Abstract
Malaria transmission entails development of the Plasmodium parasite in its insect vector, the Anopheles mosquito. Parasite invasion of the mosquito midgut is the critical first step and involves adhesion to host epithelial cell ligands. Partial evidence suggests that midgut oligosaccharides are important ligands for parasite adhesion; however, the identity of these glycans remains unknown. We have identified a population of chondroitin glycosaminoglycans along the apical midgut microvilli of Anopheles gambiae and further demonstrated ookinete recognition of these glycans in vitro. By repressing the expression of the peptide-O-xylosyltransferase homolog of An. gambiae by means of RNA interference, we blocked glycosaminoglycan chain biosynthesis, diminished chondroitin sulfate levels in the adult midgut, and substantially inhibited parasite development. We provide evidence for the in vivo role of chondroitin sulfate proteoglycans in Plasmodium falciparum invasion of the midgut and insight into the molecular mechanisms mediating parasite–mosquito interactions.
Keywords: Anopheles, glycosaminoglycans, glycosytransferase, malaria, RNAi
Malaria is caused by Plasmodium parasites, among which, Plasmodium falciparum inflicts the severest infection on human populations. The complex parasite life cycle includes developmental stages within both mammals and its obligatory insect vector, the Anopheles mosquito (1). Plasmodium gametocytes that are taken up in an infected blood meal transform into ookinetes in the mosquito midgut lumen. Ookinetes then migrate to the gut periphery where they are thought to recognize and adhere to midgut ligands. These steps directly precede cell invasion, traversal, and the differentiation of ookinetes into oocysts beneath the basal lamina. Each oocyst produces thousands of sporozoites that subsequently invade the mosquito salivary glands and are delivered to a vertebrate host during a succeeding blood meal. Clearly, ookinete invasion of midgut epithelia is the critical step for parasite establishment in the mosquito and, therefore, represents the best paradigm to develop novel transmission-blocking strategies (i.e., approaches that prevent parasite passage through the mosquito and therefore impede the subsequent cascade of secondary infections in humans).
Plasmodium ookinete molecules belonging to the Thrombospondin-related adhesive protein (TRAP) family, which includes the sporozoite surface protein, TRAP, and two ookinete proteins, the circumsporozoite and TRAP-related protein (CTRP) and the von Willebrand Factor A domain protein (WARP), have been demonstrated to bind to the glycosaminoglycan (GAG), heparin, in vitro (2). The major circumsporozoite protein (CSP) and TRAP both recognize heparan sulfate (HS) proteoglycans on the liver sinusoid, a critical step toward the establishment of parasite infection in humans (3, 4). Similarly, ookinete CTRP and WARP gene knockouts were shown to be incapable of invading the mosquito midgut, strongly implying their essential role in mosquito midgut cell invasion (5, 6). The common theme of GAG-ligand targeting by different Plasmodium stages suggest that there is conservation in the overall cell invasion strategy that occurs during parasite transit from the invertebrate to the vertebrate host and vice versa (7). However, there is no clear evidence for the existence of heparin or GAGs in the mosquito gut.
Partial evidence suggests that negatively charged oligosaccharides are present along the apical microvilli of midgut epithelial cells and may act as adhesion ligands for ookinetes, however, the identity of these glycans remain unknown (8). Clearly, the identification and characterization of mosquito ligands is needed to further understand the mechanisms that mediate parasite–vector host interactions. We have now identified one set of mosquito midgut-specific oligosaccharide ligands to be sulfated chondroitin polysaccharides and provide evidence for their essential role in P. falciparum ookinete invasion in vivo.
Results
Chondroitin Sulfate (CS) Proteoglycans Localize to the Mosquito Midgut Microvilli.
To determine whether ookinetes use GAGs as ligands for cell invasion, we first sought to resolve the presence of midgut microvillar GAGs by histochemical analysis. The cationic dye, Alcian blue stained sugar-fed mosquito midguts, thereby confirming the presence of anionic polysaccharides on microvilli apices (Fig. 1 a and b). At pH 1.0, alcian blue does not react with protonated carboxyl groups that may be present on microvilli glycoconjugates but reacts readily with ionized sulfate groups (9). MAb CS-56 (Fig. 1 c and d) and single-chain variable fragment (scFv) antibody IO3H10 staining (Fig. 1 e and f) of midgut microvilli from blood-fed mosquitoes suggests that these anionic polysaccharides are analogous to CS. Midgut sections from blood-fed mosquitoes stained with scFv RB4EA12 (10) anti-HS antibodies showed basal lamina localization of HS proteoglycans (Fig. 1 g and h) (11). CS- and HS-modified proteoglycans are differentially distributed in polarized cells in mammals, wherein CS proteoglycans predominate in the apical surface and HS proteoglycans along the basal surface (12). CSGAGs are linear heteropolysaccharides of repeating glucoronic acid (GlcA) and N-acetylgalactosamine (GalNAc) dissacharide units (GlcAβ1–3GalNAc) (13). MAb CS-56 recognizes the monosulfated GlcA-GalNAc (4S) disaccharide “A-unit” and GlcA-GalNAc (6S) “C-unit,” the disulfated GlcA (2S)-GalNAc(6S) disaccharide “D-unit”, and octasaccharides that contained an internal trisulfated tetrasaccharide “A-D core unit” composed of GlcA-GalNAc(4S)-GlcA(2S)-GalNAc(6S) in CS chains (Fig. 1i) (13). ScFv IO3H10 specifically recognizes the 6-O-sulfated groups on the CS “C unit” as well as those on the CSD and CSE units (14).
Fig. 1.
Histological analysis revealed polarized localization of CS and HS proteoglycans in An. gambiae mosquito midgut epithelial cells. (a and b) Hematoxylin and eosin-stained mosquito (sugar fed) midgut cross-sections were analyzed for the presence of acid mucopolysaccharides. Midgut sections were incubated in buffer (pH 1.0) in the absence (a) or presence (b) of the polyvalent cationic dye, Alcian blue. Red arrow indicates apical microvilli staining with the dye. (c–h) Immunofluorescence analyses of midguts from blood-fed mosquitoes (24-h PBF) indicating polarized distribution of GAG species. (c) Bright-field image of the MAb CS-56 anti-CS-stained midgut section (d) showing midgut luminal staining. (e) Bright-field image of the anti-CS scFv antibody IO3H10-stained midgut section (f). (g) Bright-field image of the anti-HS scFv antibody RB4EA12-stained midgut section (h). Note that MAb CS-56 and scFv IO3H10 both corroborate the polarized expression of CSGAG to the apical microvilli. Arrows indicate preferential staining of the luminal interface between midgut epithelial cells and the blood meal or the basal lamina of the mosquito midgut. (i) CS disaccharide unit specificity of antibodies used in the analyses. MAb CS-56 has recognition specificity for CS A, C, and D units, and trisulfated (A-D units) on octasaccharide chains, whereas scFv IO3H10 has recognition specificity for CS C, D, and E units. MV, microvilli; LUMEN, midgut interior; BM, blood meal; E, midgut epithelial cell. DAPI, which was used to stain nuclei (blue), provides apical and basal orientation for the fluorescence images. Texas red-labeled secondary antibodies were used for detection and appear red. (Scale bars, ≈100 μm.)
Ookinetes Exhibit Binding Affinity to CSGAGs.
To determine whether P. falciparum ookinetes can bind to CSGAGs, we probed an overnight ookinete culture with CSA, CSC, CSD, and CSE as well as dermatan sulfate (DS) and hyaluronic acid (HA) as controls (Fig. 2). Although the overnight cultures yielded few mature form ookinetes (≈12–16% of the total number of parasites counted were mature ookinetes, ≈60–65% were late-retort ookinetes, and the remainder were a mixture of early retorts (Stage II) and zygotes), the cultures permitted the analysis of CSGAG staining of the mature form ookinetes (the motile parasite stage in the mosquito midgut). We observed that mature ookinetes exhibit binding affinity to CSA and CSE (Fig. 2 a–f) and that recognition of the CS ligands appears to be structural-specific because ookinete binding to CSA was more diffused than CSE (Fig. 2 a and d), and affinity for CSC, CSD, or DS was not observed (data not shown). The CSD disaccharide differs from CSA, CSC, and CSE motifs by the presence of a sulfate group on C-2 of GlcA (Fig. 1i). Ookinete recognition for DS was analogous to what was observed for HA, which is nonsulfated (Fig. 2 g–i). These data suggest that the presence of GlcA and not IdoA is important for ookinete recognition because GlcA replacement by IdoA in DS disaccharides leads to a loss of affinity and that GlcA needs to be in tandem with GalNAc, because HA (which is composed of GlcA-GlcNAc disaccharides) also fails to bind to ookinetes. Similar results were also observed for P. falciparum ookinetes that developed in vivo [supporting information (SI) Fig. 6].
Fig. 2.
P. falciparum ookinetes bind to specific CS glycosaminoglycans (CSGAG). CSA (a–c) and CSE (d–f) bind to mature ookinetes, whereas HA (g–i) do not. CSC, CSD, and DS produced similar results to HA. All GAGs were biotinylated and detected with Alexa 488 conjugated to streptavidin (green). Note that CSE exhibited less diffused ookinete staining intensity than CSA. The structure of CSA, CSC, CSD, and CSE are shown in Fig. 1i, and DS and HA are described in Results. Parasite morphology, along with an antibody to the ookinete surface protein, Pfs25, was used to identify mature form ookinetes (red). DAPI was used to stain parasite nuclei (blue). (Scale bars, ≈10 μm.)
The Anopheles gambiae GAG Biosynthetic Pathway and Cloning of the Mosquito Peptide-O-Xylosyltransferase.
CSGAG dissacharide units are attached to a tetrasaccharide core composed of a GlcA, two galactose (Gal) units and a xylose (Xyl) residue (GlcAβ1–3Galβ1–3Galβ1–4Xylβ1-Ser) that is glycosidically linked to the hydroxyl group of serines on proteoglycan polypeptides (15). CSGAGs vary in the degree and pattern of sulfation through the differential action of carbon 2-O, 4-O, and 6-O sulfotransferases acting on GalNAc and the combination of A-E units (16). In mammals, the addition of Xyl by a peptide-O-xylosyltransferase (OXT) to the Ser residue on a proteoglycan core initiates the sequential formation of the tetrasaccharide core (Fig. 3a, in bold). Unlike in mammals, for Drosophila (and putatively for other dipterans) the activity of a CS GalNAc-transferase I (GalNAcT-I) as opposed to an N-acetylglucosaminyl transferase I (GlcNAcT-I) determine the initiation and progression of the chain toward either a CSGAG or HSGAG, respectively (16–18) (Fig. 3a). Anopheles homologs of Drosophila melanogaster GAG biosynthetic enzymes, including OXT, as well as the genes encoding several proteoglycans have not been characterized (16, 19). An An. gambiae genome database search using D. melanogaster sequences suggested that two predicted OXT genes (ENSANGG00000025678; ENSANGG00000003504) exist in the mosquito. However, similar to Drosophila (16), of the two homologs identified, only the transcript for the latter gene (ENSANGG00000003504) could be amplified by PCR from an An. gambiae midgut cDNA library (Fig. 3b). The complete An. gambiae O-xylosyltransferase (AgOXT1) sequence revealed a transcript size of 2.8 kb and a predicted 876-aa polypeptide (Fig. 1 b and c). As with human and Drosophila OXTs, AgOXT1 is a type II transmembrane protein, with a WSC motif glycan-binding domain (GBD) and glycosyltransferase family 14 domains (Fig. 3 b and c), but lacks the characteristic Asp-Xaa-Asp (DXD) motif present in other family members and is involved in coordination of glycan substrates (16). The functional relevance of the lack of a DXD motif in AgOXT1 is not readily apparent, although it has been shown that nematode OXT, which also lacks this motif, is enzymatically active (15).
Fig. 3.
Anopheles gambiae peptide O-xylosyltransferase (AgOXT1) mRNA transcript, predicted protein structures, and its role in GAG biosynthesis. (a) RNA-meditated interference strategy to inhibit GAG biosynthesis. We hypothesized that RNAi knockdown of the AgOXT1 will result in the depletion of GAGs because of the loss of the tetrasaccharide core common to both heparan and CS chains. (b) The single predicted transcript for AgOXT1. Gray arrows indicate primer sites used in the amplification of the double-stranded AgOXT1. (c) A comparison of the predicted protein structures of the enzyme from An. gambiae (Ag) and Drosophila melanogaster (Dm). White rectangle, transmembrane domain; cross-hatched rectangle, a DXD motif. GBD, glycan-binding domain; GlycoT14, glycosyltransferase family 14 domain; Gal, galactose; GlcA, glucoronic acid; Xyl, xylose; GalT-I, galactosyltransferase-I; GalT-II, galactosyltransferase-II; GalNAcT-I, N-acetylgalactosaminyltransferase-I; GlcNAcT-I, N-acetylglucosaminyltransferase-I.
RNAi-Mediated Depletion of Midgut CS Blocks Parasite Invasion.
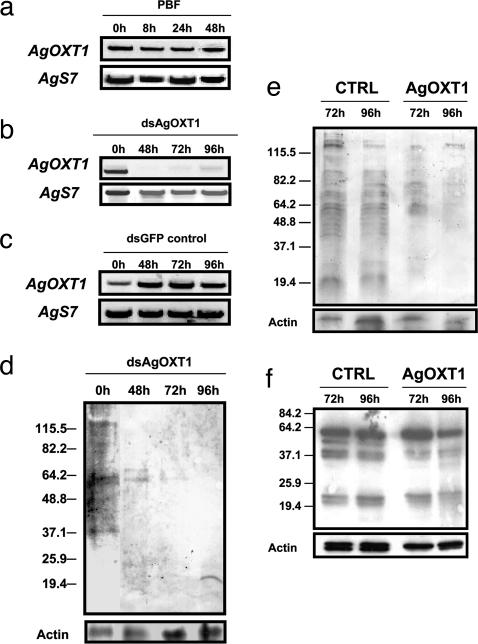
Having confirmed the presence of sulfated CSGAGs on midgut microvilli and the ability of ookinetes to bind to this class of polysaccharides, we sought to determine the role of these ligands during midgut invasion. To do so, we produced double-stranded RNA corresponding to a 720-bp coding region of AgOXT1 (Fig. 3b, gray arrows) and injected intrathoracically this construct into adult female An. gambiae mosquitoes. Total RNA was collected from blood-fed and sugar-fed adult mosquitoes at 48, 72, and 96 h after inoculation with dsRNA. AgOXT1 mRNA is constitutively transcribed during normal blood feeding (Fig. 4a). However, we found that mRNA levels were reduced significantly between 48 and 72 h after inoculation by double-stranded AgOXT1 (dsAgOXT1), followed by modest recovery at 96 h (Fig. 4b) as compared with dsRNA control-inoculated mosquitoes (Fig. 4c). The innate turnover of large proteoglycans is generally rapid. Therefore, given these results, we expected that the reduction in AgOXT1 message would lead to a loss of transferase activity and, consequently, the depletion of CSGAGs because the cycling polypeptide can no longer be modified. CS-56 and scFv IO3H10 immunoblot analyses of guts from the corresponding RT-PCR time-points (Fig. 4 d and e) supported our hypothesis, wherein we observed that CSGAGs were significantly reduced in the midgut. The multiple banding pattern observed in midguts from untreated and dsRNA control-injected mosquitoes (Fig. 4 d and e), implies the occurrence of several chondroitin proteoglycan species. However, at present, the identities of these midgut proteoglycan backbones remain unknown. Interestingly, despite AgOXT1 knockdown in the midgut, HS biosynthesis remained unaffected at both 72 and 96 h after inoculation (Fig. 4f), suggesting that, in comparison with apical CS proteoglycans, basal lamina/extracellular matrix HS proteoglycans may not turn over during the period before (0–3 days) or during experimental infection (24 h after blood feeding) with P. falciparum ookinetes. Immunofluorescence staining of the luminal face of the mosquito midgut sheets at 72 h after dsRNA injection (Fig. 5 a–d) confirmed the previous immunoblot data, wherein a marked decrease in signal was observed in dsAgOXT1-injected mosquitoes.
Fig. 4.
Intrathoracic inoculation of double-stranded AgOXT1 (dsAgOXT1) RNA into An. gambiae mosquitoes diminishes gene transcript and xylosyltransferase activity. (a–c) Semiquantitative RT-PCR analysis of AgOXT1 after blood feeding (PBF) (a) as well as several days after dsAgOXT1 inoculation (b and c). Double-stranded GFP (dsGFP) RNA was used as an injection control. Mosquito ribosomal S7 was used as a loading control. (d) MAb CS-56 immunoblot analysis of CS expression in dsAgOXT1-treated midguts (0–96 h after inoculation). (e) ScFv IO3H10 (anti-CS) immunoblot analysis of dsGFP (control) vs. dsAgOXT1-inoculated mosquito midguts at 72 and 96 h after inoculation. (f) ScFv RB4EA12 (anti-HS) immunoblot analysis of dsGFP (control) vs. dsAgOXT1-inoculated mosquito midguts at 72 and 96 h after inoculation. Antibody to β-actin was used as a loading control. Position of migration of molecular mass markers (kDa) is shown on the left.
Fig. 5.
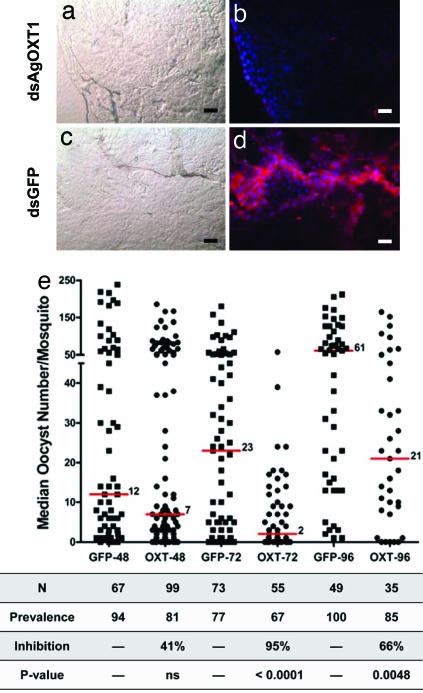
RNAi knockdown of AgOXT1 depletes CS chains on the luminal face of the mosquito midgut preventing Plasmodium falciparum establishment in the mosquito midgut. (a–d) The midguts from mosquitoes inoculated with dsAgOXT1 (a and b) or control dsGFP (c and d) were dissected 72 h after dsRNA inoculation. Each gut was sliced open to form a single layered sheet and fixed. The lumen faces toward the microscope for all images. The luminal face of treated (a) and control (c) midgut sheets shown in bright-field were stained with MAb CS-56 and detected with Texas red-conjugated secondary antibody (b and d, respectively). Note that CS proteoglycans appear red and remain present only in control-treated midgut sheets (d). DAPI was used to stain nuclei and appears blue. (Scale bars, ≈100 μm.) (e) P. falciparum transmission-blocking assay. Mosquitoes treated with dsAgOXT1 or dsGFP (control) were fed a blood meal containing P. falciparum (NF54) gametocytes at 48, 72, and 96 h after dsRNA inoculation. Eight days after infective blood feeding, the midguts were dissected, and the number of oocysts was counted. Horizontal lines indicate median oocyst number for each group. Sample size (N); Prevalence, number of infected mosquitoes in each group; Percent inhibition of median oocyst numbers was calculated as follows: [(control median oocyst no. − treatment median oocyst number/control median oocyst no.) × 100], and statistical significance (Mann–Whitney U test, α = 0.05) are shown in the table. Data are pooled from two independent experiments.
We then determined how the loss of luminal CSGAGs would affect parasite development. Control and treated dsRNA-inoculated mosquitoes were provided a P. falciparum-infected blood meal at 48, 72, and 96 h after inoculation (PI). The median Plasmodium oocyst numbers were reduced by 95% (P < 0.0001) in the dsAgOXT1 group in comparison with dsGFP controls for the 72 h PI cohorts (Fig. 5e). For the 96 h PI cohort, inhibition was reduced modestly to 66% (P = 0.0048), which may be a result of transcript/enzyme recovery as was observed for this time point by RT-PCR and immunoblot (Fig. 4 b, d, and e). We also examined mosquito midguts from dsAgOXT1-treated mosquitoes (72 h time point) at 48 h after blood feeding to determine whether early oocysts (identified by Pfs25 antibody staining) had indeed developed but either failed to mature or were lost from the midgut at day 8 (when oocysts were normally counted). As expected, early oocysts were not observed in dsAgOXT1-treated mosquito guts at 48 h, further supporting the argument that ookinete invasion of the midgut was blocked (SI Fig. 7). Furthermore, a similar level of inhibition was observed when P. falciparum gametocytes, along with MAb CS-56, were fed to mosquitoes (SI Table 1), suggesting the utility of CS proteoglycans as transmission-blocking vaccine targets (20).
Discussion
Here, we report on a role of mosquito CSGAGs in ookinete invasion of the midgut. It appears that this glycan ligand is used by Plasmodium at different stages of development in both its mosquito and vertebrate hosts (3–7). However, the CS chains that serve as ligands for Plasmodium-infected RBCs (IRBCs) on mammalian vascular endothelia are likely to be dissimilar from the CSA found in the mosquito midgut. Plasmodium-parasitized erythrocytes are sequestered in the placenta by association of parasite molecules with tissue-specific CSA (21). The CSA ligand for IRBCs in the intervillous spaces of the placenta were found to be unusually low-sulfated at position C4 (8–10% A units and 90–92% nonsulfated in placenta chains) and the absence of sulfation at C6 as compared with other tissue CSA chains (22). Immunohistological analyses of midgut CSGAGs suggest the presence of CSA, C, D, and E polysaccharides, a population of sulfated CS units that is quite different from the IRBC CSA ligand. However, given the overlap of specificity of MAb CS-56 (13), we cannot rule out that the staining pattern observed may be due primarily to recognition of CSA motifs. Furthermore, it is not clear whether the scFv IO3H10 can also recognize C4-sulfated (C4S) CSA motifs because the CSA sample used to determine the specificity of IO3H10 (14) may have included intermittent C6-sulfated (C6S) GalNAc moieties (a common occurrence in mammalian CSA chains) (18, 22). The polydispersity and spatial context (i.e., relative distribution) of CSA and CSC, CSD, or CSE on chondroitin chains vary greatly and have been found to be tissue- or species-specific (13, 14, 18, and 22). Therefore, although it remains possible that CSA may be the major sulfated chondroitin unit in the mosquito midgut, analysis of the relative position of CSA units along the chain (i.e., disaccharide repeat sequence) is essential to get a complete understanding of CSGAG structure.
Evidence for the putative importance of spatial context for CSA is highlighted by the observation that the CSA motif for IRBCs sequestering in the placenta is a dodecasaccharide domain consisting of two to four A-units interspersed with nonsulfated or C-units. These data suggest that IRBC-binding affinity for CSGAGs is highly structural-specific. We observed that mature ookinetes also bind to CSGAGs in a structural-specific manner. However, it appears that GalNAc (C4S) or (C6S), although necessary to confer binding affinity, may not be the only binding determinants on CSGAGs. It appears that sulfate and carboxylate position on GalNAc and GlcA, respectively, and not necessarily the presence of charge alone is important for binding to ookinetes. For example, epimerization of C-5 in GlcA produces the l-iduronate (IdoA) moiety present in DS (23). Although GalNAc of DS maintains the 4S modification, IdoA abrogates ookinete recognition (presumably through its axial carboxylate group). Replacing GalNAc with GlcNAc produces HA, which appears to be poorly recognized by ookinetes. Because of the inherent difficulty of producing many mature-stage ookinetes in vitro (24), it is difficult to assess CSGAG affinity by alternative methods (e.g., ELISA). However, because mature ookinetes are the final, motile stage, and mosquito CSGAGs are not present in the blood-meal bolus but are localized to the midgut apical microvilli, then we can infer from the data that mature ookinetes, which are involved in midgut adhesion, use midgut CSGAGs in the invasion process. Note that CS chains exist as repeating disaccharide polymers and that the use of biotinylated disaccharides in our binding assays may have underestimated the affinity and complexity of CSGAG recognition by ookinetes. Taken together, the results suggest that CS disaccharide sequence and relative sulfate position are critical structural determinants for ookinete recognition.
Plasmodium sporozoites have been shown to recognize liver HS proteoglycans through the major surface protein CSP and TRAP (3, 4). The related ookinete protein, CTRP can bind to heparin in vitro. However, heparin and HSs are quite dissimilar and both differ greatly from CSGAGs. Therefore, it remains to be seen whether CTRP is the cognate receptor on ookinetes for midgut CSGAGs. It should be noted that recombinant CTRP binding may not directly reflect what occurs in vivo. Alternatively, other ookinete surface lectin-like molecules may also be involved in CSGAG recognition.
Because peptide-O-xylosyltransferase is involved in the first step of GAG biosynthesis, depletion of AgOXT1 resulted in a dramatic decrease in GAG expression and marked inhibition of parasite development in the mosquito. We also targeted the GalNAcT-1 by RNAi and observed a reduction in transcript abundance without the consequent reduction in CSGAG-modified molecules in the midgut (data not shown). As expected, parasite development was also unaffected. We predict that targeting other enzymes in the biosynthetic pathway will produce similar results, presumably because of functional redundancy of related glycosyltransferases (25) in the gut.
Analysis of the role of glycans in mediating Plasmodium merozoite invasion of erythrocytes revealed the existence of a sialic acid-independent adhesion pathway (26). Preliminary evidence suggests that Plasmodium ookinetes also exhibit alternative midgut invasion mechanisms (ref. 27 and R.R.D., unpublished results). Therefore, RNAi leakiness notwithstanding, if a GAG-independent midgut invasion pathway exists, then it is possible that, for at least one parasite subpopulation, other ookinete lectin-like molecules (28) work in concert with their cognate midgut surface proteins to compensate for the loss of GAG ligands. In the context of our experiments, other midgut membrane glycan ligands that are left unaffected by RNAi knockdown include neutral and sulfated glycans and protein domains on glycolipids and glycoproteins, respectively. It is also possible that in the absence of critical sulfated glycans, protein–protein interactions may be sufficient for facilitating parasite recognition, adhesion, and invasion of midgut epithelial cells. Given the possible diversity of midgut invasion mechanisms, future work should examine different parasite–mosquito species combinations to determine the role, if any, that CSGAGs play in these interactions.
Our data has established the role of glycans as ligands that mediate parasite invasion of the mosquito midgut. However, the mechanisms by which glycans are used for cell adhesion or cell invasion are still poorly understood. The sequence and structure of midgut CS chains as well as identification of cognate lectin-like molecules on ookinetes remain to be analyzed.
After this manuscript was submitted for publication, a paper by Sinnis et al. [Sinnis P, Coppi A, Toida T, Toyoda H, Kinoshita-Toyoda A, Xie J, Kemp MM, Linhardt RJ (2007) J Biol Chem, in press] appeared that further supported our findings that chondroitin and HS polysaccharides are present in mosquito midguts.
Materials and Methods
Immunofluorescence Microscopy of Midgut Sections.
Midguts or midgut sheets from blood-fed or sugar-fed mosquitoes were fixed in 4% paraformaldehyde overnight at 4°C. Whole guts were embedded in paraffin for sectioning, and midgut sheets were used as whole mounts. Sections were blocked (PBS/3% BSA) overnight at 4°C. Each section was probed with either MAb CS-56 (1:50) (Sigma, St. Louis, MO) overnight at 4°C or the periplasmic fraction of RB4EA12 (29) containing anti-HS single-chain variable fragment (scFv) antibody (1:100) for 2 h at room temperature. MAb CS-56 was detected by Texas red-conjugated α-mouse IgM secondary antibody (Invitrogen, Carlsbad, CA). Anti-HS scFv was detected with a mouse anti-VSV tag MAb, followed by detection with Texas red-conjugated anti-mouse secondary antibody. The slides were mounted with Slowfade reagent containing DAPI (Molecular Probes/Invitrogen) and visualized by microscope.
CS–Ookinete-Binding Assays.
An 18- to 19-d-old P. falciparum NF54 gametocyte culture was transferred to a flask and diluted 1:5 in complete ookinete medium (24, 27) supplemented with 15% heat-inactivated fetal calf serum and incubated at 19°C for 24 h with rocking. Transformed ookinetes were purified by treatment with Lympholyte (Cedarlane Laboratories, Ontario, Canada) and Percoll density gradient. Parasites were smeared onto glass slides, air-dried, and then fixed in 4% paraformaldehyde for 1 h. Biotinylated CSA, CSC, CSD, CSE, DS, and HA (each at 10 μg/ml) were used to probe the slides [Saito et al. (30) and Saito et al. (31)]. CS probes were detected by Alexa-488 conjugated Streptavidin (Molecular Probes/Invitrogen). Ookinetes were identified by morphology and the use of anti-Pfs25 monoclonal antibody followed by detection using Texas red-conjugated anti-mouse secondary antibody (Molecular Probes/Invitrogen). The slides were mounted with Slowfade reagent containing DAPI (Molecular Probes/Invitrogen) and images captured as above.
Identification of Anopheles gambiae O-Xylosyltransferase Homologs.
We used the D. melanogaster amino acid sequence for peptide-O-xylosyltransferase 1 DmPOXT1 (16) to BLAST the Ensembl An. gambiae genome database v.37 (www.ensembl.org/Anopheles_gambiae/index.html). The complete sequence of all Anopheles OXT homologs was determined from ORF scanning of genomic data 5′-upstream of the predicted cDNAs found in the database. Primers corresponding to AgOXT1 (1557F, 5′-CAGCACAAGAAGCTGTTCTTTGGC-3′ and 2337R, 5′- AGCTTCCGTGACGAGAAATCGTGT-3′) were used to screen an An. gambiae cDNA library.
SDS/PAGE and Western Blot Analysis.
Mosquito midgut lysates (n = 5–10 female midguts) were fractionated on a 10% Tris-Glycine gel and transferred to nitrocellulose membranes. Transblots were probed with mouse MAb CS-56 α-CS (IgM) (Sigma) diluted 1:100 or anti-chondroitin O-6 sulfate antibodies (1:100). Primary antibodies were detected by use of alkaline phosphatase-conjugated α-mouse IgM antibody (1:5,000) and chemiluminescence by using CDP-Star substrate with Nitroblock II (PerkinElmer, Wellesley, MA).
Semiquantitative RT-PCR.
The AgOXT1 primers, 1557F and 2337R, were used to amplify a 780-bp product corresponding to the C terminus of AgOXT1 from our An. gambiae midgut cDNA library. The PCR product was cloned into the pCR2.1 TOPO (Invitrogen). For RT-PCR, 1 μg of total midgut RNA (n = 10 females) collected from each time point after blood feeding was reverse-transcribed following standard protocols. AgOXT1 transcript abundance was determined by using the primers above. The amplified An. gambiae ribosomal protein gene (AgS7) product was used as a loading control (AgS7F, 5′-TGCTGCAAACTTCGGCTAT-3′, and AgS7R, 5′-CGCTATGGTGTTCGGTTCC-3′). Amplification using Herculase polymerase (Stratagene, La Jolla, CA) was performed as follows: 94°C for 2 min, 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s, with a final extension at 72°C for 10 min (30 cycles). Cloned PCR products were confirmed by DNA sequencing.
RNA-Mediated Interference (RNAi).
AgOXT dsRNA was produced by using the primers above with the T7 polymerase promoter sequence, 5′-TAATACGACTCACTATAGGG-3′, appended to the 5′ termini of each primer and transcribed in vitro by using the Megascript RNAi kit (Ambion, Austin, TX) as per the manufacturer's protocols. The dsRNA solution was diluted to 6 μg/μl in injection buffer (10 mM Tris). An. gambiae females (5–6 d old) were anesthetized and inoculated intrathoracically with 400 ng of dsRNA or control GFP dsRNA. Mosquitoes were maintained for 2, 4, or 5 days before being fed a blood meal containing P. falciparum gametocytes (see below).
Parasite Membrane Feeding Assay.
P. falciparum gametocyte cultures from isolate NF54 were maintained as at the Johns Hopkins Malaria Research Institute Parasite Core facility. Cultures were harvested 15–17 d after initiation. The culture was washed and brought up in normal human serum (Interstate Blood Bank, Memphis, TN) plus human RBCs at 0.3% gametocytemia in a 45% hematocrit. Infective blood was delivered directly into glass, water-jacketed membrane feeders warmed to 38°C. Mosquitoes were allowed to blood feed for 20 min. The mosquitoes were maintained for 8 d, and only midguts from fully gravid females from each cage were further analyzed because egg development serves as a proxy for blood feeding to repletion. The dissected midguts were stained with 0.1% mercurochrome to visualize oocyst development (the read-out for successful parasite invasion). Three independent experiments were performed for each corresponding RNAi time point.
Statistical Analyses.
Nonparametric statistical analysis was used to evaluate the difference in median oocyst intensity between experimental and control groups (Mann–Whitney U test, one-tailed, α = 0.05) using the STATVIEW 5.0 software (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) Grants R01AI031478 and RR00052. R.R.D. is a NIH Ruth L. Kirschstein National Research Service Award scholar (5F32AI068212–02).
Abbreviations
- CS
chondroitin sulfate
- DS
dermatan sulfate
- GAG
glycosaminoglycan
- HA
hyaluronic acid
- HS
heparan sulfate
- IRBC
infected RBC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706340104/DC1.
References
- 1.Ghosh A, Edwards MJ, Jacobs-Lorena M. Parasitol Today. 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 2.Li F, Templeton TJ, Popov V, Comer JE, Tsuboi T, Torii M, Vinetz JM. J Biol Chem. 2004;279:26635–26644. doi: 10.1074/jbc.M401385200. [DOI] [PubMed] [Google Scholar]
- 3.Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. J Biol Chem. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradel G, Garapaty S, Frevert U. Mol Microbiol. 2002;45:637–651. doi: 10.1046/j.1365-2958.2002.03057.x. [DOI] [PubMed] [Google Scholar]
- 5.Templeton TJ, Kaslow DC, Fidock DA. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- 6.Yuda M, Yano K, Tsuboi T, Torii M, Chinzei Y. Mol Biochem Parasitol. 2001;116:65–72. doi: 10.1016/s0166-6851(01)00304-8. [DOI] [PubMed] [Google Scholar]
- 7.Dinglasan RR, Jacobs-Lorena M. Infect Immun. 2005;73:7797–7807. doi: 10.1128/IAI.73.12.7797-7807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinglasan RR, Fields I, Shahabuddin M, Azad AF, Sacci JB., Jr Infect Immun. 2003;71:6995–7001. doi: 10.1128/IAI.71.12.6995-7001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lev R, Spicer SS. J Histochem Cytochem. 1964;12:309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- 10.Dennissen MA, Jenniskens GJ, Pieffers M, Versteeg EM, Petitou M, Veerkamp JH, van Kuppevelt TH. J Biol Chem. 2002;277:10982–10986. doi: 10.1074/jbc.M104852200. [DOI] [PubMed] [Google Scholar]
- 11.Avnur Z, Geiger B. Cell. 1984;38:811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- 12.Tveit H, Dick G, Skibeli V, Prydz K. J Biol Chem. 2005;280:29596–29603. doi: 10.1074/jbc.M503691200. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. Glycobiology. 2005;15:593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- 14.Smetsers TF, van de Westerlo EM, ten Dam GB, Overes IM, Schalkwijk J, van Muijen GN, van Kuppevelt TH. J Invest Dermatol. 2004;122:707–716. doi: 10.1111/j.0022-202X.2004.22316.x. [DOI] [PubMed] [Google Scholar]
- 15.Mizuguchi S, Uyama T, Kitagawa H, Nomura KH, Dejima K, Gengyo-Ando K, Mitani S, Sugahara K, Nomura K. Nature. 2003;423:443–448. doi: 10.1038/nature01635. [DOI] [PubMed] [Google Scholar]
- 16.Wilson IB. J Biol Chem. 2002;277:21207–21212. doi: 10.1074/jbc.M201634200. [DOI] [PubMed] [Google Scholar]
- 17.Costa-Filho A, Souza ML, Martins RC, dos Santos AV, Silva GV, Comaru MW, Moreira MF, Atella GC, Allodi S, Nasciutti LE, et al. Insect Biochem Mol Biol. 2004;34:251–260. doi: 10.1016/j.ibmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros GF, Mendes A, Castro RA, Bau EC, Nader HB, Dietrich CP. Biochim Biophys Acta. 2000;1475:287–294. doi: 10.1016/s0304-4165(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 19.Pinto DO, Ferreira PL, Andrade LR, Petrs-Silva H, Linden R, Abdelhay E, Araujo HM, Alonso CE, Pavao MS. Glycobiology. 2004;14:529–536. doi: 10.1093/glycob/cwh070. [DOI] [PubMed] [Google Scholar]
- 20.Dinglasan RR, Valenzuela JG, Azad AF. Insect Biochem Mol Biol. 2005;35:1–10. doi: 10.1016/j.ibmb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Fried M, Domingo GJ, Gowda CD, Mutabingwa TK, Duffy PE. Exp Parasitol. 2006;113:36–42. doi: 10.1016/j.exppara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Achur RN, Valiyaveettil M, Gowda DC. J Biol Chem. 2003;278:11705–11713. doi: 10.1074/jbc.M211015200. [DOI] [PubMed] [Google Scholar]
- 23.Trowbridge JM, Gallo RL. Glycobiology. 2002;12:117R–25R. doi: 10.1093/glycob/cwf066. [DOI] [PubMed] [Google Scholar]
- 24.Carter EH, Suhrbier A, Beckers PJ, Sinden RE. Parasitology. 1987;95(Pt 1):25–30. doi: 10.1017/s0031182000057516. [DOI] [PubMed] [Google Scholar]
- 25.Ten Hagen KG, Tran DT, Gerken TA, Stein DS, Zhang Z. J Biol Chem. 2003;278:35039–35048. doi: 10.1074/jbc.M303836200. [DOI] [PubMed] [Google Scholar]
- 26.Duraisingh MT, Maier AG, Triglia T, Cowman AF. Proc Natl Acad Sci USA. 2003;100:4796–4801. doi: 10.1073/pnas.0730883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinglasan RR, Kalume DE, Kanzok SM, Ghosh AK, Muratova O, Pandey A, Jacobs-Lorena M. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0702239104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trueman HE, Raine JD, Florens L, Dessens JT, Mendoza J, Johnson J, Waller CC, Delrieu I, Holders AA, Langhorne J, et al. J Parasitol. 2004;90:1062–1071. doi: 10.1645/GE-3368. [DOI] [PubMed] [Google Scholar]
- 29.ten Dam GB, Hafmans T, Veerkamp JH, van Kuppevelt TH. J Histochem Cytochem. 2003;51:727–739. doi: 10.1177/002215540305100604. [DOI] [PubMed] [Google Scholar]
- 30.Saito A, Munakata H. Electrophoresis. 2004;25:2452–2460. doi: 10.1002/elps.200305997. [DOI] [PubMed] [Google Scholar]
- 31.Saito A, Munakata H, Satoh K. Connect Tissue Res. 2002;43:1–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.