Abstract
Interferon-τ is a major product of ovine and bovine conceptuses during the period before the trophoblast makes firm attachment to the uterine wall and begins to form a placenta. Its primary function is in preventing a return to ovarian cyclicity and hence ensuring the pregnancy continues, although it undoubtedly has other roles in ensuring receptivity of the maternal endometrium. Despite having properties similar to those of other Type 1, IFN-τ is not virally inducible and functions in a constitutive process unrelated to pathogenesis. The genes for IFN-τ (IFNT), which are confined to ruminant ungulate species, would appear to be the most recently evolved mammalian Type 1 gene family and are primarily under the transcriptional control of Ets2 and signal transduction pathways that target that transcription factor. The IFNT provide an illustration of how a gene control region can be commandeered and then refined to provide a radically changed pattern of expression.
Keywords: bovine, evolution, interferon, ovine, transcriptional control, trophoblast
Introduction
My entry into the field of interferon research was quite by chance and brought about by the unexpected discovery that the factor responsible for “rescuing” the corpus luteum (CL) during early pregnancy in sheep and cattle was a Type 1 interferon (IFN) (1-3). The CL is the normally transitory structure that develops on the ovary from the ruptured ovarian follicle following release of the oocyte and would normally regress if that oocyte failed to be fertilized and a pregnancy did not ensue. Its primary function is to produce the steroid hormone, progesterone. In most eutherian mammals, the functional lifespan of the CL must be extended during a pregnancy to provide continued production of progesterone, a steroid hormone that acts on the mucosal lining of the uterus (endometrium) to provide an environment in which the conceptus (defined here as embryo plus its surrounding membranes) can continue to develop. Therefore, the prevention of luteal regression is crucial if a pregnancy is to succeed and depends upon a biochemical signal that originates from the conceptus. In the case of cattle and sheep and related ruminants, the active factor is now known as IFN-τ, which acts locally on the maternal uterine endometrium to prevent the release of the luteolytic factor, prostaglandin F2α, at the end of the ovarian cycle when the CL is poised on the point of regression. IFN-τ probably also induces local changes in the maternal uterus to provide a local environment friendly to subsequent conceptus development. In this short paper, I describe the discovery of IFN-τ, how the genes (IFNT) encoding these IFN appear to have evolved, and the nature of the cis-regulatory control elements on the IFNT responsible for their unique pattern of expression. In closing, I speculate that although the IFN-τ would appear to provide a recently evolved mechanism for conceptus-maternal signaling, there may be analogies in other mammalian groupings, including primates.
Background
By the late 1970s, it had become clear that there was no common mechanism to explain how CL rescue occurred in different species (4, 5). In primates, where the conceptus implants into the uterine wall within a day or two of entering the uterus, a chorionic gonadotrophin (hCG in the case of the human) is secreted by the invading trophoblast, gains access to the maternal blood supply, and provides direct luteotrophic support to the CL. The situation in non-primate species is quite different. No species of mammal outside the primate order, for example, appears to even possess the gene for the β-subunit of CG. In addition, attachment of the conceptus to the uterine wall in cattle, sheep and related pecoran ruminants does not occur until the third week of pregnancy, and implantation, when it occurs, is superficial. Surprisingly, the prospective mother “knows” she is pregnant and her CL is already programmed for extension well before these placentation events are initiated. By 1980, it had become clear that the active factor responsible for CL rescue and extension of the ovarian cycle was a protein produced by the conceptus for a few days prior to when the conceptus attaches to the uterine wall, and only effective if it were introduced into the uterine lumen rather than directly into the ewe’s blood stream (4, 6). At the University of Florida, my colleagues, Fuller Bazer and Jim Godkin, and I reasoned that the protein factor was probably, therefore, secreted and produced transiently during pregnancy. Advantage was taken of the fact that it is possible to flush conceptuses from the surgically exposed uteri of early-pregnant ewes, and then to culture them as intact, whole structures. Accordingly, conceptuses were cultured under serum-free conditions in presence of a radioactive amino acid for about 24 hours, and proteins released into the medium analyzed by two-dimensional PAGE. These experiments immediately revealed that the major secretory product released by the unattached sheep conceptus was a protein of molecular weight approximately 18,000, which consisted of several forms differing slightly in isoelectric point (7). The production of this protein, which was maximal at about day 15, became undetectable after the end of the third week of pregnancy. We were able to purify this conceptus product relatively easily, show that it could be introduced into the uteri of non-pregnant ewes and cause a temporary extension of CL function, and, after raising a specific antiserum, clone its cDNA from a cDNA expression library prepared from polyadenylated conceptus RNA (reviewed in (8)). The nucleotide sequence of this cDNA revealed that it encoded a protein that was likely to be a Type 1 IFN. A parallel series of experiments with cattle embryos showed that a similar IFN-like protein was responsible for maternal recognition of pregnancy in cattle. These IFN eventually became recognized as a new family of Type 1 IFN, known as the IFN-τ (see (8, 9)).
Relationship of IFN-τ to other type 1 IFN
The IFN-τ most resemble the IFN-ω (~75 % identity), but are also quite similar to the IFN-α and IFN-β (~50% and ~25 % identity, respectively) (9). Both the IFN-τ and IFN-ω are 172 amino acids in length, i.e. about six amino acids longer than IFN-α. However, the IFN-τ have quite similar biological activities to other Type 1 IFN and bind to the Type 1 IFN receptor, through which they activate STAT transcription factors (10, 11) and up-regulate genes known to be implicated in an antiviral response (12, 13). The three dimensional structure of ovine IFN-τ4 (14) reveals that it possesses the compact 5-helix structure of IFN-α and -β (Fig.1).
Fig. 1.
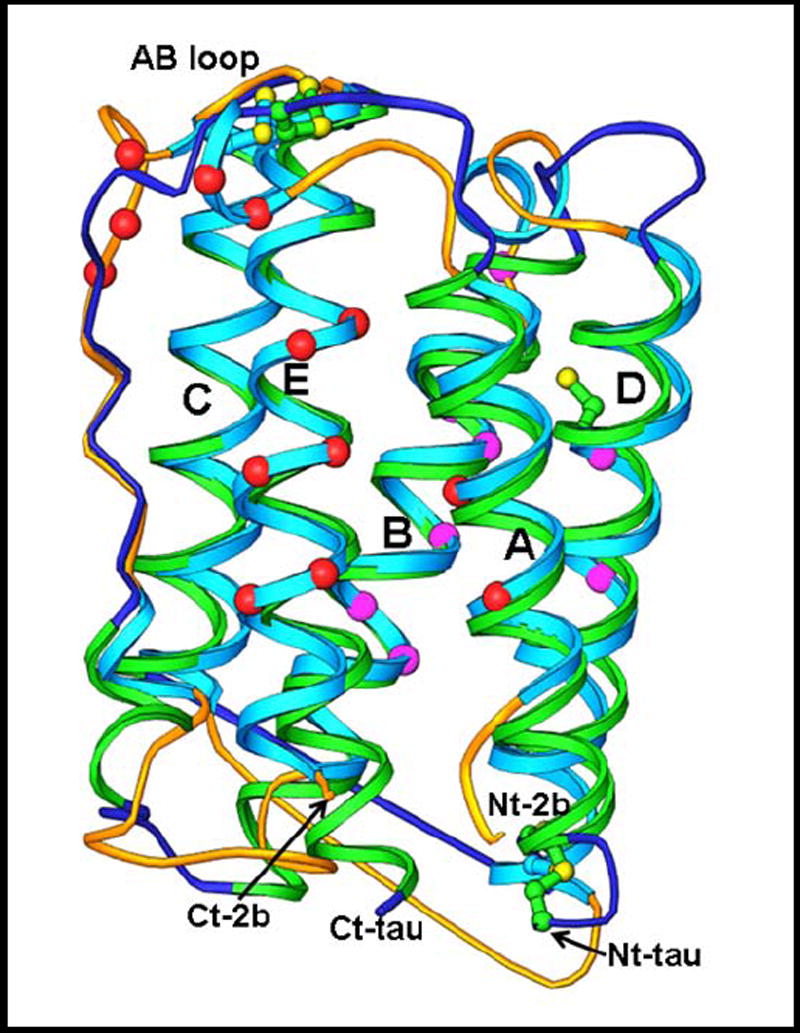
A structure comparison of human IFN-α2b and ovine IFN-τ. Shown is a ribbon diagram of IFN-α2b (α-helices cyan, loops gold) and IFN-τ (α-helices green, loops blue). Disulfide bonds are shown with sulfur atoms colored yellow. The carboxyl (Ct) and amino (Nt) termini are indicated for both structures. Note the six-residue longer carboxyl terminus of IFN-τ. Residues known to participate in IFNAR2 and IFNAR1 interactions are shown in red and magenta spheres, respectively. The figure illustrates the close similarity in the placement of the alpha helices, but the likely different conformation of the inter-helical loop regions. The figure was obtained from Dr. Mark Walters, University of Alabama-Birmingham.
Genes for the IFN-τ have been identified by Southern genomic blotting in cattle, sheep, musk oxen, goats and gazelle (all of which are Bovidae), giraffe (Giraffidae) and deer (Cervidae) (15). All of these ruminant species possess several IFNT, except for the giraffe, where it is unclear whether there is a single gene or, most probably, a group of closely related genes. Estimates of the number of IFNT in cattle ranged from a minimum of four to at least ten (15-17) but estimates are of necessity inaccurate because of the inability to distinguish which sequences represent distinct genes and which represent allelic forms. The latest version of the partially annotated bovine genome sequence only lists three genes, all linked closely to the IFNA, IFNW, and IFNB on chromosome 8 (Angela M. Walker and R. Michael Roberts, unpublished data). However, it is possible that closely related sequences have been overlooked, as the IFNT appear to be continuing to duplicate rapidly, spawning many closely related copies. For example, all the known bovine IFNT sequences have arisen within the last six million years, and the majority much more recently than that (17). Matters are even more complicated in the sheep where over 20 different sequences have been reported to GenBank. A full accounting for the cattle genes will probably require a complete walk along the relevant BACs overlapping the locus. Even then distinguishing genes with identical nucleotide sequences will be a challenge.
As mentioned above, the IFNT are most closely related to the IFNW, which themselves evolved from the IFNA about 130 million years ago, at about the time the mammals had their origins (18). The IFNT/IFNW split occurred much more recently, only 36 million years ago, and coincided with the time the ruminant artiodactyls themselves first emerged. A recent comprehensive analysis of Type 1 IFN phylogeny (19) confirms the recent evolutionary origins of the IFNT and the inference that these genes will not be found outside the ruminant order and certainly not in either mouse or man (as has been occasionally and mistakenly reported). The initial event that gave rise to the IFNT was most probably a duplication event from an existing IFNW that provided the new tau gene with a reorganized promoter and a novel 3/-end. This reorganization presumably opened up a new genetic context for transcriptional activation that directly or indirectly led to the acquisition of trophoblast expression and the loss of viral responsiveness (20).
Transcriptional control of IFNT expression
The expression of the IFNT is unique in at least three respects: lack of viral inducibility, restricted localization to embryonic trophectoderm and sustained, high level, synthesis sustained over several days. To our knowledge the IFNT are silent in the fetus, the placenta after it forms, and in all tissue of the adult animal. By contrast, expression of related Type 1 IFN genes occurs in response to virus and other pathogens in a variety of tissues, and the expression of these proteins is generally short-lived.
As with other Type 1 IFN, control of tissue and temporal expression lies in the 5/-flanking region of the intronless IFNT (21). These control regions are highly conserved across the ruminant species and lack the organized viral-response elements seen in genes for IFN-α (IFNA) and IFN-β (IFNB) (15). Two gene regions beyond the TATA box (-91 to −69, and −358 to −322) form complexes with nuclear extracts from Day 13 ovine conceptuses, a time when IFNT transcription rate per cell is at its zenith (21). Transfection experiments confirmed that the proximal-most site, is essential for gene expression. When part of this region was used as a target for yeast one-hybrid screening of a Day 13 conceptus library, the nuclear protein, Ets-2, was identified as binding to this site and to activate transcription (22). This element is adjacent to a putative AP1 site and two helical turns upstream of a binding site for the homeobox protein Dlx3. A combination of Ets2 and Dlx3 promote expression synergistically, allowing a more than 300-fold increase in expression in choriocarcinoma cells, a permissive trophoblast cell line used for transfection experiments (23). Interestingly, the potential role of Ets-2 in trophectoderm function is not limited to that of the ruminant. Mice deficient in Ets-2 exhibit defective trophectoderm function during the peri-implantation period (24), and this defect can be rescued by ectopic expression of Ets2 through transfection of retroviral vectors at the pre-implantation blastocyst stage of development (25). Moreover, Ets-2 regulates a large number of genes whose expression is a typical “signature” of trophoblast (26). Ets2-transcriptional regulation of the IFNT promoter is also silenced by Oct4, the hallmark transcription factor of pluripotent cells. We have suggested a model in which the down regulation of Oct4 that accompanies the emergence of trophectoderm releases constraints operating over the expression of IFNT, thereby placing the promoter in a permissive state for subsequent up-regulation by lineage-specific factors (27). Observations from this laboratory suggest that the Dlx3/Ets2/AP1 composite element is responsive to both the Ras/MAPK and cAMP/PKA signal transduction pathways and hence to factors that operate through these pathways. For example, Ets2-dependent transcriptional responses are regulated by both these pathways, and particularly by PKA (23). We have speculated that the sharp increase in IFN-τ production occurring as the ovine and bovine conceptus begin to elongate is triggered by progesterone-regulated growth factors released by the maternal endometrium that, in turn, bind to receptors on trophectoderm and target Ets2. In this manner, the release of IFN-τ by the conceptus is coordinated with maternal hormonal state so that both systems remain in phase.
Unique features of the IFN-τ
Ungulate species, in general, have placentae that are superficial and minimally invasive compared, for example, to those of rodents and higher primates. Although it may appear counter-intuitive, such placentae are a more recent innovation than the hemochorial placentation of the human and mouse (28) and highly efficient. However, as the structure of the placental barrier has evolved, so has the manner in which the conceptus signals to the mother. Some years ago we suggested that the IFN-τ had arisen in conjunction with the non-invasive synepitheliochorial placentation of ruminant artiodactyl species (10), possibly as an antiviral barrier, initially to protect the conceptus against infection (13), but eventually evolving to act as mediators of maternal recognition of pregnancy. The question that must be posed is whether the role of IFN-τ as a hormone of pregnancy has been achieved by their acquiring unique, or, at the very least, specialized biological properties. Alternatively, is the key to IFN-τ physiology their local production in emerging trophoblast at a critical time that allow them to intervene in the normal ovarian cycle and prevent the loss of CL function that would otherwise end the pregnancy? Certainly, the IFN-τ bind the same IFNAR1/IFNAR2 receptor used by other Type 1 IFN (29). Experiments comparing the potency of an ovine IFN-τ against another sub-type of ovine IFN, however, are not easily performed, but this laboratory has found little difference between an ovine IFN-τ and an ovine IFN-α in their respective abilities to extend estrous cycle length when infused into the uterine lumen of non-pregnant ewes (30). In cattle, limited IFN-α / IFN-τ comparisons have been attempted, but equivalent amounts of protein, rather than comparable quantities of antiviral activity, have been tested (see (31, 32). IFN-τ have excellent antiviral activities, at least the equivalent of corresponding IFN-α from the same species (33, 34). It still remains possible that IFN-τ have some specialized function, an ability for example to regulate a complement of genes in its target, the maternal endometrium, that make the action of this cytokine especially adapted to pregnancy signaling and the shut down of prostaglandin release. At present there is no convincing evidence that the IFN-τ have acquired such a specialized function. One question that has been addressed is whether the six amino acid extensions at the carboxyl tail of the IFN-τ have any significance. A recombinant form of ovine IFN-τ in which this tail had been deleted was no less potent in its ability to extend estrous cycle length than its normal length counterpart (35). The amounts used to achieve CL maintenance were comparable or lower than those secreted daily by individual conceptuses at the time of peak production (36).
Type 1 IFN and pregnancy
Although the IFN-τ are unique to the Ruminantia, a sub-order within the much larger Artiodactyla order, there are hints from transcriptional profiling experiments that a partial interferon-response occurs in humans and mice as the trophoblast makes intimate contact with the maternal endometrium and induces the formation of a deciduum, in which stromal fibroblast proliferate and form an edematous structure adjacent to the site of implantation (37-39). This response does not involve a full-blown up-regulation of many interferon-stimulated genes (ISGs), as noted in the sheep endometrium during early pregnancy (12, 13, 40), but is instead more limited. The notion that there are one or more signaling pathways common to the onset of early pregnancy across diverse taxonomic groups is an attractive one, because a process as fundamental to mammalian development as trophoblast implantation, although subject to maternal-fetal conflict and hence rapid evolutionary change(5, 41), would be expected to exhibit some vestiges of conservation. Krause & Pestka (19) in their recent review suggest that, in species of mammal that do not possess IFNT, production of another cytokine, possibly even a Type 1 IFN, such as IFN-ε, could provide the crucial signal from the growing trophoblast that prompts the local maternal endometrium to become a receptive site for the conceptus to grow and develop. What may be the conserved process in implantation is the downstream signaling pathway rather than the signaling molecule itself.
Closing comments
The discovery of IFN-τ can be considered important for several reasons. First, it described a Type 1 IFN functioning in a constitutive biological process unrelated to pathogenesis. Second, the find provided the first example of a cytokine involved in trophoblast signaling, plus an example of how a defined trophoblast product might intervene in maternal immune responses. Finally the IFNT would appear to be the most recent mammalian Type 1 gene family to have arisen; they provide a clear illustration of how a gene control region can be commandeered and then refined to provide a radically changed pattern of expression.
Acknowledgments
I thank Drs. Toshihiko Ezashi and Angela M. Walker, University of Missouri-Columbia for discussion of unpublished data, and Dr. Mark Walter, University of Alabama-Birmingham for providing Fig. 1. This work was supported by NIH grant HD21896.
Biography

R. Michael Roberts, Ph.D. University of Missouri – Columbia, Curators’ Professor of Animal Science, Biochemistry, and Veterinary Pathobiology, D.Phil., Oxford University, England
R. Michael Roberts’ career in scientific research and education began in England over 40 years ago as a botanist, but his interests gradually changed to biomedical and animal research during the 1970s while he was a member of the Department of Biochemistry and Molecular Biology at the University of Florida. Dr. Roberts joined the University of Missouri-Columbia in 1985, focusing on reproductive biology of livestock, and specifically in gene expression in trophoblast. A major discovery was the identification of interferon-tau and its role in signaling to the mother during early pregnancy. He is currently investigating (1) the effect of environmental conditions, particularly nutrition, on biasing the gender of offspring in farm species and other mammals; (2) the program whereby human embryonic stem cells can be induced to differentiate into trophoblast cells (3) an emerging signal transduction pathway involving stress-interacting protein kinase (Sin1), which interacts with the carboxyl end of the type 1 interferon receptor, IFNAR2, and interfaces downstream with the mTOR2 complex, Akt, and the RNA-binding protein, PCBP2/hnRNP-E2.
Dr. Roberts is the recipient of the Alexander Von Humboldt Award (1996) and the Wolf Prize for Agriculture (2003). Roberts was elected to the US National Academy of Sciences in 1996. In addition he is also the recipient of the 2004/2005 Scientific American “Top 50” list for accomplishments in research and technological leadership. Most recently he was awarded the 2006 Carl G. Hartman Award- The Society for Study of Reproduction’s highest award, which is given in recognition of a research career and scholarly activities in the field of reproductive biology.
Honors and Awards (selected)
Fellow World Health Organization (1977)
NATO Senior Fellowship (1977)
Sigma Xi Distinguished Professor Award, University of Florida (1983)
F. McKenzie Distinguished Professor of Reproductive Biology, University of Missouri (1988-1996)
Merit Award, National Institutes of Health (NICHD)(1990-2000)
Research Award, Society for Study of Reproduction (1990)
United States Department of Agriculture Distinguished Scientist (1992)
Researcher of the Year, College of Agriculture, University of Missouri (1995)
Milstein Award, International Society for Interferon and Cytokine Research (1995)
Member, National Academy of Sciences (elected 1996)
Alexander von Humboldt Award for Agriculture (1996)
Presidential Award (University of Missouri) for Research and Creativity (1997)
Docteur Honoris Causa (Honorary Doctorate) from the University of Liege, Belgium, (1998)
Wolf Prize for Agriculture (2003)
University of Missouri Faculty/Alumni Award (2005)
2004/2005 Scientific American “Top 50” list for accomplishments in research and technological leadership
2006 Carl G. Hartman Award- Society’s highest award and is given in recognition of a research career and scholarly activities in the field of reproductive biology
2006 Patent Recipient Awardee-University of Missouri-Columbia to be acknowledged at the Technology Transfer Showcase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imakawa K, Anthony RV, Kazemi M, Marotti KR, Polites HG, RM R. Interferon-like sequence of ovine trophoblast protein secreted by embryonic trophectoderm. Nature. 1987;330:377–379. doi: 10.1038/330377a0. [DOI] [PubMed] [Google Scholar]
- 2.Imakawa K, Hansen TR, Malathy PV, et al. Molecular cloning and characterization of complementary deoxyribonucleic acids corresponding to bovine trophoblast protein-1: a comparison with ovine trophoblast protein-1 and bovine interferon-alpha II. Mol Endocrinol. 1989;3:127–139. doi: 10.1210/mend-3-1-127. [DOI] [PubMed] [Google Scholar]
- 3.Stewart HJ, McCann SH, Barker PJ, Lee KE, Lamming GE, Flint AP. Interferon sequence homology and receptor binding activity of ovine trophoblast antiluteolytic protein. J Endocrinol. 1987;115:R13–15. doi: 10.1677/joe.0.115r013. [DOI] [PubMed] [Google Scholar]
- 4.Martal J, Lacroix MC, Loudes C, Saunier M, Wintenberger-Torres S. Trophoblastin, an antiluteolytic protein present in early pregnancy in sheep. J Reprod Fertil. 1979;56:63–73. doi: 10.1530/jrf.0.0560063. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RM, Xie S, Mathialagan N. Maternal recognition of pregnancy. Biol Reprod. 1996;54:294–302. doi: 10.1095/biolreprod54.2.294. [DOI] [PubMed] [Google Scholar]
- 6.Moor RM, Rowson LE. Local uterine mechanisms affecting luteal function in the sheep. J Reprod Fertil. 1966;11:307–310. doi: 10.1530/jrf.0.0110307. [DOI] [PubMed] [Google Scholar]
- 7.Godkin JD, Bazer FW, Moffatt J, Sessions F, Roberts RM. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13-21. J Reprod Fertil. 1982;65:141–150. doi: 10.1530/jrf.0.0650141. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev. 1992;13:432–452. doi: 10.1210/edrv-13-3-432. [DOI] [PubMed] [Google Scholar]
- 9.Roberts RM, Liu L, Alexenko A. New and atypical families of Type 1 interferons in mammals: comparative functions, structures, and evolutionary relationships. Prog Nucleic Acid Res Mol Biol. 1997;56:287–325. doi: 10.1016/s0079-6603(08)61008-9. [DOI] [PubMed] [Google Scholar]
- 10.Alexenko AP, Leaman DW, Li J, Roberts RM. The antiproliferative and antiviral activities of IFN-tau variants in human cells. J Interferon Cytokine Res. 1997;17:769–779. doi: 10.1089/jir.1997.17.769. [DOI] [PubMed] [Google Scholar]
- 11.Binelli M, Subramaniam P, Diaz T, et al. Bovine interferon-tau stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biol Reprod. 2001;64:654–665. doi: 10.1095/biolreprod64.2.654. [DOI] [PubMed] [Google Scholar]
- 12.Gray CA, Abbey CA, Beremand PD, et al. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod. 2006;74:383–394. doi: 10.1095/biolreprod.105.046656. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Green JA, Antoniou E, et al. Effect of interferon-tau administration on endometrium of nonpregnant ewes: a comparison with pregnant ewes. Endocrinology. 2006;147:2127–2137. doi: 10.1210/en.2005-1310. [DOI] [PubMed] [Google Scholar]
- 14.Radhakrishnan R, Walter LJ, Subramaniam PS, Johnson HM, Walter MR. Crystal structure of ovine interferon-tau at 2.1 A resolution. J Mol Biol. 1999;286:151–162. doi: 10.1006/jmbi.1998.2480. [DOI] [PubMed] [Google Scholar]
- 15.Leaman DW, Roberts RM. Genes for the trophoblast interferons in sheep, goat, and musk ox and distribution of related genes among mammals. J Interferon Res. 1992;12:1–11. doi: 10.1089/jir.1992.12.1. [DOI] [PubMed] [Google Scholar]
- 16.Ryan AM, Gallagher DS, Womack JE. Somatic cell mapping of omega and trophoblast interferon genes to bovine syntenic group U18 and in situ localization to chromosome 8. Cytogenet Cell Genet. 1993;63:6–10. doi: 10.1159/000133491. [DOI] [PubMed] [Google Scholar]
- 17.Alexenko AP, Ealy AD, Bixby JA, Roberts RM. A classification for the interferon-tau. J Interferon Cytokine Res. 2000;20:817–822. doi: 10.1089/10799900050151085. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RM, Ezashi T, Rosenfeld CS, Ealy AD, Kubisch HM. Evolution of the interferon tau genes and their promoters, and maternal-trophoblast interactions in control of their expression. Reprod Suppl. 2003;61:239–251. [PubMed] [Google Scholar]
- 19.Krause CD, Pestka S. Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives. Pharmacol Ther. 2005;106:299–346. doi: 10.1016/j.pharmthera.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Roberts RM, Liu L, Guo Q, Leaman DJB. The evolution of the type I interferons. J Interferon Cytokine Res. 1998;18:805–816. doi: 10.1089/jir.1998.18.805. [published erratum appears in J Interferon Cytokine Res 1999 Apr;19(4):427] [DOI] [PubMed] [Google Scholar]
- 21.Leaman DW, Cross JC, Roberts RM. Multiple regulatory elements are required to direct trophoblast interferon gene expression in choriocarcinoma cells and trophectoderm. Mol Endocrinol. 1994;8:456–468. doi: 10.1210/mend.8.4.8052267. [DOI] [PubMed] [Google Scholar]
- 22.Ezashi T, Ealy AD, Ostrowski MC, Roberts RM. Control of interferon-tau gene expression by Ets-2. Proc Natl Acad Sci USA. 1998;95:7882–7887. doi: 10.1073/pnas.95.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezashi T, Das P, Roberts RM. Reguation of IFN-tau gene expression by uterine factors: Interaction between the conceptus and maternal environment during early pregnancy in cattle and sheep. Journal of Reproduction & Development. 2006:S99–S109. [Google Scholar]
- 24.Yamamoto H, Flannery ML, Kupriyanov S, et al. Defective trophoblast function in mice with a targeted mutation of Ets2. Genes Dev. 1998;12:1315–1326. doi: 10.1101/gad.12.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada Y, Ueshin Y, Isotani A, et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007 doi: 10.1038/nbt1280. [DOI] [PubMed] [Google Scholar]
- 26.Ezashi T, Roberts RM. Regulation of Interferon-{tau} (IFN-{tau}) Gene Promoters by Growth Factors that Target the Ets-2 Composite Enhancer: A Possible Model for Maternal Control of IFN-{tau} Production by the Conceptus during Early Pregnancy. Endocrinology. 2004;145:4452–4460. doi: 10.1210/en.2004-0606. [DOI] [PubMed] [Google Scholar]
- 27.Ezashi T, Ghosh D, Roberts RM. Repression of Ets-2 induced transactivation of the interferon-tau promoter by Oct-4. Mol Cell Biol. 2001;21:7883–7891. doi: 10.1128/MCB.21.23.7883-7891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci U S A. 2006;103:3203–3208. doi: 10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld CS, Han CS, Alexenko AP, Spencer TE, Roberts RM. The Expression of interferon receptor subunits, IFNAR1 and IFNAR2, in the Ovine Uterus. Biol Reprod. 2002;6:847–853. doi: 10.1095/biolreprod.102.004267. [DOI] [PubMed] [Google Scholar]
- 30.Green MP, Spate LD, Bixby JA, Ealy AD, Roberts RM. A Comparison of the Anti-Luteolytic Activities of Recombinant Ovine Interferon-Alpha and -Tau in Sheep. Biol Reprod. 2005 doi: 10.1095/biolreprod.105.043406. biolreprod.105.043406. [DOI] [PubMed] [Google Scholar]
- 31.Spencer TE, Ott TL, Bazer FW. tau-Interferon: pregnancy recognition signal in ruminants. Proc Soc Exp Biol Med. 1996;213:215–229. doi: 10.3181/00379727-213-44053. [DOI] [PubMed] [Google Scholar]
- 32.Martal J, Chene N, Camous S, et al. Recent developments and potentialities for reducing embryo mortality in ruminants: the role of IFN-tau and other cytokines in early pregnancy. Reprod Fertil Dev. 1997;9:355–380. doi: 10.1071/r96083. [DOI] [PubMed] [Google Scholar]
- 33.Ealy AD, Green JA, Alexenko AP, Keisler DH, Roberts RM. Different ovine interferon-tau genes are not expressed identically and their protein products display different activities. Biol Reprod. 1998;58:566–573. doi: 10.1095/biolreprod58.2.566. [DOI] [PubMed] [Google Scholar]
- 34.Ealy AD, Larson SF, Liu L, et al. Polymorphic forms of expressed bovine interferon-tau genes: relative transcript abundance during early placental development, promoter sequences of genes and biological activity of protein products. Endocrinology. 2001;142:2906–2915. doi: 10.1210/endo.142.7.8249. [DOI] [PubMed] [Google Scholar]
- 35.Ealy AD, Alexenko AP, Keisler DH, Roberts RM. Loss of the signature six carboxyl amino acid tail from ovine interferon-tau does not affect biological activity. Biol Reprod. 1998;58:1463–1468. doi: 10.1095/biolreprod58.6.1463. [DOI] [PubMed] [Google Scholar]
- 36.Roberts RM. A role for interferons in early pregnancy. Bioessays. 1991;13:121–126. doi: 10.1002/bies.950130305. [DOI] [PubMed] [Google Scholar]
- 37.Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology. 2003;144:3107–3113. doi: 10.1210/en.2002-0031. [DOI] [PubMed] [Google Scholar]
- 38.Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294:445–456. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Hess AP, Hamilton AE, Talbi S, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Choi Y, Bazer FW, Spencer TE. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription 1. Endocrinology. 2003;144:5203–5214. doi: 10.1210/en.2003-0665. [DOI] [PubMed] [Google Scholar]
- 41.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]


