Abstract
Understanding the basis for the control of myometrial contractant and relaxant signaling pathways is important to understanding how to manage myometrial contractions. Signaling pathways are influenced by the level of expression of the signals and signal pathway components, the location of these components in the appropriate subcellular environment, and covalent modification. Crosstalk between these pathways regulates the effectiveness of signal transduction and represents an important way by which hormones can regulate phenotype. This review deals primarily with signaling pathways that control Ca2+ entry and intracellular release, as well as the interplay between these pathways.
Keywords: myometrium, calcium, signaling, cAMP, ion channels
1. Introduction
Interest in understanding hormonal signaling in myometrium stems from a need to control uterine contractile activity in pregnancy. Signaling pathways are influenced by the level of expression of the signals and the respective signaling components, the location of these signaling components in the appropriate subcellular environment, and covalent modification (previously reviewed in [1–5]). Hormonal influences can alter one or more of these parameters. Furthermore, more than one signaling pathway may be used to achieve the desired result, thus providing redundancy that may be essential for propagation of the species but complicating the assessment of the importance of any one pathway. This review summarizes what is currently known about the signaling pathways that control myometrial Ca2+ entry and intracellular release, as well as the interplay between these pathways.
2. Contractant and relaxant signaling pathways
2.1 Multiple pathways lead to effects on calcium homeostasis
Figure 1 depicts components involved in regulating Ca2+ homeostasis in the myometrium. Intracellular Ca2+ concentrations [Ca2+]i increase as a result of Ca2+ entry through plasma membrane cation channels and release from intracellular stores, primarily endoplasmic reticulum (ER). Many uterine contractants stimulate plasma membrane G-protein-coupled receptors (GPCRs) (see Table 1, López Bernal, 2007, this issue). These GPCRs, in turn, stimulate trimeric GTP-binding proteins (G-proteins), such as GαqGβγ, that activate multiple pathways to generate intracellular signals. Some of these signaling molecules impact Ca2+ homeostasis directly, whereas others stimulate feedback loops or initiate crosstalk between pathways that can be either positive or negative in nature and impact Ca2+ homeostasis indirectly.
Figure 1.
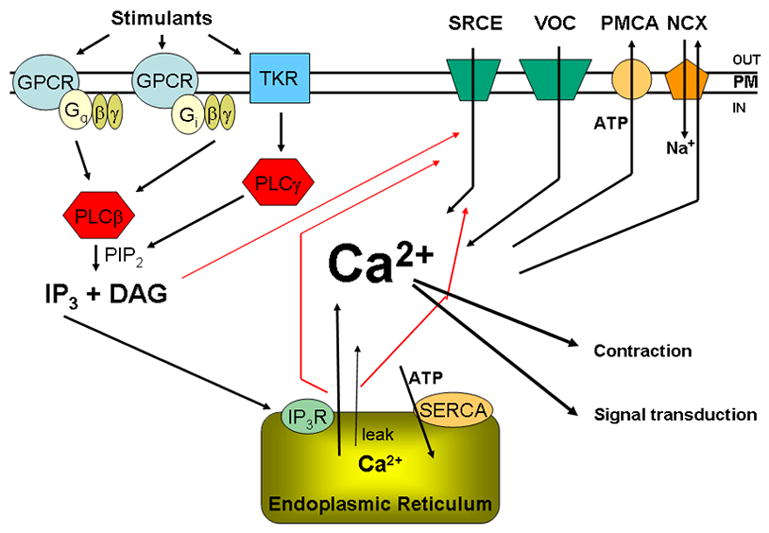
Schematic diagram showing the major components that control [Ca2+]i in myometrium. These include the contractant hormone pathways that stimulate G-protein coupled (GPCR) and tyrosine kinase (TyKR) receptors, G-proteins composed of Gα subunits (Gαq, Gαi and (not shown) Gαh), the associated Gβ and Gγ subunits, phospholipase C β and γ, which convert PIP2 to IP3 and diacylglycerol (DAG). IP3 binds to IP3 receptors (IP3R) in the endoplasmic reticulum (ER), releasing Ca2+ from this intracellular store. Another ER release mechanism involves ryanodine receptors (RyR). Other Ca2+ entry mechanisms include cation channels responsive to stimuli such as IP3R activation, DAG and release of Ca2+ from the ER (SRCE) and voltage-operated Ca2+ channels (VOC). The plasma membrane Ca2+ ATPase (PMCA) and the Na/Ca exchanger (NCX) are responsible for moving Ca2+ out of the cell; the ER Ca2+ ATPase (SERCA) pumps Ca2+ back into the ER. There is also a passive leak of Ca2+ from the ER. Ca2+ has stimulatory effects on the contractile apparatus, resulting in contraction, and also acts as an intracellular signal influencing a number of pathways in the myometrium.
In a pathway used by a number of contractants, Gαq activates phospholipase Cβ (PLCβ) isoforms, resulting in the hydrolysis of plasma membrane phosphatidylinositide (PI) bisphosphate (PIP2) to produce inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to endoplasmic reticulum (ER) IP3 receptors, stimulating an increase in release of ER Ca2+ and a rise in [Ca2+]i. Other hormones signal through receptor tyrosine kinases, directly activating PLCγ and generating the same downstream intracellular intermediates. Ca2+ is the primary charge carrier in the myometrium and is responsible for stimulating the contractile apparatus (see Taggart, 2007, this issue). Contractant hormones may indirectly stimulate a change in membrane potential, thus triggering Ca2+ entry through voltage-operated ion channels (VOC). In addition, the signals generated by PLC activation, i.e., IP3 and DAG, stimulate other types of channels and contribute to capacitative/store-operated or receptor-operated Ca2+ entry [6,7]. This type of Ca2+ entry will be broadly referred to here as signal-regulated Ca2+ entry (SRCE); it is dependent on hormone-generated signals and is potentially related to hormonal effects on contractile activity. The presence of this pathway in myometrium has been documented [8–10], but its physiological importance is only now being investigated.
[Ca2+]i concentrations decrease as a result of the actions of the plasma membrane (PMCA) and endoplasmic reticulum (SERCA) Ca2+-ATPases and Na/Ca2+ exchangers (NCX). [Ca2+]i is also affected indirectly by mechanisms such as the activation of K+ channels that hyperpolarize the cell and decrease VOC-mediated Ca2+ entry. These pathways are regulated by [Ca2+]i itself, but also by components of relaxant and contractant signaling pathways.
2.2 Contractant and relaxant signaling pathways stimulate multiple downstream actions
Myometrial contractant signaling pathways that result from stimulation of specific receptors are complex and bifurcate to influence many downstream pathways, as illustrated by the following examples.
Oxytocin
The oxytocin GPCR (OTXR) couples to several G proteins (reviewed in [2,5]). In rat myometrial cells and membranes, almost all oxytocin-stimulated increases in [Ca2+i], membrane GTPase and PLC activity were inhibited by anti-Gαq antibodies, suggesting that this was the predominant signaling pathway. Nonetheless, OTXR can also activate Gαi and can couple to Gαh to stimulate PLCδ. The relative importance of different G-protein pathways in oxytocin signaling may vary between species and cell type. The DAG and IP3 produced in response to OTXR activation each stimulate components of the SRCE mechanism. Oxytocin also stimulates the TNFα-enhanced cyclic ADP-ribose (cADPR) pathway, leading to intracellular Ca2+ release via RyR receptors [11,12].
Oxytocin stimulates additional downstream signaling pathways in myometrium, including ERK1/2 phosphorylation [2,5]. This pathway is independent of effects on [Ca2+]i. The sensitivity to pertussis toxin of oxytocin-mediated ERK stimulation in rat myometrium suggests GαiGβγ-mediated activation. However, this inhibition by pertussis toxin is reversed in human myometrial cells by a PKA inhibitor. This is inconsistent with covalent modification of Gαi, and supports involvement of Gβγ derived from Gαq activation itself, which is known to be inhibited by PKA [13]. This pathway requires the participation of the EGF receptor and Ca2+. Truncation of the C-terminus of rat OTXR resulted in loss of the ability to stimulate ERK phosphorylation but not pertussis toxin-sensitive increases in [Ca2+]i, suggesting that Gαi coupling may activate a Ca2+ release/entry pathway independent of Gαq [14]. Recently, OTXR signaling through GαiGβγ, resulting in enhanced production of cAMP, has been reported in rat pregnant myometrium [15]. Other OTR-Gαi-linked pathways resulting in ERK activation involve Src/EGR receptor and/or PLC/Src/PI3-kinase [16]. ERK activation plays a role in the regulation on contractile activity (see Taggart, 2007, this issue).
ERK activation by oxytocin has also been linked to the stimulation of cyclooxygenase-2, PGF synthase, cfos and RGS2 gene expression in myometrial cells [2,17–19]. Oxytocin also increases total protein synthesis, as well as eukaryotic elongation factor 2 dephosphorylation, in myometrial cells [20].
Prostaglandin F2α (PGF2α)
The uterus produces several prostaglandins and expresses a number of prostaglandin receptors (reviewed in [4]). PGF2α binds to FP and TP receptors; some differences in reported responses may be due to use of different signaling pathways by these two receptor types. Prostaglandin receptors can be located on both plasma and nuclear membranes and their location can change during gestation. PGF2α increases [Ca2+]i by stimulating entry and release mechanisms in human myometrium [21,22], with a significant dependence on extracellular Ca2+ [23]. PGF2α activates ERK through the SHC/SOS/Ras pathway in rat myometrial cells via a Gβγ mediated mechanism [24].
EGF
The EGF receptor possesses tyrosine kinase activity and directly stimulates PLCγ by tyrosine phosphorylation. In myometrium, this results in IP3 generation and an increase in [Ca2+]i, consistent with intracellular Ca2+ release [25]. Activation of the EGF receptor has been implicated in myometrial [Ca2+]i oscillations and also in OTXR signaling [13,26]. EGF also activates the PI-3 kinase/Akt pathway in other tissues, synergizing with PLCγ activity in mediating EGF effects [27]. EGF activation of ERK in myometrium is PKC-independent and involves the conventional Ras pathway [28]. EGF has also been implicated in myometrial cell proliferation in a pathway involving PKC and eicosanoids [29].
Relaxant signaling pathways
Myometrial relaxants such as β-adrenergic agents, CGRP and relaxin stimulate GPCR-coupled pathways that activate Gαs which activates adenylyl cyclase, generating cAMP and activating PKA. PKA is localized in various parts of the cell as a result of interaction with A-Kinase-Anchoring Proteins (AKAPs). PKA-mediated phosphorylations affect a number of the components regulating [Ca2+i], as discussed in more detail by López Bernal (2007, this issue). Another relaxant signaling pathway in the myometrium is the nitric oxide (NO) guanylyl cyclase/cGMP/protein kinase G (PKG) pathway also discussed in depth by López Bernal (2007, this issue).
Other signaling pathways
Another potentially important signaling pathway in myometrium involves sphingosine kinase-1, which produces sphingosine-1-P. Endothelin-1 affects Rho-/Rho kinase-dependent pathways via activation of this pathway [30].
Ca2+ itself is an intracellular signal that influences the activity of a number of ion channels and, through its effect on the Ca2+ binding protein calmodulin, can activate not only the contractile apparatus through myosin light chain kinase activation (Taggart, 2007, this issue), but also CaM kinases, calcineurin (protein phosphatase PP2B) and transcription factors such as NFAT. Many of these potential Ca2+-dependent mechanisms have yet to be studied in detail in the myometrium.
3. Proteins responsible for membrane calcium entry and exit pathways
3.1 Nifedipine-sensitive voltage-dependent channels
Entry of extracellular Ca2+ through L-type voltage-dependent Ca2+ channels is clearly important for sustaining myometrial contractions (reviewed in [2,5,31]). The alpha1 subunit (Cav1.1–1.4) contains the channel pore, voltage sensor and drug binding sites; beta, alpha2–delta and gamma subunits modulate channel activity [32]. The concentration of myometrial Cav1.2 alpha and beta subunit mRNAs are expressed differentially in gestation and labor and in longitudinal versus circular muscle [33–36]. Two Cav1.2 isoforms are generated by post-translational processing; the longer form, reported to generate lower inward currents but to contain the regulatory PKA phosphorylation site, predominates in late pregnant rat myometrium [37]. The link between differential expression of L-type voltage-dependent Ca2+ channel isoforms and myometrial function remains to be determined.
The role of L-type Ca2+ entry in myometrium has been assessed using agonists and channel blockers. BAY K 8644, an L-type channel agonist, markedly increased the frequency but not the amplitude of human pregnant myometrial contractions [38]. L-type channel blockers reduced both spontaneous and stimulus-induced myometrial contractions [39,40]. Nifedipine (5 nM) inhibited the amplitude of oxytocin-stimulated contractions with less effect on frequency [41] and at μM concentrations completely eliminated contractile activity [42]. The effect of nifedipine on human myometrial strips was more marked in term not-in-labor than in term-labor specimens [43]. A significant proportion of oxytocin and PGF2α-induced contractions and increases in [Ca2+]i in late pregnant guinea pig myometrium was attributed to Ca2+entry inhibited by L-type Ca blockers [44], as was the response in pregnant rat myometrial cell [45]. In contrast, no effect of nifedipine on oxytocin- stimulated increases in [Ca2+]i was observed in nonpregnant human myometrial cells [46], and L-type blockers had no effect on oxytocin-stimulated PI turnover [2,47].
Effects of contractants on myometrial membrane potential in relation to Ca2+entry are complex. Oxytocin caused a slight depolarization in myometrial cells [48], suggestive of Ca2+entry, but the mechanism was not established. In contrast, L- type current was inhibited by oxytocin in pregnant rat myometrial cells [49]. In other smooth muscles, L-type channels can be stimulated by GPCR pathways involving Gβγ, PI-3K and PKC [50]. Oxytocin has been implicated in the opening of other cation channels (consistent with SRCE) and of Ca2+-activated Cl− channels in myometrium, leading indirectly to depolarization and opening of voltage-dependent Ca2+ channels [51].
In pregnant guinea pig myometrium, PGF2α stimulated sustained depolarization and biphasic increases in [Ca2+]i at mid to late-pregnancy; nifedipine inhibited most of the former but only some of the latter [52]. Verapamil, an L-type channel inhibitor, significantly reduced PGF2α-stimulated PI turnover in late pregnant human myometrium [2,47]. In apparent contrast to a stimulation of Ca2+ entry, PGF2α has been reported to induce hyperpolarization in pregnant human myometrium, which may involve effects on the Na/K ATPase [53].
3.2 T-type cation channels
Low voltage-activated T-type Ca2+ channels have been implicated in Ca2+ oscillations, pacemaker activity and contractility in smooth muscle. Rat myometrium expresses T-type channels Cav3.1 and 3.2 mRNA (multiple splice variants) in patterns that differ between longitudinal and circular muscle and with stage of pregnancy [36,54].
T-type currents have been observed in human myometrial cells (reviewed in [55]). The relatively specific T-type channel inhibitor mibefadril exhibited effects different from those of nifedipine on human myometrial contractile activity [41] and on spontaneous and OT-stimulated mid-pregnant goat myometrial contractions [56]. In contrast, mibefradil affected rat myometrial cell contractile responses in a complex manner, suggesting effects on both Ca2+ entry and intracellular release [57].
3.3 Signal-regulated (capacitative) channels and other cation channels
Cells have additional mechanisms to allow Ca2+ entry. While, in the past, the focus was on the properties of the currents or stimuli resulting in changes in [Ca2+]i (i.e., capacitative, receptor- or store-operated, ICRAC, or non-selective cation currents, collectively termed SRCE here), as more of the proteins have been cloned, the focus has shifted to the properties of particular protein channel complexes. The TRP (transient receptor potential) channel superfamily is comprised of the TRPM, TRPC, TRPV, TRPA, TRPP, TRPML, and TRPN subfamilies. A number of the proteins in this family have been shown, in many different cell types, to contribute to nonselective cation and Ca2+ entry in response to one or more signals [6,7,58]. To date, TRPC, TRPM and TRPV proteins have been implicated in smooth muscle Ca2+ entry and responses to conditions such as hypoxia and stretch. TRPC channels are reported to influence cell migration, growth and differentiation, depolarization and modulation of membrane potential, slow wave activity and tone. The seven TRPC proteins (TRPC1-7) form separate subgroups that assemble into homo- and heterotetramers; the properties of individual channels are influenced by the nature of the subunits forming these tetramers. TRPC channels respond to a variety of stimuli, including GPCR activation, IP3 receptor activation, DAG and intracellular Ca2+ store depletion resulting from exposure to SERCA inhibitors.
Interest in the role of TRPs in myometrium stems largely from the fact that hormonal signals play a role in stimulating this type of Ca2+ entry, thus raising the possibility that TRP channels may have roles in mediating hormonal effects on uterine contractile activity. From Fig. 1, one can see that several signals resulting from activation of GPCRs linked to PLC (e.g., IP3 and DAG) could trigger SRCE in myometrium. IP3- and thapsigargin (SERCA inhibitor)-mediated ER Ca2+ release in the absence of extracellular Ca2+ has been demonstrated in primary and immortalized human myometrial cells [9,10] (Fig. 2A). Upon addition of extracellular Ca2+, these treatments evoke Ca2+ entry of a magnitude not seen in the absence of either stimulus. This entry is inhibited by SKF96365 and gadolinium, in concentrations relatively specific for SRCE, but not by nifedipine. The diacylglycerol oleyl-acetyl-glycerol (OAG) also evokes calcium entry in myometrial cells independent of the actions of PKC [59]. Therefore, all of the major stimulants of SRCE attributed to TRPC channels have been observed in myometrium. In addition, overexpression of TRPC3 in myometrial cells elicits enhanced Ca2+ entry in response to oxytocin, thapsigargin and OAG [10]. In less direct studies, a verapamil-resistant [Ca2+]i elevation consistent with oxytocin-induced SRCE was noted in human myometrial cells [22]. Oxytocin also elicited a long-lasting nonselective cation current consistent with SRCE in late pregnant rat myometrium [60]. Furthermore, the sustained phase of [Ca2+]i elevation after PGF2α-induced contractions in pregnant guinea pig myometrium was not affected by nifedipine [52], consistent with SRCE.
Figure 2.

A. Both oxytocin and thapsigargin increase PHM1 human myometrial cell [Ca2+]i, measured with Fura-2, in the absence of extracellular Ca2+ (first arrow), presumably by release of Ca2+ from intracellular stores. When extracellular Ca2+ is increased to 1 mM, a second increase in [Ca2+]i is observed in cells treated with oxytocin or thapsigargin (SRCE), but not if cells are not exposed to these agents (not shown). SRCE was inhibited by SKF96365 or gadolinium. Data from [10] with permission. B. Relative expression of TRPCs in human myometrial cells, expressed relative to TRPC4 (HMC: primary non-pregnant human myometrial cells; PHM1 and PHM2: immortalized myometrial cells prepared from myometrial samples from two pregnant women. Modified from data in [63]. C. Expression of myometrial Orai1, Stim1, PMCA isoforms and SK3 K+ channel mRNAs, determined by quantitative RT-PCR in PHM1 cell RNA by the ∆∆Ct method, normalized to HMBS and expressed relative to TRPC1 mRNA [63]. Data represent mean of determinations using two different RNA preparations (J. Phillips and B. Sanborn, unpublished observations).
TRPC mRNA and protein have been detected in rat and human myometrium [9,61–63]. In human myometrium and myometrial cells, the relative abundance of TRPC mRNAs is TRPC1=TRPC4>TRPC6≫TRPC3>TRPC5>TRPC7 [63] (Fig. 2B). TRPC2 is a pseudogene in humans. Interestingly, rat myometrium expresses predominantly hTRPC4 mRNA, although all the other TrpCs except TrpC3 were also detected [62].
Expression of TRPC proteins may be regulated in ways that affect myometrial responsiveness. One study reports no significant differences in TRPC1, TRPC3, TRPC4, TRPC6 or TRPC7 mRNAs between human not-in-labor and in-labor samples but significant increases in TRPC3, TRPC4 and TRPC6 protein concentrations after the onset of labor [64]. In another study, fundal myometrium TRPC4 mRNA decreased between not-in-labor and in-labor samples, with no changes in the other TRPC mRNAs and no change in TRPC1, TRPC3, TRPC4, or TRPC6 membrane protein [63]. Only TRPC4 and TRPC5 mRNA and protein changed during gestation in rat myometrium, decreasing after d13 [62]; no changes in TRPC mRNAs were seen with labor. Clearly, complex relationships exist between TRPC mRNA and protein expression in myometrium that are yet to be fully understood.
To date, understanding of the functional role of TRPs in Ca2+ homeostasis and contraction has come largely from other smooth muscle systems. Endothelial-dependent regulation of vascular tone is altered in TrpC4−/− mice, whereas TrpC6 −/− mice exhibit increased vascular muscle contractility attributable to upregulation of TrpC3 channels in these animals [65,66]. TRPCs have been implicated as mechanosensors linked to PP2B/NFAT signaling pathways responsible for gene activation [67]. In human myometrium, interleukin 1-β increased exclusively TRPC3 protein, suggesting a possible role for this protein in labor [64]. Recently, mechanical stretch of late pregnant human myometrial cells in culture has been found to enhance basal and store-operated Ca2+ entry and inward currents, accompanied by increases in TRPC3 and TRPC4 mRNA and TRPC3 protein [68]. This study provides additional evidence that labor-associated events could alter the expression of TRPCs and enhance SRCE in labor.
Other mechanisms have been proposed for store-operated Ca2+ entry. One involves the Stim1 protein, which acts as an ER Ca2+ sensor and interacts with Orai1, a plasma membrane protein implicated in Ca2+ entry [69,70]. Stim1 is reported to bind to TRPC1, TRPC2 and TRPC4 and to stimulate TRPC1 channel activation [70]. Orai1 interacts with TRPC3 and TRPC6; low concentrations of Orai1 enhance store-operated Ca2+ entry in cells overexpressing these TRPCs, whereas high concentrations inhibit inward current Ca2+ entry, suggesting that Orai1 may act as a regulatory subunit of TRP channels [71]. Stim1 and Orai1 are expressed in human myometrium (Fig. 2C), but their function there remains to be elucidated. An alternative store-operated mechanism involves production of a Ca2+ influx factor, Ca-dependent phospholipase A2 activation, and generation of lysophospholipids that activate store-operated channels [72]; this has not been studied in myometrium.
It is also very likely that, in addition to the channels discussed here, other types of cation entry channels exist in myometrium. Clearly, this is an important area for future investigation.
3.4 Calcium pumps, exchangers and intracellular release channels
PMCAs, SERCAs and NCXs
The plasma membrane Ca2+ ATPase (PMCA) extrudes Ca2+ from the cell, thus lowering [Ca2+]i (Fig. 1). PMCA has been detected in human myometrium and expression is reported to increase in labor [73]. PMCA1b, 4a and 4b mRNA/or protein are detectable in rat uterus [74]; there is significant expression of PMCA1 and PMCA4b but not PMCA4a mRNA in human myometrial cells (Fig. 2C). Both PMCA4 and NCX have been implicated in attenuation of depolarization-induced [Ca2+]i transients and associated membrane currents in mouse and rat myometrial cells [75,76].
The ER Ca2+ ATPase (SERCA) is of critical importance in refilling the ER Ca2+ store (Fig. 1). SERCA isoforms 2a and 2b have been detected in myometrium; the refilling of the ER Ca2+ store is essential for sustained myometrial contractions and plays an important role in modulating myometrial intracellular [Ca2+]i (see Wray, 2007, this issue).
Ryanodine receptors and IP3Rs
Human myometrium expresses all 3 RyR mRNAs, with RyR3 being the most abundant; expression is regulated during pregnancy [77,78] (see also Wray 2007, this issue). Nonetheless, there are only variable reports of effects of ryanodine or caffeine on myometrial [Ca2+]i and no evidence of Ca2+ sparks that would be indicative of Ca2+ -induced Ca2+ release, suggesting an absence of productive coupling. However, RyR may be important for establishing localized Ca2+ elevations in myometrial cells (Wray, 2007, this issue). Human myometrium expresses IP3R1 and rat myometrium expresses IP3R1-3, which presumably mediate IP3-stimulated Ca2+ release from the ER [77,78].
4. Crosstalk between contractant and relaxant pathways
There is significant potential for crosstalk between contractant and relaxant signaling pathways in the systems described here. This crosstalk may regulate the effectiveness of a given pathway as well as the subcellular localization of the signaling components themselves. This may be an important way in which hormones regulate and fine tune the control of contractile activity at various stages in gestation.
4.1 Pathway crosstalk at the level of GPCRs, G proteins and effectors
Both PKC, stimulated by contractant pathways, and PKA and PKG, stimulated by relaxant pathways, exert effects on components of their own and other signaling pathways. PKA and PKC phosphorylation can affect GPCR desensitization and recycling directly or indirectly. G-proteins, RGS proteins and adenylyl cyclase isoforms are also phosphorylation targets (López Bernal, 2007, this issue).
Further downstream in the Gαq pathway, PLCβ1 is phosphorylated and inhibited by PKC but not PKA, PLCβ2 is phosphorylated and inhibited by PKA, and PLCβ3 is phosphorylated and inhibited by PKA, PKC and PKG (reviewed in [2,5]). Notably, in addition to regulation by negative crosstalk from the PKA and PKG pathways, the effect of PKC on PLCβ constitutes potential negative feedback by the OTR pathway itself. Both PKCα and PKCδ have been identified as downstream components of oxytocin receptor activation in pregnant human myometrium [79]. In rat myometrium, PLCβ1, PLCβ2 and PLCβ3 increase at term [80] whereas PLCβ2 was not detected in human myometrial cells [81]. Prelimnary data indicate that phosphorylation of PLCβ3 on Ser1105 occurs in intact myometrial cells in response to both activation of PKA and PKC pathways (Zhong and Sanborn, unpublished observations), raising the possibility that rapid regulation of hormonal signaling responses impacts temporal fluctuations in [Ca2+i] and influences the pattern of contractile activity. A recent report confirms the importance of the PKA inhibitory pathway in the regulation of myometrial PLC and provides evidence for a Gβγ-mediated enhancement of this effect by oxytocin in pregnancy [15].
Importantly, cAMP and cGMP are less potent inhibitors of contractile pathways at the end of gestation. There are multiple reasons for this, ranging from changes in the ability to generate and sustain the signals to changes in pathway component expression and in the sublocalization of the signaling components. These are discussed in more detail elsewhere (England, 2007, this issue; López Bernal, 2007, this issue).
4.2 Pathway crosstalk at the level of ion channels and calcium pumps
Calcium channels
Cav1.2a and 1.2b subunits are phosphorylated on comparable Ser residues by CaMKII, PKA and PKC [82]. PKA-mediated phosphorylation shifts the Cav1 channel into a gating mode where its activation occurs under physiological conditions. Smooth muscle Cav1.2b currents are inhibited by 8-Br-cGMP, while PKC has variable effects on channel activity, ranging from activation to inhibition [32]. The divergent effects may depend on the type of PKC, the specific channel splice variant expressed or phosphorylation of other subunits. PKC elicited a stimulatory effect on L-type Ca2+ currents in pregnant rat myometrium [83].
In other systems, the activity of T-type channels, particularly Cav3.2, which predominates in myometrium, is modulated by Gβγ, PKA, PKG, PKC, CaMKII and tyrosine kinases [84]. Whether this type of regulation has functional significance in myometrium remains to be determined. The role of covalent modification in TRP channel function is not fully understood at present.
Other channels
ATP-dependent, BKCa, small conductance (SK), voltage-gated (Kv) and 2-pore K+ channels that promote membrane repolarization are also regulated in part by the action of kinases stimulated in Gαs, Gαq and Gβγ signaling pathways. All of these types of K+ channels are expressed in myometrium and, in many cases, regulation is dependent on stage of pregnancy (see England, 2007, this issue). Ca2+-activated Cl− channels are stimulated by GPCR activation but inactivated by CaMKII; Ca2+-activated chloride inward currents have been observed in myometrium (England, 2007, this issue).
Calcium pumps and exchangers
Potential phosphorylation sites for PKC and PKA have been identified in all PMCAs. Phosphorylation of PMCA1b by PKA increased both affinity for Ca2+ and catalytic activity, whereas phosphorylation by PKC increased maximal activity. SERCA activity in smooth muscle can be enhanced by PKA-mediated inhibition of the endogenous inhibitor phospholamban [85]. Whether similar effects pertain and have functional significance in the myometrium remains to be determined. NCX has been reported to be phosphorylated in smooth muscle, but there is no information on this regulation in myometrium.
IP3 and ryanodine receptors
IP3R1 is phosphorylated by PKA, PKG, PKC, CaMKII, ERK and tyrosine kinases with variable effects on Ca2+ release, but the effect of these signaling pathways has not been studied extensively in myometrium. In myometrium and other smooth muscle, IP3Rs are associated with a PKG substrate (IRAG); overexpression of IRAG suppresses ER Ca2+ release, consistent with a relaxation mechanism [86]. While RyR2 is phosphorylated by PKA, there is little information about phosphorylation of RyR3, which predominates in myometrium.
4.3 Importance of subcellular localization
Many events involved in signal transduction are dependent on subcellular co-localization of the components into microdomains and “signalplexes”. Some GPCRs, G-proteins, effectors, PKA and PKC, PMCA and PIP2 have been localized to membrane lipid microdomains [87,88]. Some TRPC proteins associate with caveolin-1, lipid rafts, Gαq/11, PLCs, IP3R and scaffolding proteins [58,89,90]. L-type Ca channels exist in and are stimulated by a complex containing the β2-adrenergic receptor, a G-protein, adenylyl cyclase, PKA and protein phosphatase 2A [91]. PLCβ3 interacts with E3ARP, a protein that enhances GPCR stimulation of PLC and with other scaffolding proteins [92]. The plasma membrane PKA anchoring protein AKAP79 binds cell membranes via interaction with PIP2 and associates with GPCRs and other scaffolding proteins [93]. AKAP79 also binds PKC and PP2B, thus facilitating localized phosphorylation/dephosphorylation cycles. Besides influencing subcellular localization, PIP2 can also influence activity of a number of signaling components affecting [Ca2+]i [94]. PMCA, NCX, KATP and other inward rectifier channels have been reported to be activated by PIP2, whereas TRP and IP3R are inhibited [58,94].
Although estrogen has been reported to down-regulate caveolin and caveolae in rat myometrium, caveolin concentrations increase in late pregnancy [95]. Caveolin may help coordinate myometrial signaling pathways, as evidenced by the observation that a caveolin-1 peptide inhibits carbachol-stimulated myometrial contractions [96]. OTXR can exist in and outside of membrane microdomains, and signaling to different pathways is determined by location [97,98]. The inhibitory effect of PKA on PI turnover in myometrium is AKAP-dependent [99]. The PKA/plasma membrane AKAP79 association decreases markedly near term in rat myometrium, coincident with a decrease in inhibitory effects of cAMP on contractant-stimulated PI turnover that occurs in the membrane [100]. Effects are significant but less dramatic in human myometrium near or at term [101] (see also López Bernal, 2007, this issue).
The presence of closely associated signaling complexes supports the concept of the generation, in response to stimuli, of localized changes in signals such as Ca2+, cyclic nucleotides, PIP2 and IP3 in the myometrium that can rapidly influence effectors in the immediate environment. The challenge in understanding the function of such signaling is to demonstrate that it makes an impact on myometrial physiology.
5. Conclusions
While a number of the pathway components of hormonal signal transduction relating to myometrial Ca2+ homeostasis have been identified and some of the respective roles defined, there are still many undefined components. In addition, the challenge remains understanding both the regulation of the assembly and activity of these signaling pathways, the interrelationship of the complex crosstalk and feedback loops that integrate signals over time, and the relation of these influences to the pattern and control of uterine contractile activity. Only in doing so can we understand fully how uterine contractions are regulated and better determine how to control them.
Acknowledgments
The assistance of Jennifer Phillips in the preparation of this manuscript is gratefully acknowledged. Supported in part by HD09618 and HD38970.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Challis J. Mechanism of parturition and preterm labor. Obstet Gynecol Surv. 2000;55:650–660. doi: 10.1097/00006254-200010000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Sanborn BM. Hormones and calcium: mechanisms controlling uterine smooth muscle contractile activity. The Litchfield Lecture Exp Physiol. 2001;86:223–237. doi: 10.1113/eph8602179. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Bernal A. Mechanisms of labour - biochemical aspects. BJOG. 2003;110:39–45. doi: 10.1046/j.1471-0528.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 4.Myatt L, Lye S. Expression, localization and function of prostaglandin receptors in myometrium. Prostaglandins Leukot Essent Fatty Acids. 2004;70:137–148. doi: 10.1016/j.plefa.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Sanborn BM, Ku CY, Shlykov SG, Babich LG. Molecular signaling through G protein coupled receptors and the control of intracellular calcium in myometrium. J Soc Gynecol Investig. 2005;12:479–487. doi: 10.1016/j.jsgi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Putney JW., Jr Physiological mechanisms of TRPC activation. Pflugers Arch. 2005;451:29–34. doi: 10.1007/s00424-005-1416-4. [DOI] [PubMed] [Google Scholar]
- 7.Minke B. TRP channels and Ca2+ signalling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monga M, Campbell DF, Sanborn BM. Oxytocin-stimulated capacitative calcium entry in human myometrial cells. Am J Obstet Gynecol. 1999;181:424–429. doi: 10.1016/s0002-9378(99)70573-9. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Gupta A, Shlykov SG, Corrigan R, Tsujimoto S, Sanborn BM. Multiple Trp isoforms implicated in capacitative calcium entry are expressed in human pregnant myometrium and myometrial cells. Biol Reprod. 2002;67:988–994. doi: 10.1095/biolreprod.102.004119. [DOI] [PubMed] [Google Scholar]
- 10.Shlykov SG, Yang M, Alcorn JL, Sanborn BM. Capacitative cation entry in human myometrial cells and augmentation by hTrpC3 overexpression. Biol Reprod. 2003;69:647–655. doi: 10.1095/biolreprod.103.015396. [DOI] [PubMed] [Google Scholar]
- 11.Thompson M, Barata da Silva H, Zielinska W, White T, Bailey J, Lund F, et al. Role of CD38 in myometrial Ca2+ transients: modulation by progesterone. Am J Physiol Endocrinol Metab. 2004;287:E1142–E1148. doi: 10.1152/ajpendo.00122.2004. [DOI] [PubMed] [Google Scholar]
- 12.Dogan S, Deshpande D, White T, Walseth T, Kannan M. Regulation of CD 38 expression and function by steroid hormones in myometrium. Mol Cell Endocrinol. 2006;246:101–106. doi: 10.1016/j.mce.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Zhong M, Yang M, Sanborn BM. Extracellular signal-regulated kinase 1/2 activation by myometrial oxytocin receptor involves Gα(q)Gβγ and epidermal growth factor receptor tyrosine kinase activation. Endocrinology. 2003;144:2947–2956. doi: 10.1210/en.2002-221039. [DOI] [PubMed] [Google Scholar]
- 14.Hoare S, Copland JA, Strakova Z, Ives K, Jeng Y-J, Hellmich MR, et al. The proximal portion of the COOH terminus of the oxytocin receptor is required for coupling to Gq, but not Gi. Independent mechanisms for elevating intracellular calcium concentrations from intracellular stores. J Biol Chem. 1999;274:28682–28689. doi: 10.1074/jbc.274.40.28682. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Lutz S, Steffens F, Korth M, Wieland T. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: implications for myometrial contractility. Mol Endocrinol. 2007;21:740–752. doi: 10.1210/me.2006-0220. [DOI] [PubMed] [Google Scholar]
- 16.Rimoldi V, Reversi A, Taverna E, Rosa P, Francolini M, Cassoni P, et al. Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene. 2003;22:6054–6060. doi: 10.1038/sj.onc.1206612. [DOI] [PubMed] [Google Scholar]
- 17.Park E, Echetebu C, Soloff S, Soloff M. Oxytocin stimulation of RGS2 mRNA expression in cultured human myometrial cells. Am J Physiol Endocrinol Metab. 2002;282:E580–E584. doi: 10.1152/ajpendo.00437.2001. [DOI] [PubMed] [Google Scholar]
- 18.Franczak A, Woclawek-Potocka I, Oponowicz A, Kurowicka B, Kotwica G. Oxytocin stimulates prostaglandin F2alpha secretion and prostaglandin F synthase protein expression in porcine myometrial tissue. Reprod Biol. 2004;4:177–184. [PubMed] [Google Scholar]
- 19.Oldenhof AD, Shynlova OP, Liu M, Langille BL, Lye SJ. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C1530–C1539. doi: 10.1152/ajpcell.00607.2001. [DOI] [PubMed] [Google Scholar]
- 20.Devost D, Girotti M, Carrier M, Russo C, Zingg H. Oxytocin induces dephosphorylation of eukaryotic elongation factor 2 in human myometrial cells. Endocrinology. 2005;146:2265–2270. doi: 10.1210/en.2004-1428. [DOI] [PubMed] [Google Scholar]
- 21.Luckas JJ, Taggart MJ, Wray S. Intracellular calcium stores and agonist-induced contractions in isolated human myometrium. Am J Obstet Gynecol. 1999;181:468–476. doi: 10.1016/s0002-9378(99)70580-6. [DOI] [PubMed] [Google Scholar]
- 22.Fu X, Liu Y, Ciray N, Olovsson M, Ulmsten U, Gylfe E. Oxytocin-induced oscillations of cytoplasmic Ca2+ in human myometrial cells. Acta Obstet Gynecol Scand. 2000;79:174–179. [PubMed] [Google Scholar]
- 23.Molnar M, Hertelendy F. Signal transduction in rat myometrial cells: comparison of the actions of endothelin-1, oxytocin and prostaglandin F2 alpha. Eur J Endocrinol. 1995;133:467–474. doi: 10.1530/eje.0.1330467. [DOI] [PubMed] [Google Scholar]
- 24.Ohmichi M, Koike KKA, Masuhara K, Ikegami H, Ikebuchi Y, Kanzaki T, et al. Role of mitogen-activated protein kinase pathway in prostaglandin F2a-induced rat puerperal uterine contraction. Endocrinology. 1997;138:3103–3111. doi: 10.1210/endo.138.8.5305. [DOI] [PubMed] [Google Scholar]
- 25.Anwer K, Monga M, Sanborn BM. Epidermal growth factor increases phosphoinositide turnover and intracellular free calcium in an immortalized human myometrial cell line independent of the arachidonic acid metabolic pathway. Am J Obstet Gynecol. 1996;174:676–681. doi: 10.1016/s0002-9378(96)70449-0. [DOI] [PubMed] [Google Scholar]
- 26.Barhoumi R, Awooda I, Mouneimne Y, Safe S, Burghardt R. Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol Lett. 2006;165:133–141. doi: 10.1016/j.toxlet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Wu J, Wang Z. Akt binds to and phosphorylates phospholipase C-gamma1 in response to epidermal growth factor. Mol Biol Cell. 2006;17:2267–2277. doi: 10.1091/mbc.E05-10-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robin P, Boulven I, Bole-Feysot C, Tanfin Z, Leiber D. Contribution of PKC-dependent and -independent processes in temporal ERK regulation by ET-1, PDGF, and EGF in rat myometrial cells. Am J Physiol Cell Physiol. 2004;286:C798–C806. doi: 10.1152/ajpcell.00465.2003. [DOI] [PubMed] [Google Scholar]
- 29.Kornyei J, Li X, Lei Z, Rao C. Analysis of epidermal growth factor action in human myometrial smooth muscle cells. J Endocrinol. 1995;146:261–270. doi: 10.1677/joe.0.1460261. [DOI] [PubMed] [Google Scholar]
- 30.Leiber D, Banno Y, Tanfin Z. Exogenous sphingosine 1-phosphate and sphingosine kinase activated by endothelin-1 induced myometrial contraction through differential mechanisms. Am J Physiol Cell Physiol. 2007;292:C240–C250. doi: 10.1152/ajpcell.00023.2006. [DOI] [PubMed] [Google Scholar]
- 31.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38:397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Keef K, Hume J, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–C1746. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 33.Mershon J, Mikala G, Schwartz A. Changes in the expression of the L-type voltage-dependent calcium channel during pregnancy and parturition in the rat. Biol Reprod. 1994;51:993–999. doi: 10.1095/biolreprod51.5.993. [DOI] [PubMed] [Google Scholar]
- 34.Tezuka N, Ali M, Chwalisz IK, Garfield RE. Changes in transcripts encoding calcium channel subunits of rat myometrium during pregnancy. Am J Physiol. 1995;269:C1008–C1017. doi: 10.1152/ajpcell.1995.269.4.C1008. [DOI] [PubMed] [Google Scholar]
- 35.Collins P, Moore J, Lundgren D, Choobineh E, Chang S, Chang A. Gestational changes in uterine L-type calcium channel function and expression in guinea pig. Biol Reprod. 2000;63:1262–1270. doi: 10.1095/biolreprod63.5.1262. [DOI] [PubMed] [Google Scholar]
- 36.Ohkubo T, Kawarabayashi T, Inoue Y, Kitamura K. Differential expression of L- and T-type calcium channels between longitudinal and circular muscles of the rat myometrium during pregnancy. Gynecol Obstet Invest. 2005;59:80–85. doi: 10.1159/000082333. [DOI] [PubMed] [Google Scholar]
- 37.Helguera G, Olcese R, Song M, Toro L, Stefani E. Tissue-specific regulation of Ca2+ channel protein expression by sex hormones. Biochim Biophys Acta. 2002:59–66. doi: 10.1016/s0304-4165(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 38.Poli E, Rusagara J, Coruzzi G, Bertaccini G. Dihydropyridine receptors in the pregnant human uterus in vitro. Pharmacology. 1989;39:309–316. doi: 10.1159/000138614. [DOI] [PubMed] [Google Scholar]
- 39.Ulmsten U, Andersson K, Forman A. Relaxing effects of Nifedipine on the nonpregnant human uterus in vitro and in vivo. Obstet Gynecol. 1978;52:436–441. [PubMed] [Google Scholar]
- 40.Forman A, Andersson K, Maigaard S. Effects of calcium channel blockers on the female genital tract. Acta Pharmacol Toxicol (Copenh) 1986;58:183–192. doi: 10.1111/j.1600-0773.1986.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 41.Young R, Zhang P. Inhibition of in vitro contractions of human myometrium by mibefradil, a T-type calcium channel blocker: support for a model using excitation-contraction coupling, and autocrine and paracrine signaling mechanisms. J Soc Gynecol Investig. 2005;12:e7–e12. doi: 10.1016/j.jsgi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Phillippe M, Basa A. Effects of sodium and calcium channel blockade on cytosolic calcium oscillations and phasic contractions of myometrial tissue. J Soc Gynecol Investig. 1997;4:72–77. [PubMed] [Google Scholar]
- 43.Longo M, Jain V, Vedernikov Y, Hankins G, Garfield R, Saade G. Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Hum Reprod. 2003;9:159–164. doi: 10.1093/molehr/gag023. [DOI] [PubMed] [Google Scholar]
- 44.Maiggard S, Forman A, Brogaard-Hansen K, Andersson K. Inhibitory effects of nitrendipine on myometrial and vascular smooth muscle in human pregnant uterus and placenta. Acta Pharmacol Toxicol (Copenh) 1986;59:1–10. doi: 10.1111/j.1600-0773.1986.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 45.Arnaudeau S, Lepretre N, Mironneau J. Oxytocin mobilizes calcium from a unique heparin-sensitive and thapsigargin-sensitive store in single myometrial cells from pregnant rats. Pflugers Arch. 1994;4284287:51–59. doi: 10.1007/BF00374751. [DOI] [PubMed] [Google Scholar]
- 46.Holda JR, Oberti C, Perez-Reyes E, Blatter LA. Characterization of an oxytocin-induced rise in [Ca2+]i in single human myometrium smooth muscle cells. Cell Calcium. 1996;20:43–51. doi: 10.1016/s0143-4160(96)90049-4. [DOI] [PubMed] [Google Scholar]
- 47.Maka F, Breuiller-Fouche M, Geny B, Ferre F. Prostaglandin F2 alpha stimulates inositol phosphate production in human pregnant myometrium. Prostaglandins. 1993;45:269–283. doi: 10.1016/0090-6980(93)90052-9. [DOI] [PubMed] [Google Scholar]
- 48.Mironneau J. Effects of oxytocin on ionic currents underlying rhythmic activity and contraction in uterine smooth muscle. Pflugers Arch. 1976;363:113–118. doi: 10.1007/BF01062278. [DOI] [PubMed] [Google Scholar]
- 49.Inoue Y, Shimamura K, Sperelakis N. Oxytocin actions on voltage--dependent ionic channels in pregnant rat uterine smooth muscle cells. Can J Physiol Pharmacol. 1992;70:1597–1603. doi: 10.1139/y92-229. [DOI] [PubMed] [Google Scholar]
- 50.Callaghan B, Zhong J, Keef K. Signalling pathway underlying stimulation of L-type Ca2+ channels in rabbit portal vein myocytes by recombinant Gβ{γ} subunits. Am J Physiol Heart Circ Physiol. 2006;291:H2541–H2546. doi: 10.1152/ajpheart.00420.2006. [DOI] [PubMed] [Google Scholar]
- 51.Arnaudeau S, Lepretre N, Mironneau J. Oxytocin mobilizes calcium from a unique heparin-sensitive and thapsigargin-sensitive store in single myometrial cells from pregnant rats. Pflugers Arch. 1994;428:51–59. doi: 10.1007/BF00374751. [DOI] [PubMed] [Google Scholar]
- 52.Coleman HA, Hart JD, Tonta MA, Parkington HC. Changes in the mechanisms involved in uterine contractions during pregnancy in guinea-pigs. J Physiol (Lond) 2000;523:785–798. doi: 10.1111/j.1469-7793.2000.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parkington HC, Tonta MA, Brennecke SP, Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol. 1999;181:1445–1451. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 54.Ohkubo T, Inoue Y, Kawarabayashi T, Kitamura K. Identification and electrophysiological characteristics of isoforms of T-type calcium channel Ca(v)3.2 expressed in pregnant human uterus. Cell Physiol Biochem. 2005;16:245–254. doi: 10.1159/000089850. [DOI] [PubMed] [Google Scholar]
- 55.Sanborn BM. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig. 2000;7:4–11. doi: 10.1016/s1071-5576(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 56.Mandi G, Sarkar S, Mishra S, Raviprakash V. Effects of calcium channel blocker, mibefradil, and potassium channel opener, pinacidil, on the contractile response of mid-pregnant goat myometrium. Indian J Exp Biol. 2005;43:795–801. [PubMed] [Google Scholar]
- 57.Asokan K, Sarkar S, Mishra S, Raviprakash V. Effects of mibefradil on uterine contractility. Eur J Pharmacol. 2002;455:65–71. doi: 10.1016/s0014-2999(02)02487-1. [DOI] [PubMed] [Google Scholar]
- 58.Ambudkar I. Ca2+ signaling microdomains: platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25–32. doi: 10.1016/j.tips.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Shlykov SG, Sanborn BM. Stimulation of interacellular Ca 2+ oscillations by diacylglycerol in human myometrial cells. Cell Calcium. 2004;36:157–164. doi: 10.1016/j.ceca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Shimamura K, Kusaka M, Sperelakis N. Oxytocin induces an inward current in pregnant rat myometrial cells. Can J Physiol Pharmacol. 1994;72:759–763. doi: 10.1139/y94-108. [DOI] [PubMed] [Google Scholar]
- 61.Dalrymple A, Slater DM, Beech D, Poston L, Tribe RM. Molecular identification and localization of Trp homologues, putative calcium channels, in pregnant human uterus. Mol Hum Reprod. 2002;8:946–951. doi: 10.1093/molehr/8.10.946. [DOI] [PubMed] [Google Scholar]
- 62.Babich LG, Ku CY, Young HW, Huang H, Blackburn MR, Sanborn BM. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- 63.Ku C, Babich L, Word R, Zhong M, Ulloa A, Monga M, et al. Expression of transient receptor channel proteins in human fundal myometrium in pregnancy. J Soc Gynecol Investig. 2006;13:217–225. doi: 10.1016/j.jsgi.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Dalrymple A, Slater DM, Poston L, Tribe RM. Physiological induction of transient receptor potential canonical proteins, calcium entry channels, in human myometrium: influence of pregnancy, labor, and interleukin-1 β. J Clin Endocrinol Metab. 2004;89:1291–1300. doi: 10.1210/jc.2003-031428. [DOI] [PubMed] [Google Scholar]
- 65.Freichel M, Philipp S, Cavalie A, Flockerzi V. TRPC4 and TRPC4-deficient mice. 2004:189–203. [PubMed] [Google Scholar]
- 66.Dietrich A, Mederos Y, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bush E, Hood D, Papst P, Chapo J, Minobe W, Bristor M, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 68.Dalrymple A, Mahn K, Poston L, Songu-Mize E, Tribe R. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13:31–39. doi: 10.1093/molehr/gal110. [DOI] [PubMed] [Google Scholar]
- 69.Smyth J, DeHaven W, Jones B, Mercer J, Trebak M, Vazquez G, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 70.Huang G, Zeng W, Kim J, Yuan J, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 71.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong D, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Csutora P, Zarayskiv V, Peter K, Monje F, Smani T, Zakharow S, et al. Activation mechanism for CRAC current and store-operated Ca2+ entry: calcium influx factor and Ca2+-independent phospholipase A2beta-mediated pathway. J Biol Chem. 2006;281:34926–34935. doi: 10.1074/jbc.M606504200. [DOI] [PubMed] [Google Scholar]
- 73.Tribe RM, Moriarty P, Poston L. Calcium homeostatic pathways change with gestation in human myometrium. Biol Reprod. 2000;63:748–755. doi: 10.1095/biolreprod63.3.748. [DOI] [PubMed] [Google Scholar]
- 74.Penniston J, Enyedi A. Modulation of the plasma membrane Ca2+ pump. J Membr Biol. 1998;165:101–109. doi: 10.1007/s002329900424. [DOI] [PubMed] [Google Scholar]
- 75.Matthew A, Kupittayanant S, Burdyga T, Wray S. Characterization of contractile activity and intracellular Ca2+ signalling in mouse myometrium. J Soc Gynecol Investig. 2004;11:207–212. doi: 10.1016/j.jsgi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 76.Shmigol A, Eisner D, Wray S. Properties of voltage-activated [Ca2+]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol. 1998;511:803–811. doi: 10.1111/j.1469-7793.1998.803bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin C, Chapman KE, Thornton S, Ashley RH. Changes in the expression of myometrial ryanodine receptor mRNAs during human pregnancy. Biochim Biophys Acta. 1999;1451:343–352. doi: 10.1016/s0167-4889(99)00104-4. [DOI] [PubMed] [Google Scholar]
- 78.Mesonero JE, Tanfin Z, Hilly M, Colosetti P, Mauger JP, Harbon S. Differential expression of inositol 1,4,5-trisphosphate receptor types 1, 2, and 3 in rat myometrium and endometrium during gestation. Biol Reprod. 2000;63:532–537. doi: 10.1095/biolreprod63.2.532. [DOI] [PubMed] [Google Scholar]
- 79.Bermeo M, Fomin V, Ventolini G, Gibbs S, McKenna D, Hurd W. Magnesium sulfate induces translocation of protein kinase C isoenzymes alpha and delta in myometrial cells from pregnant women. Am J Obstet Gynecol. 2004;191:1051–1052. doi: 10.1016/j.ajog.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Mhaouty-Kodja S, Houdeau E, LeGrand C. Regulation of myometrial phospholipase C system and uterine contraction by beta-adrenergic receptors in midpregnant rat. Biol Reprod. 2004;70:570–576. doi: 10.1095/biolreprod.103.021535. [DOI] [PubMed] [Google Scholar]
- 81.Dodge KL, Sanborn BM. Evidence for inhibition by protein kinase A of receptor/G alpha(q)/phospholipase C (PLC) coupling by a mechanism not involving PLCbeta2. Endocrinology. 1998;139:2265–2271. doi: 10.1210/endo.139.5.5963. [DOI] [PubMed] [Google Scholar]
- 82.Catterall W. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 83.Kusaka M, Sperelakis N. Stimulation of Ca2+ current by phorbol esters in rat myometrial cells is dependent on intracellular Ca2+ concentration. Reprod Fertil Dev. 1996;8:1147–1152. doi: 10.1071/rd9961147. [DOI] [PubMed] [Google Scholar]
- 84.Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 85.Paul RJ, Shull GE, Kranias EG. The sarcoplasmic reticulum and smooth muscle function: evidence from transgenic mice. Novartis Found Symp. 2002;246:228–238. doi: 10.1002/0470853050.ch17. [DOI] [PubMed] [Google Scholar]
- 86.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase lbeta. Nature. 200;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- 87.Ostrom RS, Post SR, Insel PA. Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving Gx1. J Pharm Exp Therapeutics. 2000;294:407–412. [PubMed] [Google Scholar]
- 88.Daniel E, El-Yazbi A, Cho W. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med. 2006;10:529–544. doi: 10.1111/j.1582-4934.2006.tb00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwiatek A, Minshall R, Cool D, Skidgel R, Malik A, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 91.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A b2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav 12. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 92.Suh P, Hwang J, Ryu S, Donowitz M, Kim J. The roles of PDZ-containing proteins in PLC-b-mediated signaling. Biochem Biophy Res Comm. 2001;288:1–7. doi: 10.1006/bbrc.2001.5710. [DOI] [PubMed] [Google Scholar]
- 93.Gardner L, Naren A, Bahouth S. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2006;282:5085–5099. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- 94.Hilgemann D, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001. 2001:RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 95.Turi A, Kiss AL, Mullner N. Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int. 2001;25:785–794. doi: 10.1006/cbir.2001.0769. [DOI] [PubMed] [Google Scholar]
- 96.Taggart MJ, Leavis P, Feron O, Morgan KG. Inhibition of PKCalpha and rhoA translocation in differentiated smooth muscle by a caveolin scaffolding domain peptide. Exp Cell Res. 2000;258:72–81. doi: 10.1006/excr.2000.4891. [DOI] [PubMed] [Google Scholar]
- 97.Gimpl G, Fahrenholz F. Human oxytocin receptors in cholesterol-rich vs. cholesterol-poor microdomains of the plasma membrane. Eur J Biochem. 2000;267:2483–2497. doi: 10.1046/j.1432-1327.2000.01280.x. [DOI] [PubMed] [Google Scholar]
- 98.Reversi A, Rimoldi V, Brambillasca S, Chini B. Effects of cholesterol manipulation on the signaling of the human oxytocin receptor. Am J Physiol Requl Integr Comp Physiol. 2006;291:R861–R869. doi: 10.1152/ajpregu.00333.2006. [DOI] [PubMed] [Google Scholar]
- 99.Dodge KL, Carr DW, Sanborn BM. Protein kinase A anchoring to the myometrial plasma membrane is required for cyclic adenosine 3′,5′-monophosphate regulation of phosphatidylinositide turnover. Endocrinology. 1999;140:5165–5170. doi: 10.1210/endo.140.11.7133. [DOI] [PubMed] [Google Scholar]
- 100.Dodge KL, Carr DW, Yue C, Sanborn BM. A role for AKAP (A kinase anchoring protein) scaffolding in the loss of a cyclic adenosine 3′,5′-monophosphate inhibitory response in late pregnant rat myometrium. Mol Endocrinol. 1999;13:1977–1987. doi: 10.1210/mend.13.12.0378. [DOI] [PubMed] [Google Scholar]
- 101.Ku CY, Word A, Sanborn BM. Differential expression of protein kinase A, AKAP79 and PP2B in pregnant human myometrial membranes prior to and during labor. J Soc Gynecol Investig. 2005;12:1509–1515. doi: 10.1016/j.jsgi.2005.04.002. [DOI] [PubMed] [Google Scholar]


