 5D) but to a much lesser degree compared with soil-grown plants (Fig. 3A); that is, the mutant showed a reduced level of PSII-LHCII SCs (PSII SC bands 1, 2, and 5) and of the band containing PSI monomer + PSII dimer (PSI, PSII-D). Interestingly, immunoblot analysis revealed no significant differences between wild-type and cyp38-2 mutant plants in the content of components of the PSII, PSI, CFO-CF1, and Cytb6 f complexes: D1, PsaD + PsaF, CF1α, and Cytf + Rieske (Fig. 5E). This similarity suggested that the reduced level of certain individual subunits observed in the soil-grown plants was caused by secondary effects resulting from reduced nutrition status. The analyses indicated that, provided a source of carbon, the cyp38 mutant resembled the wild type in being able to synthesize individual protein components of the photosynthetic membrane complexes. However, unlike the wild type, the mutant did not assemble the PSII SCs in the same pattern as the wild type, thereby leading to decreased accumulation of the complexes.
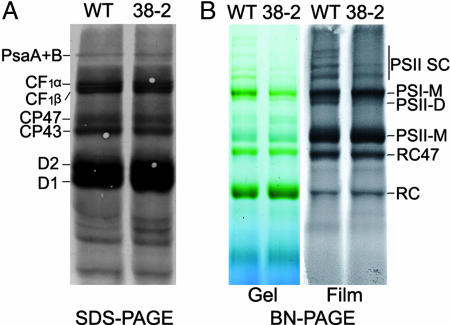
To distinguish between synthesis and assembly, we monitored the biosynthesis and assembly of subunit proteins in photosynthetic complexes directly by radiolabeling. Detached primary leaves from sucrose-grown plants were fed [35S]Met, and cycloheximide was applied to inhibit the synthesis of nucleus-encoded proteins so that only chloroplast-encoded proteins were labeled (22). When the thylakoid membranes were resolved by SDS/PAGE, the protein patterns of the mutant and wild-type plants were similar with respect to content and density of the individual bands (Psa A + B, CF1α, CF1β, CP47, CP43, D2, and D1) (Fig. 6A). However, when the membrane preparations were analyzed by BN/PAGE, much lower levels of newly formed PSII SCs were observed in the cyp38 mutant relative to wild type, especially in the gel exposed to film (Fig. 6B). In addition, a band corresponding to PSII dimer (PSII-D) in the wild type was essentially missing in the mutant. Based on the protein radiolabeling results, we concluded that the ability of the mutant to synthesize thylakoid membrane proteins was similar to the wild type. However, the cyp38 mutant plants were impaired in the assembly of the PSII SC.
Fig. 6.
In vivo synthesis of plastid-encoded membrane proteins. (A) Primary leaves from wild-type (WT) and cyp38-2 (38-2) sucrose-grown plants were fed [35S]Met in the presence of cycloheximide. After a 20-min incubation, thylakoid membrane proteins (5 μg of chlorophyll) were isolated and separated by 12.5% SDS/PAGE. Proteins were then transferred to a nitrocellulose membrane and visualized autoradiographically. (B) After 20 min of labeling leaves with [35S]Met (as in A), thylakoid membrane complexes (5 μg of chlorophyll) were separated by BN/PAGE. (Left) BN/PAGE pattern. (Right) Autoradiography image of the BN/PAGE. PSI-M, PSI monomer; PSII-D, PSII dimer; RC47, PSII reaction center complex without CP43 subunit (41); RC, PSII reaction center complex without CP47 and CP43 subunits (41).
cyp38 Mutants Also Display Defects in the Stability of PSII SCs.
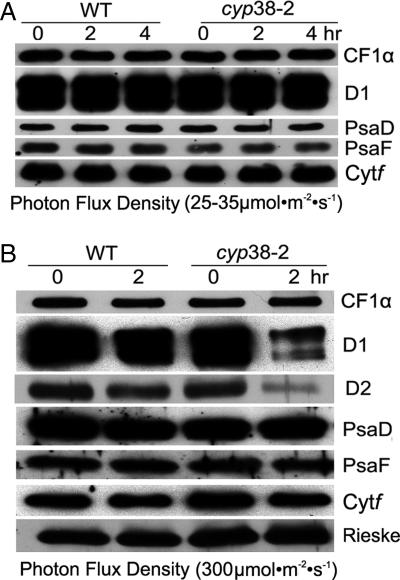
The in vivo radiolabeling experiments demonstrated defects in the PSII assembly in the mutant, but did not exclude the possibility that the mutant PSII SCs had a shorter half-life compared with wild type. To test whether the CYP38 protein is important for PSII maintenance, we monitored the levels of several thylakoid membrane proteins by immunoblotting. We used leaves from sucrose-grown plants, in which protein biosynthesis was inhibited with lincomycin (chloroplast-encoded proteins) and cycloheximide (nuclear-encoded proteins). Under these conditions, a reduced level of a particular protein reflects its degradation. Under low light, the level of proteins examined (CF1α, D1, PsaD, PsaF, and Cytf) did not significantly decrease in either the mutant or wild type (Fig. 7A). By contrast, under high light (300 μmol·m−2·s −1), the levels of the D1 and D2 components of PSII were dramatically reduced in the mutant, whereas the levels of members of the other complexes were similar (monitored by CF1α, PsaD, PsaF, Cytf, and Rieske) (Fig. 7B). The results suggest that D1 and D2 were degraded to a greater extent in high light in the absence of CYP38 possibly because of a defect in the assembly of the PSII SC. Therefore, they emerge as targets highly susceptible to photodamage as in earlier studies (24, 25).
Fig. 7.
Half-life of thylakoid membrane proteins in wild-type and cyp38 plants. (A) Arabidopsis leaves from sucrose-grown wild-type (WT) and cyp38-2 plants were incubated with lincomycin and cycloheximide for 30 min and illuminated under 25–35 μmol·m−2·s−1 for 0, 2, and 4 h. Thylakoid membrane proteins (1 μg of chlorophyll) were resolved by SDS/PAGE, and the gel was immunoblotted to assess protein half-life. (B) Conditions as in A, except that leaves were illuminated under 300 μmol·m−2·s−1 for 0 and 2 h.
Concluding Remarks.
Because the structures of the photosynthetic membrane complexes of chloroplasts have been described in x-ray crystallography analyses (26, 27), it becomes increasingly important to understand the mechanisms underlying their assembly and maintenance. To this end, it is essential to identify and understand the proteins that function in the assembly and maintenance of the supermolecular complexes that catalyze this energy conversion in nature. Earlier work has shown that proteins functional in the translation and import of subunit proteins are required for the biogenesis of photosynthetic complexes (4, 28). However, the proteins that function specifically in the assembly of the complexes have long been a mystery and are only now beginning to be identified (29–31).
Recent studies on immunophilins of the thylakoid lumen have begun to uncover information central to the problem. Thus, potential functions of luminal immunophilins have been linked to the biogenesis of photosynthetic complexes. One, FKBP13, interacts with the Rieske protein in the accumulation of the cytochrome b6f complex (32). Another, FKBP20-2, is required for the accumulation of PSII SCs (13). Finally, as seen here, the other type of immunophilin, CYP38, is linked to the assembly and maintenance of PSII. Owing to their extensive presence in the thylakoid lumen, it seems likely that other immunophilins will be found to participate in the biogenesis and maintenance of photosynthetic complexes.
Materials and Methods
Plant Materials and Growth Conditions.
Wild-type (ecotype Columbia-0) and cyp38 T-DNA insertion Arabidopsis thaliana mutant (ecotype Columbia-0) seeds were obtained from Syngenta (Research Triangle Park, NC). For soil-grown plants, sown seeds were cold-treated for 2 days and then grown under long-day conditions (16-h illumination of 25–35 μmol·m−2·s−1 and 8-h dark cycle). For plate-grown plants, seeds were surface-sterilized with 70% ethanol for 5 min and 50% bleach for 10 min and then washed four times with sterilized distilled water. The washed seeds were plated on 0.8% agar plates containing half-strength MS medium with or without 2% sucrose under the same growth conditions as soil-grown plants.
Identification of T-DNA Insertional Mutants and Complementation with Transgenic Plants.
Three mutant lines were obtained from Syngenta: Garlic 204 32, Garlic 206 38, and Garlic 321 44. Homozygous lines were isolated by confirming the T-DNA insert by PCR. DNA isolation and T-DNA border information were obtained according to ref. 33. RT-PCR was used to verify expression levels in homozygous plants. RNA isolation, cDNA synthesis, and RT-PCR were performed according to standard procedures (34). For complementation by genomic DNA, a 6-kb genomic DNA fragment containing the AtCYP38 gene and the 2.5-kb upstream and 900-bp downstream sequences were amplified by PCR and cloned behind the CaMV 35S promoter in the binary vector pCAMBIA1300. For complementation by cDNA, the coding region of the gene was amplified by RT-PCR from total RNA and cloned into pCAMBIA1301 without the GUS gene. These constructs were further transferred into cyp38-2 plants by using the floral dip method (35).
Chlorophyll Fluorescence Measurements.
Chlorophyll fluorescence parameters were measured with an FMS2 fluorometer (Hansatech, Norfolk, UK) with intact leaves of wild-type and mutant plants grown for 7 weeks in soil under low-light conditions (25–35 μmol·m−2·s−1) (13). Fv/Fm was defined as (Fm − Fo)/Fm, and ΦPSII was defined as (F′m − Fs)/F′m. NPQ was calculated as (Fm − F′m)/F′m; 1-qL is equal to (F′o/Fs)(F′m − Fs)/(F′m − F′o). Fm is the maximum fluorescence in the dark-adapted state; F′m is the maximum fluorescence in any light-adapted state; Fs is the steady-state fluorescence in the light; Fo is the minimal fluorescence in the dark-adapted state; and F′o is the minimal fluorescence in any light-adapted state.
Preparation of Total Protein Sample and Thylakoid Membranes.
Arabidopsis leaf tissues were homogenized at 4°C with a mortal and pestle in 0.33 M sorbitol/10 mM EDTA/50 mM Hepes (pH 8.0). The homogenate was filtered through two layers of Miracloth (Calbiochem, La Jolla, CA), and a small portion of the filtrate was taken as a total protein sample. The remaining portion of the filtrate was used to prepare thylakoid membranes (36).
Immunoblotting Analysis.
Western blotting was performed as before (37). In brief, protein samples corresponding to equal amounts of chlorophyll were electrophoresed through 12.5% SDS/PAGE, and protein was transferred to a nitrocellulose filter. The filter was incubated with antibodies generated against the indicated thylakoid membrane proteins and visualized by using the ECL immunodetection procedure (Amersham Biosciences, Pittsburgh, PA).
Half-Life of Thylakoid Membrane Proteins.
Expanding leaves from 4- to 5-week-old Arabidopsis plants (wild type and cyp38-2) grown on the MS plates with 2% sucrose were vacuum-infiltrated with 100 μg/ml lincomycin and 20 μg/ml cycloheximide as indicated to block chloroplast- and nucleus-encoded protein synthesis, respectively (38). After 30-min incubation, the leaves were illuminated under growth conditions for 0, 2, and 4 h or under 300 μmol·m−2·s−1 for 2 h. After treatment, thylakoid membranes were isolated, and the content of the PSII proteins was determined by immunoblot analysis.
BN/PAGE and 2D SDS/PAGE.
BN/PAGE was performed according to refs. 13 and 39. For 2D analysis, excised BN/PAGE lanes were soaked for 30 min in 2× SDS sample buffer supplied with 5% β-mercaptoethanol. Each lane with denatured proteins was placed on top of 1.5-mm-thick 12% SDS/PAGE and electrophoresized at room temperature with a constant current of 20 mA. After electrophoresis, gels were stained with silver (40).
In Vivo Radiolabeling of Chloroplast Proteins.
Primary leaves from plants supplied with sucrose for 4 weeks were used for in vivo protein radiolabeling according to refs. 22 and 38, with minor modifications. Isolated thylakoid membranes were subjected to either SDS/PAGE or BN/PAGE with equal chlorophyll loading. The SDS/PAGE was transferred to a nitrocellulose membrane and visualized autoradiographically. BN/PAGE were dried directly with a gel drier (BioRad, Hercules, CA) and exposed to x-ray film.
Acknowledgments
We thank Drs. A. Melis, S. Park, and R. Malkin (University of California, Berkeley) for providing antibodies and K. Niyogi (University of California, Berkeley) for obtaining and interpreting the chlorophyll fluorescence data. This work was supported by U.S. Department of Agriculture grants (to B.B.B. and S.L.) and U.S. Department of Energy grants (to S.L.).
Abbreviations
- PSI
photosystem I
- PSII
photosystem II
- SC
supercomplex
- CYP
cyclophilin
- FKBP
FK506-binding protein
- NPQ
nonphotochemical quenching
- BN
blue native.
Footnotes
The authors declare no conflict of interest.
References
- 1.Nelson N, Yocum CF. Annu Rev Plant Biol. 2006;57:521–565. doi: 10.1146/annurev.arplant.57.032905.105350. [DOI] [PubMed] [Google Scholar]
- 2.Barber J. Biochem Soc Trans. 2006;34:619–631. doi: 10.1042/BST0340619. [DOI] [PubMed] [Google Scholar]
- 3.Shi L, Schroder WP. Biochim Biophys Acta. 2004;1608:75–96. doi: 10.1016/j.bbabio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Wollman FA, Minai L, Nechushtai R. Biochim Biophys Acta. 1999;1411:21–85. doi: 10.1016/s0005-2728(99)00043-2. [DOI] [PubMed] [Google Scholar]
- 5.Peltier JB, Emanuelsson O, Kalume DE, Ytterberg J, Friso G, Rudella A, Liberles DA, Soderberg L, Roepstroff P, von Heijne G, von Wijk KJ. Plant Cell. 2002;14:211–236. doi: 10.1105/tpc.010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schubert M, Petersson UA, Haas BJ, Funk C, Schroder WP, Kieselbach T. J Biol Chem. 2002;277:8354–8365. doi: 10.1074/jbc.M108575200. [DOI] [PubMed] [Google Scholar]
- 7.Spetea C, Hundal T, Lundin B, Heddad M, Adamska I, Andersson B. Proc Natl Acad Sci USA. 2004;101:1409–1414. doi: 10.1073/pnas.0308164100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber SL. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 9.Luan S. Bot Bull Acad Sin. 1998;39:217–223. [Google Scholar]
- 10.Buchanan BB, Luan S. J Exp Bot. 2005;56:1439–1447. doi: 10.1093/jxb/eri158. [DOI] [PubMed] [Google Scholar]
- 11.Romano P, Gray J, Horton P, Luan S. New Phytol. 2005;166:753–769. doi: 10.1111/j.1469-8137.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 12.He Z, Li L, Luan S. Plant Physiol. 2004;134:1248–1267. doi: 10.1104/pp.103.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima A, Lima S, Wong JH, Phillips RS, Buchanan BB, Luan S. Proc Natl Acad Sci USA. 2006;103:12631–12636. doi: 10.1073/pnas.0605452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkel-Shirley B. Curr Opin Plant Biol. 2002;5:218–223. doi: 10.1016/s1369-5266(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 15.Fulgosi H, Vener AV, Altschmied L, Herrmann RG, Andersson B. EMBO J. 1998;17:1577–1587. doi: 10.1093/emboj/17.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vener AV, Rokka A, Fulgosi H, Andersson B, Herrmann RG. Biochemistry. 1999;38:14955–14965. doi: 10.1021/bi990971v. [DOI] [PubMed] [Google Scholar]
- 17.Rokka A, Aro EM, Herrmann RG, Andersson B, Vener AV. Plant Physiol. 2000;123:1525–1536. doi: 10.1104/pp.123.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta JD, Li Q, Thomson AB, Hunt AG. Plant Mol Biol. 1998;37:729–734. doi: 10.1023/a:1006000213403. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell K, Johnson GN. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 20.Muller P, Li XP, Niyogi KK. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer DM, Johnson G, Kiirats O, Edwards GE. Photosynth Res. 2004;79:209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- 22.Meurer J, Plucken H, Kowallik K, Westhoff P. EMBO J. 1998;17:5286–5297. doi: 10.1093/emboj/17.18.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plucken H, Muller B, Grohmann D, Westhoff P, Eichaker LA. FEBS Lett. 2002;532:85–90. doi: 10.1016/s0014-5793(02)03634-7. [DOI] [PubMed] [Google Scholar]
- 24.Baena-Gonzalez E, Aro E. Philos Trans R Soc London Ser B. 2002;357:1451–1460. doi: 10.1098/rstb.2002.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melis A. Trends Plants Sci. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- 26.Nelson N, Ben-Sham A. Nat Rev Mol Cell Biol. 2004;5:971–982. doi: 10.1038/nrm1525. [DOI] [PubMed] [Google Scholar]
- 27.Merchant S, Sawaya MR. Plant Cell. 2005;17:648–663. doi: 10.1105/tpc.105.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis P, Robinson C. Curr Biol. 2004;14:1064–1067. doi: 10.1016/j.cub.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 29.Nakamoto SS, Hamel PP, Merchant S. Biochimie. 2000;82:603–614. doi: 10.1016/s0300-9084(00)00605-2. [DOI] [PubMed] [Google Scholar]
- 30.Rochaix JD. FEBS Lett. 2002;529:34–38. doi: 10.1016/s0014-5793(02)03181-2. [DOI] [PubMed] [Google Scholar]
- 31.Melkozernov AN, Barber J, Blankenship RE. Biochemistry. 2006;45:331–345. doi: 10.1021/bi051932o. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R, Mould R, He Z, Luan S. Proc Natl Acad Sci USA. 2002;99:15806–15811. doi: 10.1073/pnas.222550399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. Plant Cell. 2002;14:2985–2994. doi: 10.1105/tpc.004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. [Google Scholar]
- 35.Clough SJ, Bent AF. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Choi YD, Voytas D, Rodermel S. Plant J. 2000;2:303–313. doi: 10.1046/j.1365-313x.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 37.Fu A, Park S, Rodermel S. J Biol Chem. 2005;280:42489–42496. doi: 10.1074/jbc.M508940200. [DOI] [PubMed] [Google Scholar]
- 38.Peng L, Ma J, Chi W, Guo J, Zhu S, Lu Q, Lu C, Zhang L. Plant Cell. 2006;18:955–969. doi: 10.1105/tpc.105.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asakura Y, Hirohashi T, Kikuchi S, Belcher S, Osborne E, Yano S, Terashima I, Barkan A, Nakai M. Plant Cell. 2003;16:201–214. doi: 10.1105/tpc.014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwenkert S, Umate P, Dal Bosco C, Volz S, Mlcochova L, Zoryan M, Eichacker LA, Ohad I, Herrmann RG, Meurer J. J Biol Chem. 2006;281:34227–34238. doi: 10.1074/jbc.M604888200. [DOI] [PubMed] [Google Scholar]
- 41.Rokka A, Suorsa M, Saleem A, Battchikova N, Aro EM. Biochem J. 2005;388:159–168. doi: 10.1042/BJ20042098. [DOI] [PMC free article] [PubMed] [Google Scholar]