Abstract
The widespread emergence of human and wildlife diseases has challenged ecologists to understand how large-scale agents of environmental change affect host–pathogen interactions. Accelerated eutrophication of aquatic ecosystems owing to nitrogen and phosphorus enrichment is a pervasive form of environmental change that has been implicated in the emergence of diseases through direct and indirect pathways. We provide experimental evidence linking eutrophication and disease in a multihost parasite system. The trematode parasite Ribeiroia ondatrae sequentially infects birds, snails, and amphibian larvae, frequently causing severe limb deformities and mortality. Eutrophication has been implicated in the emergence of this parasite, but definitive evidence, as well as a mechanistic understanding, have been lacking until now. We show that the effects of eutrophication cascade through the parasite life cycle to promote algal production, the density of snail hosts, and, ultimately, the intensity of infection in amphibians. Infection also negatively affected the survival of developing amphibians. Mechanistically, eutrophication promoted amphibian disease through two distinctive pathways: by increasing the density of infected snail hosts and by enhancing per-snail production of infectious parasites. Given forecasted increases in global eutrophication, amphibian extinctions, and similarities between Ribeiroia and important human and wildlife pathogens, our results have broad epidemiological and ecological significance.
Keywords: amphibian decline, emerging disease, environmental change
Emerging infections of humans and wildlife are often closely associated with anthropogenic alterations of the ecological and evolutionary relationships between hosts and pathogens, including climate change, biological invasions, land use change, and pollution (1–5). Owing to the complexity of pathogen–host–environment interactions, however, experimental evidence linking environmental change and increased infection is often lacking, leaving the ecosystem drivers of many emerging diseases unknown (5–7). In aquatic ecosystems, one of the most profound forms of ecological change is eutrophication, which is caused by anthropogenic inputs of nitrogen (N) and/or phosphorus (P) associated with agriculture, livestock, erosion, sewage waste, and atmospheric deposition (8–10). Because N and P often limit primary production, their addition causes marked shifts in ecosystem conditions (8, 9, 11).
Ecological theory (12) and limited field studies (see refs. 13 and 14) suggest that, unlike many environmental stressors, eutrophication will broadly enhance infection and the pathology of human and wildlife parasites. Nevertheless, experimental evidence linking nutrient enrichment and parasitism is largely absent, and the mechanisms through which eutrophication affects disease emergence remain poorly understood (12–15). Considering forecasted increases in global agricultural production and fertilizer application (10), as well as the persistence of anthropogenic P in agricultural soils and aquatic ecosystems (16), eutrophication will almost certainly become an increasingly severe problem in the coming century (12). This underscores the importance of understanding the mechanisms linking eutrophication and host–pathogen interactions and of balancing nutrient-mediated agricultural gains with concurrent increases in disease risk (11, 14).
Here we evaluated how elevated nutrient inputs leading to eutrophication affected the transmission and pathology of Ribeiroia ondatrae, a trematode parasite implicated in recent outbreaks of severe limb deformities in North American amphibians. This multihost parasite, which sequentially infects freshwater snails, larval amphibians, and waterbirds [see supporting information (SI) Fig. 4], has been causally linked to high frequencies of malformations (10–90%) in amphibian populations, including missing limbs, extra limbs, and malformed limbs (2, 17–21) (Fig. 1). Furthermore, infection and the resulting deformities substantially reduce amphibian survival, potentially contributing to widespread population declines and extirpations. However, the ecological drivers of parasite abundance, and the reasons for the apparent increase in amphibian deformities, have remained controversial (2, 19).
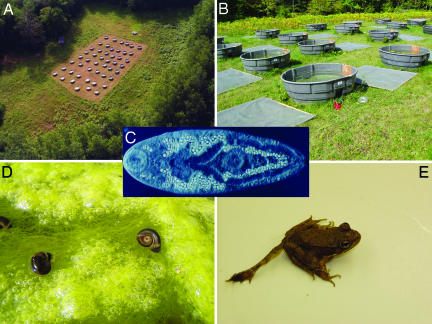
Fig. 1.
Experimental setup. Bird's eye (A) and local (B) views of outdoor mesocosms used to investigate effects of nutrient enrichment on host–parasite interactions. Nutrients (N and P) and trematode eggs were added to mesocosms in a factorial experiment to understand how anthropogenic eutrophication influenced transmission of a multihost pathogen. The pathogenic trematode R. ondatrae (C, excysted metacercariae) uses pulmonate snails (D) as first intermediate hosts and amphibians as second intermediate hosts. Nutrient enrichment was hypothesized to promote algal growth, leading to an increase in the density and biomass of herbivorous snail hosts, thereby enhancing parasite transmission into snails. Infected snails with high resource availability were also expected to produce more parasite cercariae, increasing the risk of amphibian infection and pathology. In amphibians, Ribeiroia infection induces severe limb malformations (E) by disturbing the developing limb field.
We experimentally tested the hypothesis that eutrophication enhances Ribeiroia transmission and identified the mechanisms responsible for this phenomenon. Uniquely, our experiment explicitly examines how nutrient enrichment affects transmission of a disease-causing parasite among multiple hosts within a complex life cycle. We hypothesized that, by increasing algal production, eutrophication would promote parasite infection through two, potentially complementary mechanisms. First, higher resource availability will increase the population growth of susceptible snail hosts (22), leading to enhanced parasite transmission and a higher density of infected snails (12). Second, higher resource levels will reduce infected snail mortality, increase snail body size, and enhance host vigor (15, 23–25), promoting parasite secondary production within infected individuals. Thus, a higher density of infected snails and a greater per-snail production of parasites should jointly drive an increase in amphibian infection and disease risk.
Results
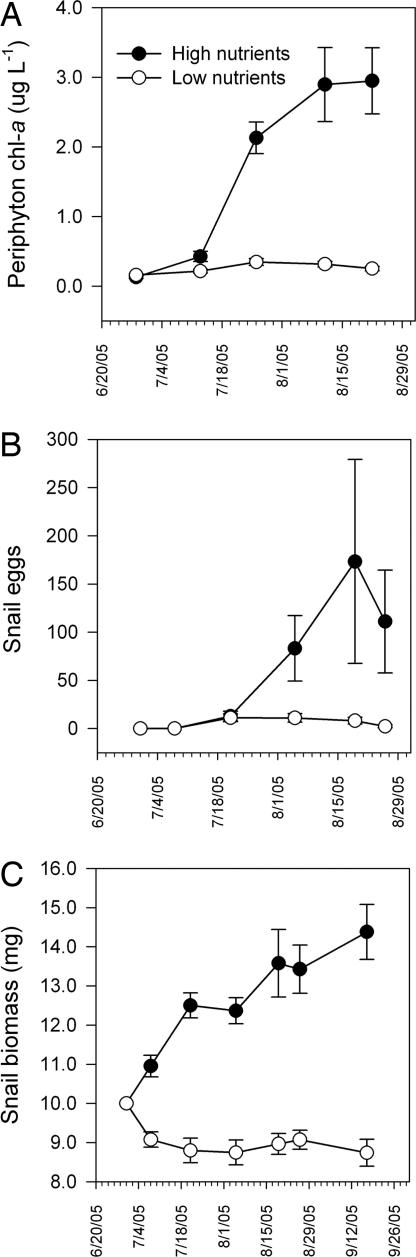
Nutrient additions significantly enhanced all primary response variables (Figs. 2 and 3). Except where otherwise noted, values were log10-transformed before analyses. Eutrophication promoted growth of periphytic algae [measured as chlorophyll a (chl a)], which exhibited a monotonic increase in high nutrient mesocosms during the experiment (Fig. 2A). We also found significant effects for time and the time-by-nutrient interaction on phytoplankton chl a [repeated-measures ANOVA (RM-ANOVA), time: Greenhouse–Geisser adjusted F[3.397,101.9] = 6.832, P < 0.0001; time-by-nutrients: Greenhouse–Geisser adjusted F[3.397,101.9] = 3.052, P = 0.027]. However, nutrients had a significant between-subjects effect only on periphyton chl a (F[1,30] = 75.930, P < 0.0001), reflecting the consistently stronger influence of nutrient enrichment on periphyton relative to phytoplankton.
Fig. 2.
Effects of nutrient enrichment on algal and snail growth. Nutrient enrichment significantly enhanced periphyton chl a (A) (RM-ANOVA, nutrients: F[1,30] = 75.93, P < 0.0001; time × nutrients: Greenhouse–Geisser corrected F[2.061,120] = 23.747, P < 0.0001), snail egg production (B) (RM-ANOVA, nutrient status: F[1,30] = 9.803, P = 0.004; time × nutrients: Greenhouse–Geisser adjusted F[2.58,77.39] = 6.92, P = 0.001), and dry mass of the snail host population (P. trivolvis) (C) (RM-ANOVA, nutrients: F[1,30] = 68.46, P < 0.0001; time × nutrients: Greenhouse–Geisser corrected F[2.96, 88.88] = 21.802, P < 0.0001). Snail density was converted to total dry mass by using the following equation (mass in grams = 0.0002 × [length in mm]2.7232; R2 = 0.96). Values are mean ± 1 SE and are pooled among parasite egg treatments, because parasite level did not significantly affect measured response variables.
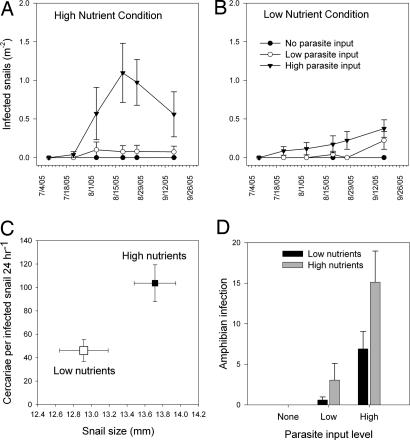
Fig. 3.
Influence of eutrophication on Ribeiroia infection in snails and amphibians. Effects of Ribeiroia egg input level on the density of infected snail hosts (P. trivolvis) under high-nutrient conditions (A) and under low-nutrient conditions (B) (parasite input: F[2,30] = 18.917, P < 0.0001; nutrients: F[1,30] = 11.079, P = 0.001; parasite × nutrients: F[2,30] = 8.368, P = 0.001). (C) Influence of nutrient condition and snail size (log10-transformed) on the per capita production of cercariae by infected snails (with snails nested within mesocosm and date sampled; mixed-model analysis, nutrients: F[1,31.477] = 5.20, P = 0.03; snail size: F[1,87.09] = 28.604, P < 0.0001). (D) Mean abundance of Ribeiroia metacercariae within larval green frogs as a function of Ribeiroia egg input level and nutrient condition (ANOVA, parasite input: F[2,28] = 19.27, P < 0.0001; nutrient input: F[1,28] = 5.289, P = 0.02). No metacercariae were recovered from amphibians in the “no-parasite” treatment. Values are mean ± 1 SE.
Increases in periphytic algae led to enhanced egg production and a greater population biomass of herbivorous snails, regardless of parasite input level (Fig. 2 B and C). Total snail biomass increased by nearly 50% in the high-nutrient condition but declined by 10% among low-nutrient mesocosms (Fig. 2C). Results were comparable if we used mean snail density rather than biomass. Correspondingly, snail egg masses in high-nutrient mesocosms had, on average, twice as many eggs as those in the low-nutrient condition, highlighting the enhanced fecundity of snails in this condition (mixed model, nutrients: F[1,56.17] = 15.88, P < 0.0001; mean eggs per egg mass ± 1 SE: high nutrients, 24.8 ± 1.3, low nutrients, 11.8 ± 1.5; n = 115). Parasite input level did not affect snail biomass (RM-ANOVA, F[2,30] = 0.009, P = 0.60), snail egg production (F[2,30] = 0.001, P = 0.99), or chl a levels (F[2,30] < 1.772, P > 0.18).
Nutrient level and Ribeiroia egg input jointly determined the density of infected snails among treatments (Fig. 3 A and B). Increases in parasite egg input caused an increase in infected snail density; however, this effect was significantly enhanced by nutrient addition (Fig. 3 A and B). Comparable results were achieved if we used the total number of cercariae produced per sampling date or the prevalence of infection (arcsin square-root-transformed) among snails >10 mm as response variables (see SI Fig. 5). To evaluate the effect of eutrophication on cercarial production among actively shedding snails, we conducted a mixed-model analysis with nutrient status and parasite input level as fixed factors, snail size as a covariate, and cercarial production as the response variable. Snail subjects were nested within mesocosms and within sampling date. Eutrophication significantly enhanced per capita production of cercariae (Fig. 3C), whereas parasite input and the parasite-by-nutrient interaction had no detectable effects. Pooling across dates and parasite conditions, infected snails from high-nutrient mesocosms produced, on average, twice as many cercariae as snails from low-nutrient mesocosms (Fig. 3C). Infected snail size was also a significant covariate in predicting cercarial production (Fig. 3C), suggesting that nutrient-mediated increases in host size facilitated increases in parasite production.
Ribeiroia egg input level and nutrient status each positively affected infection abundance in amphibians, which ranged from 0 to 48 metacercariae (n = 338) (Fig. 3D). Owing to increases in infected snail density and in per-snail release of cercariae, larval amphibians in eutrophic treatments exhibited a 2- to 5-fold increase in infection relative to amphibians in low-nutrient mesocosms. Because green frogs normally require >1 yr to metamorphose at this latitude, limb development among most animals was insufficient to evaluate malformation status. Nevertheless, these infection levels are well within the range of values known to induce limb malformations in amphibians (17). Nutrient status also enhanced amphibian size (total length, ANOVA, F[1,28] = 10.728, P = 0.004), whereas parasite treatment negatively affected amphibian survival (arcsin square-root-transformed) (ANOVA, F[2,15] = 4.247, P = 0.03) (see SI Fig. 6).
Discussion
Ecologists and epidemiologists are increasingly challenged to understand how large-scale agents of environmental change affect host–pathogen interactions (1, 4–7). Our study is the first to experimentally link aquatic eutrophication and transmission of a multihost parasite. In freshwater ecosystems, eutrophication is a widespread and growing problem with sharply negative effects on water quality, but the indirect effects of nutrient pollution on human and wildlife diseases are largely unexplored (8, 9, 11–14). Parasite-induced malformations in amphibians, which may have increased in prevalence and severity in recent decades (17–21), cause elevated mortality and morbidity in affected populations, but heretofore the environmental drivers of increased infection were largely unknown (but see ref. 2). By explicitly manipulating the inputs of nutrients and parasite eggs into experimental mesocosms, our results emphasize the importance of interactions among eutrophication, host dynamics, and parasite transmission. Importantly, the effects of eutrophication not only increased infection among first intermediate hosts (snails), but cascaded through the parasite's life cycle to increase amphibian infection, thereby elevating the risk of mortality and malformation.
Eutrophication promoted parasite infection and amphibian disease risk through two related but distinct mechanisms. First, increases in primary production resulting from nutrient addition enhanced the growth, reproduction, and survival of herbivorous snails, increasing the availability of first intermediate hosts for Ribeiroia miracidia (Fig. 2). In turn, this led to an increase in the density and prevalence of infected snails (Fig. 3 A and B). Second, eutrophication caused infected snails to nearly double their individual production of cercariae relative to infected snails in low-nutrient mesocosms (Fig. 3C). This likely occurred because of enhanced snail survival and growth under high-resource conditions (older and larger snails generally produce more parasites) or because of an increased capacity to translate snail resources into parasite secondary production (12, 15, 23–25). Importantly, although nutrient-mediated increases in infected host size contributed strongly to the observed increase in cercarial production, nutrient input level also had direct positive effects on cercarial release, suggesting that our results reflect changes in both host size and parasite productivity (26). Taken together, the increase in infected snails and in the per capita production of cercariae led to significant increases in Ribeiroia metacercariae among co-occurring amphibian larvae (Fig. 3D). Considering that most theoretical models of trematode parasites assume equivalent production of cercariae among infected hosts regardless of environmental conditions, these results have important implications for understanding how environmental change indirectly affects pathogen transmission. Although the long developmental period of Rana clamitans (≈1 yr) precluded inclusion of limb malformations as a response variable, the strong relationship between Ribeiroia infection and malformation frequency in amphibians (2, 17–21) suggests that the observed increase in infection can reasonably be expected to increase malformation likelihood.
In nature, the relationship between eutrophication and parasitism is likely to be variable and more complex than reported here, but several lines of evidence suggest that the proposed linkage between nutrient enrichment and Ribeiroia extends beyond the results of our experiment: (i) Available field data support correlations among eutrophication, snail host biomass, and Ribeiroia infection (19–21). (ii) Definitive hosts (especially birds) are often attracted to eutrophic environments, thereby increasing the input of parasite eggs deposited through feces (13, 26). In our experiment, nutrient enrichment and parasite inputs were independent variables, effectively decoupling this positive feedback. If definitive hosts are also more likely to consume infected amphibians as a result of their malformations (17), this will further amplify the effect of eutrophication on Ribeiroia abundance. However, definitive host abundance and activity will also depend strongly on the availability of suitable habitat, suggesting that the effects of eutrophication on infection will be context-dependent (27). Eutrophic wetlands embedded within environments favorable to bird hosts (e.g., rangeland), for example, may be more likely to support Ribeiroia and malformation epidemics than similarly eutrophic wetlands surrounded by less hospitable bird habitat (e.g., row crop agriculture). (iii) Finally, although our experiment included a simplified aquatic community, limited evidence suggests that inclusion of higher trophic levels, including snail and amphibian predators, may enhance infection through shifts in host behavior and life history strategies. By achieving size refugia, planorbid snails, such as those used by Ribeiroia, can achieve competitive dominance in high-nutrient environments (22), whereas amphibian larvae exposed to chemical cues from predators will often reduce their activity, thereby increasing their risk of trematode parasite infection (28). Nevertheless, given the inherent complexity and variability of natural communities, further study is needed to understand under what conditions eutrophication is likely to enhance infection and pathology. The current experiment included only two levels of nutrients (ambient vs. enriched), leaving open the question of whether the response of parasitism to eutrophication is linear or more complex, limiting the extent to which these results can be extrapolated over a broader range of nutrient levels.
An increase in Ribeiroia infection and malformations owing to progressive eutrophication could pose a serious risk to affected amphibian populations. Ribeiroia is a pathogenic parasite, causing direct and indirect (e.g., via malformations) mortality in amphibians (e.g., ref. 16 and this study). Although our experiment was short-term, sustained increases in the levels of Ribeiroia infection may precipitate decline or collapse of the amphibian population over longer time scales. In natural environments, the overwintering of infected snails may compound the effects of eutrophication among years. Indeed, by the end of our experiment, the number of prepatent (immature) snails infected with Ribeiroia more than doubled the total number of infected snails, forecasting substantially higher densities of infected snails for the following year. Because Ribeiroia causes reproductive castration in infected snails (21), high levels of infection may eventually reduce the snail population, possibly leading to cyclical patterns in Ribeiroia abundance and amphibian malformations. Moreover, eutrophic habitats frequently have high levels of contaminants such as pesticides or heavy metals, which may compromise the immune resistance of amphibians and further increase their susceptibility to parasite infection (2, 29), provided that such contaminants do not adversely affect the abundance of infected snail or bird hosts. Thus, as natural wetlands continue to be altered or destroyed, amphibians, now the most imperiled class of vertebrates worldwide (30), may be increasingly forced to use marginal, often eutrophic habitats that may be hotspots for disease.
Our results have broad applicability to other multihost parasites and their hosts. Recent increases in a variety of human and wildlife multihost parasites have been linked to eutrophication, including cholera, salmonid whirling disease, West Nile virus, coral diseases, and malaria (13, 14, 31–33). Trematode parasites similar to Ribeiroia that use snails as intermediate hosts also infect humans, ranging from the nuisance, but relatively innocuous, cercarial dermatitis to the pathogenic schistosomiasis, which is estimated to afflict 200 million people across Africa and Asia (35). If the life cycles of Schistosoma spp. are similarly affected by eutrophication, forecasted increases in agricultural nutrient applications in developing countries where schistosomiasis is endemic could hinder or inhibit efforts to control this disease. Ultimately, parasites that use multiple hosts through their life cycles are embedded in food webs with many connections to environmental drivers such as climate change, nutrient mobilization, and biotic exchange (1, 5, 6, 36). Such large-scale drivers can have substantial impacts on parasites and their hosts, with consequences for plant, animal, and human health. By understanding these impacts we will be better able to forecast disease risk in a changing world.
Methods
Experimental Design.
To evaluate how eutrophication affected Ribeiroia transmission among hosts throughout its life cycle, we conducted a 2 × 3 factorial experiment manipulating the inputs of nutrients (ambient and elevated) and parasite eggs (none, low, and high) in mesocosms containing a community of pulmonate snails, larval amphibians, zooplankton, and algae (Fig. 1). Experiments were conducted in 1,200-liter mesocosms established outdoors near the University of Wisconsin Trout Lake Station (Fig. 1). Mesocosms were randomly assigned to condition and replicated six times for a total of 36 mesocosms. We initially seeded mesocosms with 1,000 liters of lake water, 22 kg of commercial “play sand” as substrate, and 30 g of CaCO3 to promote snail shell growth. To provide inocula of algae and zooplankton, we added 50 ml of lake sediment and 30 ml of concentrated zooplankton and phytoplankton from each of five local wetlands.
We stocked mesocosms with 50 randomly selected uninfected snails (Planorbella trivolvis) collected and pooled from three local wetlands (mean size ± 1 SE = 12.8 ± 0.15 mm). To avoid inadvertent introduction of infected snails into the experiment, we isolated all snails individually into 50-ml vials for 24 h and subsequently examined the associated water for trematode cercariae. We dissected an additional 200 snails to ensure that prepatent (immature) infections of any trematode were rare (<0.5%) and that Ribeiroia was completely absent. We collected green frog (R. clamitans) egg masses (n = 3) from three wetlands in northern Wisconsin and allowed them to hatch and develop in the laboratory [stage 26 (37)] before adding 75 randomly selected individuals to each mesocosm on July 20, 2005. Mesocosms were covered with 1-mm mesh lids to minimize colonization of unintended flora and fauna.
Experimental Manipulation: Nutrient and Parasite Additions.
We experimentally enhanced N and P in half of the mesocosms by adding 4.7 g of NH4NO3 and 0.4 ml of 85% H3PO4, respectively, and left remaining mesocosms at ambient nutrient concentrations. These one-time additions were selected to achieve initial concentrations of ≈1,800 μg·liter−1 of N and 200 μg·liter−1 of P for 945 liters of water (20:1 molar ratio). One week later, after algae had begun to use nutrient resources, average total unfiltered concentrations of N and P in the high- and low-nutrient treatments were 873 ± 31.4 μg·liter−1 N and 101.8 ± 3.1 μg·liter−1 P (high nutrients) and 467.3 ± 2.1 μg·liter−1 N and 10.3 ± 0.4 μg·liter−1 P (low nutrients), respectively. These levels are well within the range of values observed in natural amphibian habitats (19, 20). A recent survey of amphibian habitats in Wisconsin found nutrient concentrations of 2,859 μg·liter−1 N and 348 μg·liter−1 P for agricultural (eutrophic) wetlands and 1,235 μg·liter−1 N and 43 μg·liter−1 P for forested wetlands (R.B.H., unpublished data).
To obtain embryonated eggs of Ribeiroia, we experimentally infected laboratory rats (n = 10) with 50 metacercariae isolated from infected amphibians. After 2 weeks, rat fecal matter was collected on wet paper towels, soaked in spring water for 24 h, filtered through a sieve series, and incubated in the dark at 28°C for 3 weeks (38). Water was aerated continuously and changed weekly. We estimated Ribeiroia egg density ± 1 SE by counting the numbers of embryonated eggs in five 20-μl aliquots examined at ×200 magnification. We added eggs to mesocosms biweekly at one of three levels: 0 (control), 150 ± 11 (low egg input), and 1,500 ± 111 (high egg input). Eggs were placed into 1-liter plastic chambers suspended at the mesocosm surface and allowed to hatch and subsequently seek out susceptible snail hosts. We used suspended chambers to ensure that eggs were exposed to sunlight, which stimulates hatching, and equipped chambers with four openings covered by a 1-mm mesh screen to allow hatching parasite miracidia to escape the chamber and enter the mesocosm. After hatching, parasite miracidia were allowed to locate and infect susceptible snail hosts, which, after a period of intrasnail maturation, developed into parasite rediae. Rediae, in turn, produced mobile cercariae that emerged nightly from infected snails and actively infected larval amphibians, wherein they formed metacercariae. For mesocosms in the “no-parasite” treatment, we added a comparable volume (0.02–0.2 ml) of sieved and incubated feces from uninfected rats to equalize any nutrient additions administered through fecal material.
Sampling Schedule and Data Collection.
Biweekly throughout the summer, we quantified levels of algal growth, snail density and reproduction, snail infection prevalence, and the daily, per-snail release of cercariae. In this manner we evaluated the effects of eutrophication on Ribeiroia transmission into snails (miracidia to rediae) and from snails into amphibians (cercariae to metacercariae) (see SI Fig. 4). We collected phytoplankton chl a from open water samples (2 liters) and filtered it onto 45-μm glass fiber filters, whereas we isolated periphyton chl a from 5 × 2.8-cm strips of flagging tape suspended on the sides of each mesocosm. Filtered samples were frozen for 24 h, extracted in methanol, homogenized, and centrifuged. Chlorophyll values were measured on a spectrophotometer. Predominantly, we were interested in the effects of nutrient addition on periphyton rather than phytoplankton growth, because the former provides an important food resource for herbivorous snails. Moreover, we expected that, within our experiments, phytoplankton standing stock would be controlled by zooplankton grazing, rather than by nutrient limitation.
Over this same time period, we visually enumerated the numbers of snails, snail egg masses, and eggs per egg mass within each mesocosm. To avoid unintentional bias in data collection, mesocosms were numerically coded without any indication of treatment condition. We classified snails into four size categories (1–5 mm, 5.1–10 mm, 10.1–15 mm, and >15 mm) that could consistently be classified visually (>95% observer accuracy) and determined the number of eggs per snail egg mass by counting eggs from up to a maximum of 10 randomly selected egg masses on 11 × 17-cm Plexiglas sheets placed within each mesocosm. Beginning on July 7, 2005, we estimated snail infection prevalence and daily cercarial production by randomly selecting 25 adult snails (>10 mm) from each mesocosm and isolating them individually into 50-ml centrifuge tubes allowed to float overnight within mesocosms. Released cercariae were identified and enumerated to provide estimates of snail infection prevalence and per capita production of Ribeiroia cercariae. All snails were returned to mesocosms the following morning. In total, 5,342 snails were examined for cercarial release, of which 94 were confirmed infected with Ribeiroia. Infected snails released an average ± 1 SE of 83.6 ± 11.2 (range: 2–651).
Winter conditions did not allow us to maintain the experiment between years, and we destructively sampled the mesocosms on September 15. We dissected all snails >5 mm that did not release cercariae to determine the number of prepatent (immature) infections and measured (total length in millimeters) all surviving amphibian larvae. We necropsied 10 larvae from each mesocosm (including individuals from the “no-parasite” treatments) to quantify Ribeiroia metacercariae.
Supplementary Material
Acknowledgments
We dedicate this research to the memory of Dr. Daniel Robert Sutherland (1952–2006). For logistical support we thank T. Kratz, S. Knight, L. Winn, and the Wisconsin Department of Natural Resources. For assistance with experiment establishment and maintenance we thank E. Preu, A. Schiller, M. Pecore, J. Vehrs, G. Sass, J. Rusak, K. Skogen, and the Wisconsin Department of Natural Resources Division of Forestry Fire, especially the late S. Matula. K. Cottingham, K. Lunde, V. McKenzie, R. Ostfeld, V. Smith, and A. Townsend provided comments helpful in shaping the manuscript. This research was funded, in part, by grants from the National Science Foundation (DEB-0411760 and DEB-0217533), the Anna Grant Birge Fund, and the Juday family.
Abbreviations
- chl a
chlorophyll a
- RM-ANOVA
repeated-measures ANOVA.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707763104/DC1.
References
- 1.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. Science. 2002;296:2160–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 2.Kiesecker JM. Proc Natl Acad Sci USA. 2002;99:9900–9904. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope K, Masuoka P, Rejmánková E, Grieco J, Johnson S, Roberts D. Ecol Appl. 2005;15:1223–1232. [Google Scholar]
- 4.Patz JS, Campbell-Lendrum D, Holloway T, Foley JA. Nature. 2005;438:310–317. doi: 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- 5.Daszak P, Cunningham AA, Hyatt AD. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 6.Ostfeld R, Keesing F, Eviner V, editors. Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Princeton: Princeton Univ Press; 2007. [Google Scholar]
- 7.National Research Council. [Accessed September 1, 2006];Grand Challenges in Environmental Sciences. 2001 Available at www.nap.edu/books/0309072549/html.
- 8.Schindler DW. Limnol Oceanogr. 2006;51:356–363. [Google Scholar]
- 9.Smith VH, Joye SB, Howarth RW. Limnol Oceanogr. 2006;51:351–355. [Google Scholar]
- 10.Millennium Ecosystem Assessment. [Accessed May 1, 2007];Millennium Ecosystem Reports. 2005 Available at www.maweb.org.
- 11.Townsend AR, Howarth RW, Bazzaz FA, Booth MS, Cleveland CC, Collinge SK, Dobson AP, Epstein PR, Holland EA, Keeny DR, et al. Front Ecol Environ. 2003;1:240–246. [Google Scholar]
- 12.Lafferty KD, Holt RD. Ecol Lett. 2003;6:654–664. [Google Scholar]
- 13.Johnson PTJ, Carpenter SR. In: Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems. Ostfeld R, Keesing F, Eviner V, editors. Princeton: Princeton Univ Press; 2007. [Google Scholar]
- 14.McKenzie VJ, Townsend AR. EcoHealth. 2007 in press. [Google Scholar]
- 15.Smith VH, Jones TP, II, Smith MS. Front Ecol Environ. 2005;3:268–274. [Google Scholar]
- 16.Bennett EM, Carpenter SR, Caraco NF. BioScience. 2001;51:227–234. [Google Scholar]
- 17.Johnson PTJ, Lunde KB, Ritchie EG, Launer AE. Science. 1999;284:802–804. doi: 10.1126/science.284.5415.802. [DOI] [PubMed] [Google Scholar]
- 18.Sessions SK, Franssen RA, Horner VL. Science. 1999;284:800–802. doi: 10.1126/science.284.5415.800. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PTJ, Chase JM. Ecol Lett. 2004;7:521–526. [Google Scholar]
- 20.Johnson PTJ, Lunde KB, Thurman EM, Ritchie EG, Wray SW, Sutherland DR, Kapfer JM, Frest TJ, Bowerman J, Blaustein AR. Ecol Monogr. 2002;72:151–168. [Google Scholar]
- 21.Johnson PTJ, Sutherland DR, Kinsella JM, Lunde KB. Adv Parasitol. 2004;57:191–253. doi: 10.1016/S0065-308X(04)57003-3. [DOI] [PubMed] [Google Scholar]
- 22.Chase JM. Oikos. 2003;101:187–195. [Google Scholar]
- 23.Pulkkinen K, Ebert D. Ecology. 2004;85:823–833. [Google Scholar]
- 24.Keas BE, Esch GW. J Parasitol. 1997;83:96–104. [PubMed] [Google Scholar]
- 25.Sandland GJ, Minchella DJ. Oecologia. 2003;134:479–486. doi: 10.1007/s00442-002-1127-x. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer MV, Canfield DE., Jr Hydrobiologia. 1994;279-280:107–119. [Google Scholar]
- 27.Gagne SA, Fahrig L. Landscape Ecol. 2007;22:205–215. [Google Scholar]
- 28.Thiemann GW, Wassersug RJ. Biol J Linn Soc. 2000;71:513–528. [Google Scholar]
- 29.Forson DD, Storfer A. Ecol Appl. 2006;16:2325–2332. doi: 10.1890/1051-0761(2006)016[2325:airsit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 31.Bruno JF, Petes LE, Harvell CD, Hettinger A. Ecol Lett. 2003;6:1056–1061. [Google Scholar]
- 32.Cottingham KL, Chiavelli DA, Taylor RA. Front Ecol Environ. 2003;1:80–86. [Google Scholar]
- 33.Reiskind MH, Walton ET, Wilson ML. J Med Entomol. 2004;41:650–656. doi: 10.1603/0022-2585-41.4.650. [DOI] [PubMed] [Google Scholar]
- 34.Rejmánková E, Grieco J, Achee N, Masuoka P, Pope K, Roberts D, Higashi RM. In: Disease Ecology: Community Structure and Pathogen Dynamics. Collinge SK, Ray CR, editors. New York: Oxford Univ Press; 2006. [Google Scholar]
- 35.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 36.Lafferty KD, Dobson AP, Kuris AM. Proc Natl Acad Sci USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosner KL. Herpetelogica. 1960;16:183–190. [Google Scholar]
- 38.Huizinga HW. Exp Parasitol. 1973;33:350–364. doi: 10.1016/0014-4894(73)90039-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.