Summary
Detection of a DNA double strand break (DSB) is necessary to initiate DSB repair. Several proteins including the MRX/N complex, Tel1/ATM (ataxia-telangiectasia-mutated), and Mec1/ATR (ATM and Rad3-related) have been proposed as sensors of DNA damage, yet how they recognize the breaks is poorly understood. DSBs occur in the context of chromatin, implicating factors capable of altering local and/or global chromatin structure in the cellular response to DNA damage, which includes DSB sensing. Emerging evidence indicates that ATP-dependent chromatin-remodeling complexes function in DNA repair [1-3]. Here we describe an important and novel early role for the RSC ATP-dependent chromatin remodeler linked to DSB sensing in the cell's DNA damage response. RSC is required for full levels of H2A phosphorylation by facilitating the recruitment of Tel1/ATM and Mec1/ATR to the break site. Consistent with these results, we also show that Rsc2 is needed for efficient activation of the Rad53-dependent checkpoint, as well as for Cohesin's association with the break site. Finally, Rsc2 is needed for the DNA damage-induced changes in nucleosome structure surrounding the DSB site. Together, these new findings functionally link RSC to DSB sensing, highlighting the importance of ATP-dependent chromatin-remodeling factors in the cell's early response to DNA damage.
Results and Discussion
RSC interacts with Tel1/ATM and Mec1/ATR kinases under DNA damaging conditions
We and others showed recently that the RSC ATP-dependent chromatin remodeler functions in both the HR and NHEJ repair of DSBs [4];[5]. In particular, we showed that RSC is involved in a postsynaptic step of HR. However, RSC's rapid recruitment to DSBs, comparable to that of the earliest responding DNA repair factors, and it's interactions with Mre11, the earliest DSB sensor prompted us to investigate an early role for RSC in repair. Should RSC play a role in sensing DSBs we reasoned that it would interact with one or more of the earliest repair factors recruited to DSBs, including the phosphoinositide (PI)-3-kinase-related protein kinase sensors Tel1/ATM and Mec1/ATR. To test this, we initially compared the growth of cells lacking both RSC2 and TEL1 to those lacking either gene on rich complete (YPD) medium and on YPD medium containing the DNA damaging agent methylmethane sulfonate (MMS). We found that the rsc2Δ tel1Δ double mutants grew perceptively worse than the single mutants on YPD medium and that the synthetic interactions were dramatically enhanced in the presence of MMS (Figure 1A). Similarly, we tested whether Rsc2 interacts with the essential Mec1 cell cycle checkpoint protein by comparing growth of single and double mutants. Importantly, growth of the rsc2Δ mec1-1 sml1-1 mutant (the sml1-1 mutation suppresses the lethality conferred by mec1-1 [6]) on MMS was significantly worse than that of the parental rsc2Δ and mec1-1 mutants (Figure 1B). Interestingly, synergistic interactions between tel1Δ1 and mec1-1 in the presence of DNA damaging agents have been described previously [7]. Together, these results suggest that Rsc2 interacts synthetically with both Tel1 and Mec1 under DNA damaging conditions, consistent with the idea that RSC and the ATM and ATR kinases are either in a single or parallel but compensatory DNA damage response pathways.
Figure 1. Rsc2 exhibits synthetic enhancement interactions with Tel1 and Mec1.

(A) Tenfold serial dilutions of wild-type (BY4741), rsc2Δ (BLY908), tel1Δ (BLY959), and rsc2Δ tel1Δ (BLY962) cells were spotted onto YPD medium and YPD medium containing 0.01% MMS. Cells were incubated at 30°C for 1 to 2 days. (B) Tenfold serial dilutions of wild-type (BLY46), sml1-1 (BLY1042), rsc2Δ (BLY1041), rsc2Δ sml1-1 (BLY1045), mec1-1 sml1-1 (BLY207) and rsc2Δ mec1-1 sml1-1 (BLY921) strains were spotted onto YPD medium and YPD medium containing 0.03% MMS and incubated at 30°C for 1 to 2 days.
RSC is required to maintain normal levels of DSB-induced phosphorylated H2A
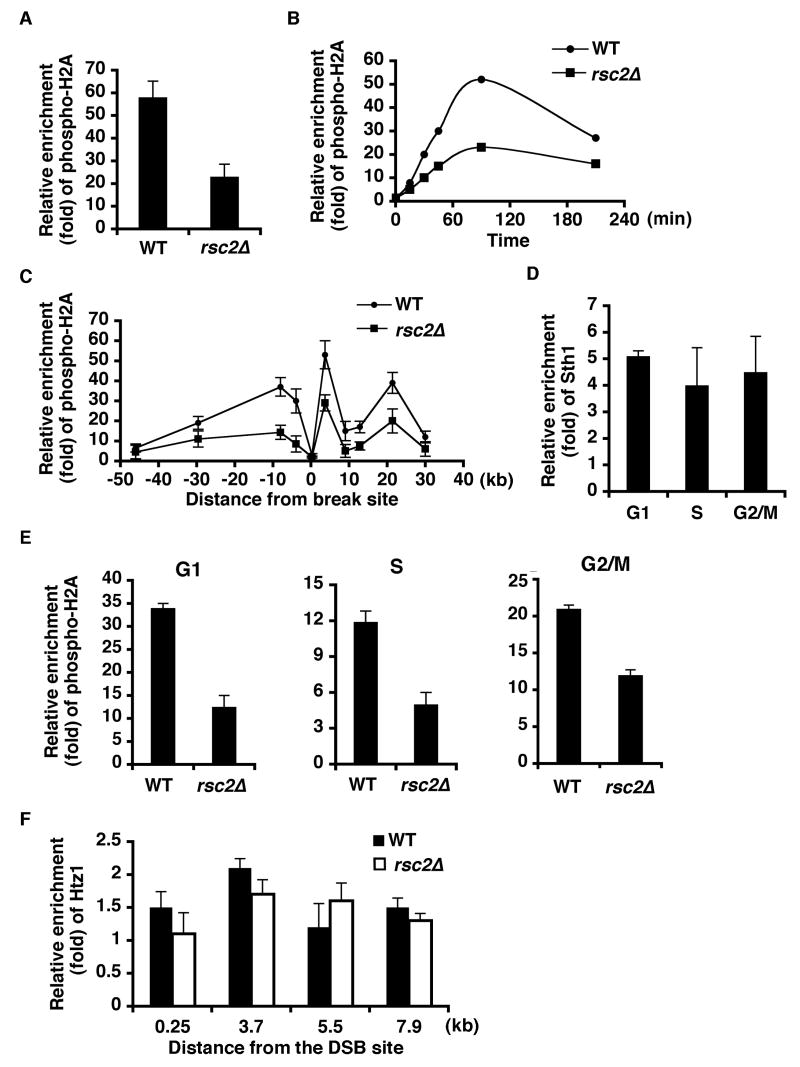
Since RSC interacts genetically with Tel1 and Mec1 under DNA damaging conditions, and RSC's recruitment and histone H2A's phosphorylation at a DSB are among the earliest events detected following formation of the lesion, we hypothesized that RSC is necessary for the Tel1/Mec1-dependent phosphorylation of histone H2A. To test this, we first compared levels of H2A phosphorylation in isogenic wild-type and mutant cells lacking Rsc2 following DSB induction. Strikingly, we found that the enrichment of DNA damage-induced phospho-H2A near the HO DSB was reduced approximately 3-fold in the absence of Rsc2 compared with wild-type cells (Figure 2A). Additionally, levels of MMS-induced phosphorylated-H2A were also significantly reduced in rsc2Δ cells (Figure 4E), consistent with the ChIP data. As a control, we found that levels of H2A were unaffected in the absence of Rsc2 (Figure S1A in the Supplemental Data). In these and subsequent experiments, DSB efficiency was comparable in wild-type and rsc2Δ cells (Figure S2) (data not shown). The reduced H2A phosphorylation in rsc2Δ cells was detected as early as 30 min following DSB induction, persisted for 210 min (Figure 2B), and was observed in a large chromatin domain flanking the DSB, a 30-kb region to the right of the DSB site and a 40-50-kb region to the left of the DSB (Figure 2C). The requirement of Rsc2 for full levels of H2A phosphorylation within 30 min of HO induction, and the phospho-H2A-independent recruitment of Sth1 to the break site (Figure S3), together with our previous result showing that RSC is recruited rapidly to the break site [4], strongly suggests that RSC's ATP-dependent remodeling activity functions ahead of H2A phosphorylation, in contrast to another related ATP-dependent chromatin remodeler, INO80, whose recruitment is phospho-H2A-dependent [8] [9].
Figure 2. Rsc2 is required for full level H2A phosphorylation in response to a DSB.
(A) Wild-type (BLY836) and rsc2Δ (BLY857) cells were collected before and 1.5 h after DSB induction for ChIP assays, using anti-phospho-H2A.X antibody (Upstate). Enrichment of phospho-H2A at the break site was quantified using primers specific for a sequence 3.7 kb from the break site by real-time PCR analysis, normalized to non-DSB induction samples and to an internal control sequence (ACT1). (B) Cells were collected at the indicated time points and processed for ChIP using anti-phospho-H2A.X antibody as described in (A). (C) ChIP samples were prepared exactly as in (A) and the recruitment of phospho-H2A to a 30-kb region to the right of the DSB site (assayed at 0.25 kb, 3.7 kb, 8.9 kb, 12.8 kb, 21.4 kb, and 30 kb) and a 40-50-kb region to the left of the DSB (assayed at 3.9 kb, 8 kb, 29.6 kb, and 46 kb) were monitored using specific primer sets. The means and standard deviations of three independent ChIP experiments were plotted (as in subsequent experiments). (D) Wild-type (BLY754) cells were arrested in G1, S, and G2/M phases before DSB induction. ChIP assays were performed with anti-Sth1 antibody and samples prepared for real-time PCR using primers monitoring a sequence 250 bp from the break site. (E) G1, S, and G2/M wild-type (BLY1046) and rsc2Δ (BLY1048) cells were prepared as in (D) and ChIP assays carried out as in (A). (F) Htz1's association with several positions adjacent to a DSB (0.25 kb, 3.7 kb, 5.5 kb, and 7.9 kb) was monitored 1.5 h after DSB induction in wild-type (BLY836) and rsc2Δ (BLY857) cells by ChIP assays using anti-Htz1 antibody (Abcam).
Figure 4. Rsc2 is required for Cohesin's efficient association with the break site and for checkpoint activation.
(A) Enrichment of Mcd1 at sites 0.25, 0.37, 5.5, and 8.9 kb to the right, and 3.9, 5.6, and 7.5 kb to the left of the DSB site was monitored in cycling wild-type (BLY837) and rsc2Δ (BLY861) cells expressing Mcd1HA6 1.5 h after HO induction by ChIP. (B) S phase wild-type (BLY837) and rsc2Δ (BLY861) cells were collected and processed for ChIP as in part (A). (C) G2/M phase wild-type (BLY837) and rsc2Δ (BLY861) cells were collected and processed for ChIP as in part (A). (D) Rad9's recruitment to 0.25 kb and 0.37 kb from the break site was analyzed in G1 phase wild-type (BLY1046), hta S129* (BLY1047), and rsc2Δ (BLY1048) cells expressing Rad9-HA by ChIP, and quantified by real-time PCR. (E) Mid-logarithmic wild-type (BY4741) and rsc2Δ (BLY908) cells were incubated in 0.2% MMS medium for 1 h. Cell extracts from untreated and MMS-treated cells were subjected to immunoblot analysis using anti-Rad53 and anti-Sth1 antibodies.
DSB-induced phospho-H2A was shown previously to be dependent on both Tel1 and Mec1 in cycling cells, while its formation in G1-arrested cells was largely dependent on Tel1 [10]. Since RSC functions in both NHEJ and HR pathways [4, 5], which predominate in G1 and post S phases, respectively, we next asked when during the cell cycle RSC is required for full level H2A phosphorylation. Initially, we determined that Sth1, RSC's essential ATPase subunit, associates with chromatin 0.25 kb from the DSB site at similar levels throughout the cell cycle (Figure 2D). To determine whether RSC is needed for formation of phospho-H2A during the cell cycle, we compared relative enrichment of phospho-H2A in mating pheromone-induced G1 phase arrested, HU-induced S phase arrested, and nocodazole-induced G2/M phase arrested wild-type and rsc2Δ cells. In G1 phase, H2A phosphorylation is reduced nearly three-fold in rsc2Δ cells compared to wild-type cells. In S and G2/M cell cycle stages, H2A phosphorylation levels are also decreased by approximately two-fold (Figure 2E). Together these results suggest that RSC is required for full levels of H2A phosphorylation in response to DNA damage in a large 70-80-kb region surrounding the DSB throughout the cell cycle.
Loss of phospho-H2A in cells lacking Rsc2 is not due to its replacement by Htz1
Similar to cells lacking RSC, cells lacking Ino80, the ATPase subunit of the INO80 chromatin-remodeling complex also fail to maintain high levels of phospho-H2A. Ino80 is thought to act antagonistically to the Swr1 remodeler at chromatin flanking DSBs, replacing the Htz1 histone variant with H2A, while Swr1 catalyzes the substitution of phosphorylated H2A with Htz1. In cells lacking Ino80, Htz1 replaces histone H2A [11]. Therefore, we investigated whether the loss of histone H2A phosphorylation surrounding the DSB in rsc2Δ cells is due to the substitution of H2A with Htz1 [11]. Notably, we found that Htz1 enrichment at chromosomal sites near the DSB was comparable in rsc2Δ and wild-type cells (Figure 2F), suggesting that Rsc2 and Ino80 regulate phosphorylated H2A levels by distinct mechanisms.
Rsc2 is required for efficient recruitment of Tel1 and Mec1 to DNA break sites and for DNA end processing
Rsc2's synthetic genetic interactions with Tel1 and Mec1 under DNA damaging conditions (Figure 1) and Rsc2's requirement for phosphorylation of H2A throughout the cell cycle (Figure 2E) are consistent with roles for RSC function in both the Tel1 and Mec1 DNA repair pathways. To test the hypothesis that recruitment of either Tel1 or Mec1 to sites adjacent to the DSB requires RSC function, we monitored relative enrichment levels of Tel1-HA or Mec1-HA at two sites near the HO-induced DSB in wild-type and rsc2Δ cells. Importantly, we found that recruitment of both Tel1 and Mec1 was reduced approximately 2-fold in cells lacking Rsc2 (Figures 3A and 3B). Levels of both Tel1-HA and Mec1-HA were comparable in wild-type and rsc2Δ cells, ruling out the possibility that Rsc2 regulates expression of Tel1 or Mec1 proteins (Figures 3A and 3B). We also tested Sth1's association with the break site in a tel1Δ mec1Δ sml1Δ mutant and found that its enrichment was comparable to that in wild-type cells (Figure S3), suggesting that RSC functions upstream of Tel1 and Mec1. These results suggest that Rsc2 regulates DSB-induced phosphorylation of H2A by promoting the recruitment of both the Tel1 and Mec1 kinases to the DNA break site.
Figure 3. Rsc2 is required for the efficient recruitment of Tel1, Mec1, and Rfa1 to the break site.
(A) (left) Wild-type (BLY877) and rsc2Δ (BLY922) cells expressing Tel1-HA were immunoprecipitated with anti-HA antibody in ChIP assays to analyze the enrichment of Tel1 at sites 1 kb and 2 kb from the break site 1.5 h following induction of the single break as described [25] except that enriched DNAs were quantified by real-time PCR analysis. (right) Tel1 and Sth1 protein levels were measured in wild-type and rsc2Δ strains by immunoblot analysis using anti-HA and anti-Sth1 antibodies. (B) (left) ChIP was carried out with wild-type (BLY876) and rsc2Δ (BLY923) strains expressing Mec1-HA as described in (A). (right) Mec1 protein levels in wild-type and rsc2Δ strains were evaluated as in part (A). (C) RPA's enrichment on ssDNA 0.25 kb, 3.7 kb, 4.3 kb, 5.5 kb, 6.6 kb, and 7.9 kb from the break site was monitored before and 1 h after DSB induction in wild-type and rsc2Δ cells by ChIP using anti-Rfa1 antibody (a gift from S. Brill).
While both Mre11 and Tel1/ATM are recruited to DSBs before DSB end resection [12], Mec1/ATR is localized later to the resected ssDNA and its recruitment requires the essential ssDNA-binding protein replication protein A (RPA) [12-14], a key activator of the DNA damage checkpoint response [12, 13]. We found that the lack of Rsc2 significantly reduced recruitment of Rfa1, the largest subunit of RPA, to ssDNA at various positions from the HO-induced DSB (Figure 3C), without affecting levels of the Rfa1 protein (Figure S1B), suggesting that Rsc2 is needed for the efficient conversion of dsDNA to ssDNA at DSBs. To test this, we performed a quantitative real-time PCR-based method to measure the amount of ssDNA at the DSB site. Indeed, we found that in rsc2Δ cells, the relative amount of ssDNA is significantly reduced compared with wild-type cells (Figure S4) and reduction was first detected one hour following DSB induction. Interestingly, these results indicate that Rsc2 functions much earlier in end-processing than the related Ino80 chromatin remodeler [9], further suggesting functional differences among different remodelers in DSB repair.
RSC function is required for efficient enrichment of the DNA damage-induced Cohesin domain at DSBs
Phosphorylated H2A recruits several repair factors to the break site including the Cohesin sister chromatid-pairing complex [15];[16]. RSC's requirement for the association of Cohesin with chromosomal arms [17] and involvement of both RSC and Cohesin in HR repair [4];[15];[18] prompted us to test whether RSC is also necessary for loading Cohesin at DSBs. Significantly, levels of Mcd1 (an essential subunit of Cohesin) enrichment at several sites within a 20-kb region of chromatin flanking the HO-DSB were reduced 2- to 4-fold in the absence of Rsc2 (Figure 4A), and we determined that this reduction was not due to a lower abundance of Mcd1 in these cells (Figure S1C). Since Cohesin is recruited de novo to DSBs in post-G1 phase cells [15], we repeated these ChIP experiments in early S and G2/M phase cells. As in cycling cells, Cohesin's recruitment to the region surrounding the break site was reduced approximately 3-fold in both S and G2/M phase rsc2Δ mutants (Figures 4B and 4C). As a control, we compared the enrichment of Cohesin at the DSB site before HO induction in wild-type and rsc2Δ cells, and found that it was comparable (Figures 4A, B, and C). Under the same non-HO inducing conditions, we found that enrichment of Cohesin at the chV556kb chromosomal arm site was RSC-dependent, as we have shown before (data not shown) [17]. Therefore, we conclude that the requirement of Rsc2 for the efficient loading of Cohesin is DNA damage-specific. These findings suggest that RSC is required for Cohesin's DSB-induced enrichment at a 20-kb region of chromatin surrounding the DSB, either by promoting H2A phosphorylation or through a more direct mechanism.
Rad9's recruitment to DSBs and activation of the Rad53 cell cycle checkpoint protein in response to DNA damage are defective in the absence of Rsc2
Since Tel1/ATM and Mec1/ATR are also required for the phosphorylation of multiple downstream targets, including the Rad53/CHK2 checkpoint kinase, we predicted that Rsc2 plays a role in checkpoint activation. Phosphorylation of H2A was recently shown to be required for activation of the G1 checkpoint, in particular for Rad9's recruitment to DSBs as well as activation of Rad53 [19]. Therefore, we initially compared Rad9's enrichment at DSBs in G1 phase in wild-type, H2A-S129A (which blocks H2A phosphorylation), and rsc2Δ cells. We found that in rsc2Δ cells Rad9 enrichment near the DSB was reduced nearly to the levels observed in H2A-S129A cells (Figure 4D), although Rad9's expression levels were unchanged in these cells (Figure 4D). Since Rad9 is required for Rad53's phosphorylation in response to a DSB [20] and Rad53 is a downstream target of the Tel1 and Mec1 kinases, we reasoned that Rsc2 would be required for full activation of Rad53. Indeed, levels of the multi-phosphorylated forms of Rad53 are significantly reduced in rsc2Δ cells under MMS treatment (Figure 4E). Therefore, consistent with its roles in regulating both phosphorylation of H2A and the recruitment of Tel1 and Mec1 to the break site, Rsc2 is required for efficient checkpoint activation under DNA damaging conditions,.
Rsc2 is required for chromatin remodeling at a DSB
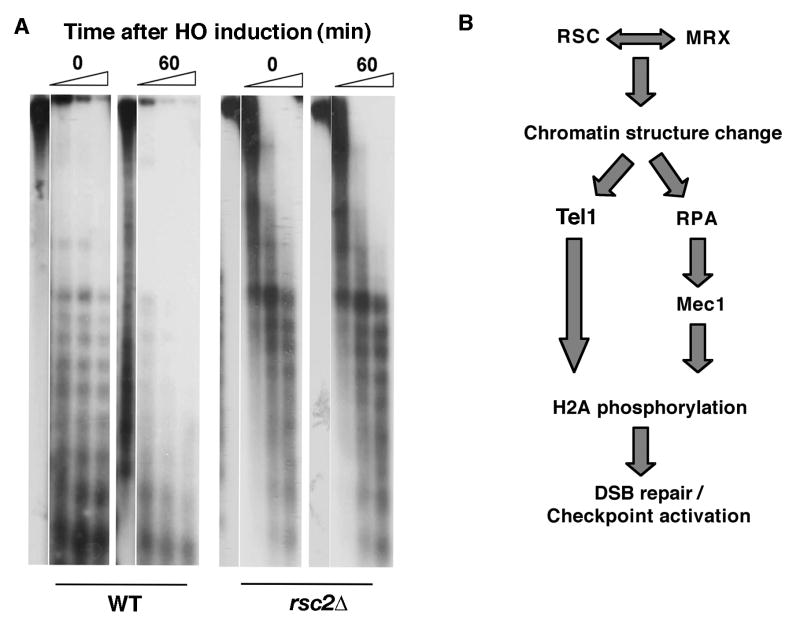
RSC's early recruitment to the HO-induced DSB suggests that it remodels nucleosomes near the DNA lesion. To test this we compared chromatin integrity at the MAT locus in wild-type and rsc2Δ cells before and after DSB induction by assaying sensitivity to micrococcal nuclease. In wild-type cells we observed a loss of the nucleosome ladder within 60 min of DSB induction, suggesting that nucleosomes are repositioned upon DSB induction (Figure 5A), consistent with a previous report [21]. In striking contrast, in the absence of Rsc2, no alteration of nucleosome structure was observed within 60 min of DSB formation (Figure 5A), in agreement with a recent study [22]. These data suggest that the DSB-induced remodeling of nucleosomes is Rsc2-dependent. In addition, RSC's remodeling of chromatin at the break site is independent of Tel1 or Mec1, since DSB-induced chromatin remodeling is not compromised in a tel1Δ mec1Δ sml1Δ strain (Figure S5). Together with a previous report showing that H2A phosphorylation is not required for chromatin remodeling at a DSB site [21], we conclude that RSC remodels DSB chromatin independently of phospho-H2A, Tel1, and Mec1.
Figure 5. Rsc2 functions in remodeling chromatin structure at the break site and functions as a DSB sensor.
(A) Nuclei of wild-type (BLY836) and rsc2Δ (BLY857) cells were prepared before or 1 h after HO induction and chromatin was digested with micrococcal nuclease (MNase) and subjected to Southern blot analysis with a probe specific to the MAT locus. Triangles indicate increasing times of MNase digestion. (B) A model for RSC function in the early cellular response to DNA damage.
Conclusion
Detection of DNA damage is critical for the cell's ability to activate checkpoints and efficiently repair the damage. Our findings link RSC to DNA damage sensing and the DNA damage checkpoint response. We propose a model in which RSC is recruited to the break site as soon as a single DSB is generated (Figure 5B). At the DSB, RSC cooperates with the MRX DNA damage sensor [5] [22] to remodel chromatin. Indeed, as reported earlier, RSC requires Mre11 for its recruitment to DSBs [5] and we and others have also shown that Mre11's recruitment to DSBs is dependent on RSC (Figure S6) [22], thereby facilitating recruitment of additional sensors, including Tel1 and Mec1, to the restructured DNA break site. RSC's role in facilitating RPA's enrichment on ssDNA may also contribute to Mec1's recruitment. Tel1 and Mec1 then promote further changes in chromatin by regulating phosphorylation of H2A (Figure 5B). H2A phosphorylation ensures checkpoint (e.g., Rad53) activation and recruitment of repair factors, including Cohesin (whose DSB-induced enrichment also relies on Rad53) [15]. Our data argue strongly that RSC participates directly in the DDR, although changes in transcription factor or repair factor levels mediated by RSC may also contribute in part to the efficiency of the DDR. Interestingly, an ATP-dependent chromatin-remodeling activity with remarkably similar properties was recently identified at DSBs in human cells [23]. Although human SWI/SNF, like RSC, is also required for H2A phosphorylation, it is dispensable for recruitment of ATM and ATR and for checkpoint activation [24], and therefore regulates H2AX phosphorylation differently than RSC. In addition, the evidence presented here linking RSC to early DSB sensing distinguishes RSC from the related INO80 ATP-dependent chromatin remodeler and suggests that different remodelers function at different steps in DSB repair. Thus, in addition to a role in the post-synapsis repair of DSBs described previously by us [4], RSC also plays an important early role in detecting double-strand breaks, remodeling chromatin at the DSB, and activating DNA damage checkpoints. An early nucleosome-remodeling function is likely to be redundant given its importance in initiating the DNA damage response, and the several other chromatin remodelers so far implicated in the DDR suggest possible compensatory chromatin-remodeling activities. This work advances our understanding of how DSBs are sensed and highlights the functional importance of ATP-dependent chromatin remodelers in the very early stages of DSB repair.
Supplementary Material
Acknowledgments
We thank S. Brill, B. Cairns, and P. Sung for generous gifts of anti-Rfa1, anti-Sth1, and anti-Mre11 antibodies, respectively; R. Rothstein, K. Sugimoto, S. Marcand, D. Koshland, and S. Kron for strains and plasmids; and M. O'Connell for help with elutriation. This work was supported by NIH grant R01 GM567000.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osley MA, Tsukuda T, Nickoloff JA. ATP-dependent chromatin remodeling factors and DNA damage repair. Mutat Res. 2007 doi: 10.1016/j.mrfmmm.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao Y, Shen X. Chromatin remodeling in DNA double-strand break repair. Curr Opin Genet Dev. 2007;17:126–131. doi: 10.1016/j.gde.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.van Attikum H, Gasser SM. ATP-dependent chromatin remodeling and DNA double-strand break repair. Cell Cycle. 2005;4:1011–1014. doi: 10.4161/cc.4.8.1887. [DOI] [PubMed] [Google Scholar]
- 4.Chai B, Huang J, Cairns BR, Laurent BC. Distinct roles for the RSC and Swi/Snf ATP-dependent chromatin remodelers in DNA double-strand break repair. Genes Dev. 2005;19:1656–1661. doi: 10.1101/gad.1273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim EY, Ma JL, Oum JH, Yanez Y, Lee SE. The yeast chromatin remodeler RSC complex facilitates end joining repair of DNA double-strand breaks. Mol Cell Biol. 2005;25:3934–3944. doi: 10.1128/MCB.25.10.3934-3944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- 7.Morrow DM, Tagle DA, Shiloh Y, Collins FS, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 8.Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, Shen X. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004;119:767–775. doi: 10.1016/j.cell.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 9.van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell. 2004;119:777–788. doi: 10.1016/j.cell.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, Haber JE, Lichten M. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol. 2004;14:1703–1711. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papamichos-Chronakis M, Krebs JE, Peterson CL. Interplay between Ino80 and Swr1 chromatin remodeling enzymes regulates cell cycle checkpoint adaptation in response to DNA damage. Genes Dev. 2006;20:2437–2449. doi: 10.1101/gad.1440206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Costanzo V, Shechter D, Lupardus PJ, Cimprich KA, Gottesman M, Gautier J. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol Cell. 2003;11:203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 14.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 15.Unal E, Arbel-Eden A, Sattler U, Shroff R, Lichten M, Haber JE, Koshland D. DNA damage response pathway uses histone modification to assemble a double-strand break-specific cohesin domain. Mol Cell. 2004;16:991–1002. doi: 10.1016/j.molcel.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 16.van Attikum H, Gasser SM. The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol. 2005;6:757–765. doi: 10.1038/nrm1737. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Hsu JM, Laurent BC. The RSC Nucleosome-Remodeling Complex Is Required for Cohesin's Association With Chromosome Arms. Mol Cell. 2004;13:739–750. doi: 10.1016/s1097-2765(04)00103-0. [DOI] [PubMed] [Google Scholar]
- 18.Strom L, Lindroos HB, Shirahige K, Sjogren C. Postreplicative recruitment of cohesin to double-strand breaks is required for DNA repair. Mol Cell. 2004;16:1003–1015. doi: 10.1016/j.molcel.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri A, Wysocki R, Jobin-Robitaille O, Altaf M, Cote J, Kron SJ. Yeast G1 DNA damage checkpoint regulation by H2A phosphorylation is independent of chromatin remodeling. Proc Natl Acad Sci U S A. 2006;103:13771–13776. doi: 10.1073/pnas.0511192103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney FD, Yang F, Chi A, Shabanowitz J, Hunt DF, Durocher D. Saccharomyces cerevisiae Rad9 acts as a Mec1 adaptor to allow Rad53 activation. Curr Biol. 2005;15:1364–1375. doi: 10.1016/j.cub.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 21.Tsukuda T, Fleming AB, Nickoloff JA, Osley MA. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature. 2005;438:379–383. doi: 10.1038/nature04148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim EY, Hong SJ, Oum JH, Yanez Y, Zhang Y, Lee SE. RSC mobilizes nucleosomes to improve accessibility of repair machinery to the damaged chromatin. Mol Cell Biol. 2007;27:1602–1613. doi: 10.1128/MCB.01956-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruhlak MJ, Celeste A, Dellaire G, Fernandez-Capetillo O, Muller WG, McNally JG, Bazett-Jones DP, Nussenzweig A. Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol. 2006;172:823–834. doi: 10.1083/jcb.200510015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Park EJ, Lee HS, Kim SJ, Hur SK, Imbalzano AN, Kwon J. Mammalian SWI/SNF complexes facilitate DNA double-strand break repair by promoting gamma-H2AX induction. Embo J. 2006;25:3986–3997. doi: 10.1038/sj.emboj.7601291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakada D, Matsumoto K, Sugimoto K. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 2003;17:1957–1962. doi: 10.1101/gad.1099003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.