Abstract
Objective
To determine the association between individual ovarian dimensions, advancing age and declining ovarian reserve in an infertile population.
Design
An ongoing prospective observational study.
Setting
Academic infertility practice.
Patients
69 premenopausal women presenting for the evaluation and management of infertility.
Interventions
Transvaginal ultrasound assessment of the ovarian dimensions (length, width and overall diameter in mm) and evaluation of the ovarian reserve status by measuring serum levels of follicle stimulating hormone and estradiol in the early follicular phase of the menstrual cycle.
Main Outcomes
Relationship between the individual ovarian dimensions with age (years) and ovarian reserve-OR (reflected by historical maximal FSH levels).
Results
A statistically significant decrease in ovarian size parameters accompanied advancing age (ovarian width, r= − 0.30, p= 0.01, ovarian length, r = − 0.24, p= 0.04, and the mean overall diameter, r= − 0.30, p= 0.01). FSH levels demonstrated a significant and linear correlation with age (r=0.39, p<0.01). Increasing levels of FSH (and hence declining OR) were associated with a significant decline in the mean ovarian width (r= − 0.39, p<0.01), the length (r= −0.38, p<0.01) and the overall mean ovarian diameter (− 0.42, p<0.01), Patients with a known diagnosis of diminished ovarian reserve demonstrated significantly reduced ovarian dimensions compared to patients with other infertility etiologies. Multivariate linear regression analysis confirmed individual ovarian measurements (width, length and overall diameter) as independent predictors of OR (FSH levels) after adjusting for parameters that are known to influence ovarian size, i.e. age, smoking status, BMI and anovulation history. Ovarian width emerged as the strongest predictor of OR (largest β coefficient and hence the strongest association with OR status) compared to ovarian length and the mean ovarian diameter.
Conclusion
Single ovarian dimensions are reliable predictors of advancing age and declining OR status in premenopausal infertile women. The magnitude of this association is most robust for the ovarian width.
Keywords: Ovarian reserve, infertility, Ovarian diameter, Ovarian size, FSH
Introduction
Ovarian reserve (OR), a term that has evolved in the era of assisted reproductive technology, refers to the residual oocyte-granulosa cell repertoire that, at any given age, is available for procreation. Both quantitative and qualitative deteriorations in the oocyte complement, and therefore a waning OR, are recognized phenomenon associated with advancing age (1–5). Although a decline in OR thus accompanies chronological aging, an acceleration in this process appears to underlie in a sub set of premenopausal women. This latter occurrence is evidenced by suboptimal responses to attempts at ovarian stimulation, as seen in a proportion of young women undergoing treatments for infertility, and by the recent appreciation that this subset of women may be destined for transgression into menopause at a younger age (6–8).
A spectrum of markers prognostic of OR are validated to varying degrees in the infertile population. These include biochemical markers (Follicular Stimulating Hormone (FSH), Estradiol (E2) Inhibin B, Anti-Mullerian Hormone (AMH), FSH: LH ratio) (9–19), and ovarian morphometeric markers (ovarian volume, antral follicle count, AFC and mean ovarian diameter) (11, 20–23) assessed in the early follicular phase (basal) of the menstrual cycle. Dynamic assessment of OR (clomiphene citrate challenge test (CCCT) (11, 24–26), exogenous FSH ovarian response test (EFORT) (27), GnRH Analogue Stimulation Test (GAST) (28) improves sensitivity of OR assessment, albeit at the expense of inconvenience and increasing cost (14–17, 24–26, 29).
Despite the limitations (absence of a clear cut off level signifying diminished OR (DOR), inter cycle variability (30) and inter-laboratory variability depending on assay used) basal FSH levels are commonly utilized for OR assessment in routine clinical practice (9–11). Furthermore, historical elevated basal FSH levels, despite subsequent normalization, have been shown to be predictive of poor outcome following IVF (31).
In a recent study, Fratterelli et al., validated a simplified 2-diameteral formula (V= (D1 + D2)/2) for the assessment of OR (32). These authors demonstrated that mean ovarian diameter (length + width/2) provided a comparable degree of predictability of OR as the ovarian volume in infertile women undergoing ART (32). Taking this approach a step further, we set out to evaluate if the simple assessments of individual ovarian diameters (length and width) in the early follicular phase of menstrual cycle, as currently employed in our clinical practice, reliably reflects OR status (as reflected by historical maximal FSH and chronological age) in our patient population. We furthermore aimed to independently assess the strength of associations between OR and the individual ovarian parameters (length and width) after adjusting for factors that are know to influence ovarian diameters.
Materials and Methods
Infertile patients attending a tertiary care infertility practice in the early follicular phase of menstrual cycle were offered participation in a prospective observational study from April 2004–July 2005. The study was approved by the Clinical Center for Investigations (CCI) at Albert Einstein College of Medicine (AECOM) and the IRB at Montefiore Medical Center. Inclusion criteria comprised female partners of infertile couples attending our facility for management of infertility and presenting in the early follicular phase of the menstrual flow (cycle days 1–3). Written and signed informed consents were obtained.
At each visit, patients underwent evaluation of ovarian morphometry by TVUS (Aloka 1400, Phillips, 7.5 MHz). Serum samples were collected in the early follicular phase (days 1–3) of the menstrual cycle. Samples were stored at −80° C until the assessment of markers of ovarian reserve (FSH and E2) by electro-chemiluminescence immunoassays (ELICA, Roche Elecsys 1010, Roche Diagnostics, Indianapolis, IN; Estradiol II, minimal detection limit 5pg/ml, intra-assay CV 5.7% and inter-assay CV 6.2% and FSH, minimal detection limit 0.10 mIU/ml, intra-assay CV 1.8 % and interassay CV 5.3 %). Patient’s height (inches) and weight (pounds) were recorded and body mass index (BMI) was calculated (kg/m²).
Ultrasound evaluation of the ovaries was performed as per routine clinical practice, by members of the clinical team that included trained RN’s and REI fellows in training, under the direct supervision of board certified or eligible Reproductive Endocrinologist attending physicians. Ovarian images were procured in the saggital and coronal planes and the frozen image reflecting the largest ovarian dimensions was utilized for the measurement of ovarian length and width (cm) as per standard clinical practice. Mean values were calculated for ovarian length (right ovarian length + left ovarian length/ 2), width (right ovarian width + left ovarian width /2) and overall ovarian diameter (mean length + mean width/2).
The patients were asked to complete a questionnaire enquiring about demographic information (race/education/ethnicity)/medical and social (smoking/alcohol) histories. The etiology for infertility was abstracted from patient records, as were the maximal reported values for early follicular phase FSH for each patient. The assessment of OR was based on the maximal FSH levels (historical or current) available for each patient and the chronological age (years).
Statistics
The main outcomes of interest were the independent associations of individual ovarian measurements (mean ovarian width, length and overall diameter) with OR (FSH and age) in the premenopausal infertile women. Data were analyzed and confirmed to meet the Gaussian distribution for subsequent assessments by Pearson’s correlation and student’s t test analyses to determine the associations between OR parameters and the individual ovarian measurements. Assessments of the associations between the individual ovarian measurements and a known diagnosis of diminished ovarian reserve were made using a two tailed Student’s t-test.
Multivariate linear regression models were created to determine the relationship between the individual ovarian parameters and FSH, after adjusting for confounders that have been known to influence ovarian dimensions, specifically age, smoking status (33, 34), a diagnosis of anovulation (35–37) and the BMI (38–40). Because of the significant correlation between the individual ovarian measurements, and therefore the risk of collinearity if incorporated within a single regression model, separate linear regression analyses were conducted incorporating one of the three measurements at a time to assess the magnitude of association (β coefficient) between the individual ovarian measurements and FSH levels. STATA (Intercooled STATA 8.2 for Windows, StataCorp, TX) was utilized for analysis and a p value of <0.05 was considered statistically significant.
Results
Sixty nine patients were enrolled over the study period. Table 1 demonstrates the patient demographics and characteristics. The continuous data are reported as mean ± SD, (range). The mean age of patients was 34.53 ± 4.50 (21–44). The mean value for the maximal reported FSH levels in the study population was 8.46 mIU/ml ± 3.70 (3.47– 20).
Table 1.
Describes the patient characteristics and ovarian reserve parameters.
| Variable | Mean ± SD (range) |
|---|---|
| Age (years) | 34.53 ± 4.50 (21– 44). |
| BMI (Kg/m²) | 27.15 ± 6.96 (19.11– 56.12) |
| FSH† (mIU/ml) | 8.46 ± 3.70 (3.47– 20) |
| Ovarian Length (cm) | 4.60 ± 1.01 (2.66–7.90) |
| Ovarian Width (cm) | 2.96 ± 0.84 (1.65– 5.68) |
| Overall Ovarian Diameter (cm) | 5.27 ± 1.24 (3.25–9.31) |
Historical maximal FSH level for the patient
Demographic information was available for 61/69 (88.40%). The majority of patients were Caucasians (65.57%); Asians and Blacks constituted 11.48% and 16.39% of the participants respectively and ethnicity was unknown for 6.56%. The commonest etiologies for infertility were anovulation (24.64%, 17/69) and male factor (21.74%, 15/69), followed by diminished ovarian reserve (14.49%, 10/69, unexplained infertility (15.94%, 11/69), tubal disease (13.04%, 9/69,), more than one etiology (5.80%, 4/69) and other diagnoses not specified (4.35%, 3/69). None of the enrollees had undergone a prior unilateral oopherectomy. Fifteen percent of the patients reported a history of smoking.
Adequate visualization of both ovaries was achieved in all the cases and the average values (cm) for the individual mean ovarian measurements were: length, 4.60 ± 1.01 (2.66–7.90), width, 2.96 ± 0.84 (1.65– 5.68) and overall diameter, 5.27 ± 1.24 (3.25–9.31). In a sub sample of patients (n=8), following the initial ovarian measurements performed by the clinical staff members, repeat measurements were performed by a single investigator (LP) to assess the Inter-observer variations in ovarian dimensions. A reassuring inter-observer consistency in the individual ovarian measurements was confirmed (r = 0.95, p<0.001 for ovarian length, r = 0.90 and p=0.002 for ovarian width), with CV’s of 3.90 % and 1.64% for length and width respectively.
A decrease in the ovarian size parameters (length, width and overall diameter) accompanied advancing age; this relationship was statistically significant for each of the three ovarian dimensions, i.e. ovarian width (r= −0.30, p=0.01), ovarian length (r=−0.24, p=0.04),and the mean overall diameter (−0.30, p=0.01), Figure1. FSH levels demonstrated a significant and linear correlation with age (r=0.39, p<0.01). Increasing levels of FSH (and hence declining OR) were associated with a significant decline in the mean ovarian width (r= −0.39, p<0.01), the mean length (r= −0.38, p<0.01) and the overall mean ovarian diameter (−0.42, p<0.01), Figure 2. The association between FSH levels and individual ovarian measurements was thus comparable to that of FSH with age. Patients diagnosed with diminished ovarian reserve demonstrated significantly lower parameters for each of the ovarian dimensions (ovarian width 2.39 ± 0.78, p<0.01, length 3.95 ± 0.74, p=0.01 and mean ovarian diameter 4.36 ± 1.13, p<0.01) compared to patients with other etiologies for infertility (ovarian width 3.09 ± 0.80, length 4.74 ± 1.00 and mean ovarian diameter 5.46 ± 1.18.
Figure 1.
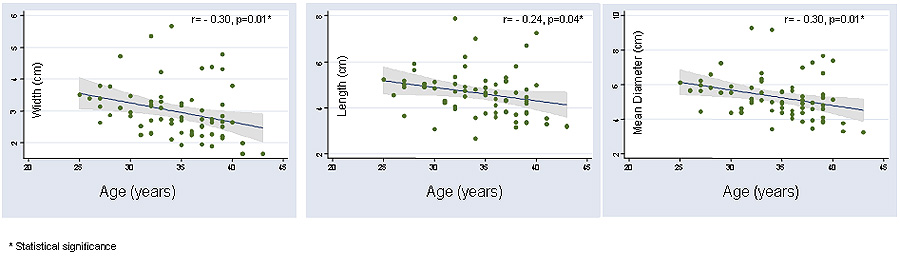
Significant and inverse correlation is demonstrated between advancing age and declining individual ovarian dimensions.
Figure 2.
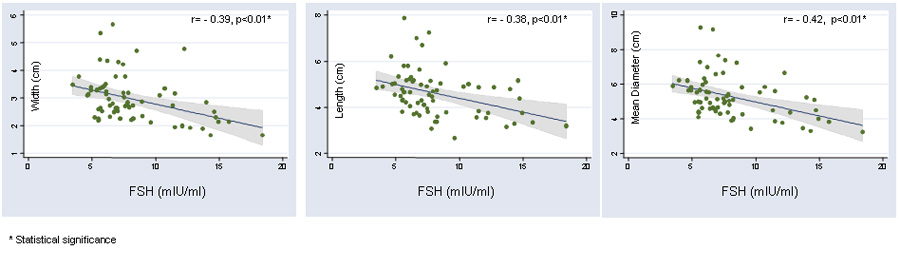
Significant and inverse correlation is demonstrated between declining ovarian reserve (increasing FSH levels) and individual ovarian dimensions.
Multivariate linear regression analysis confirmed the individual ovarian measurements (width/length and overall diameter) as independent predictors of FSH levels after adjusting for parameters that are recognized to influence the ovarian size, i.e. age (as demonstrated earlier by the data presented), smoking status, BMI and anovulation history. The adjusted analyses demonstrated the ovarian width as a stronger predictor of FSH levels (larger β coefficient and hence stronger association with OR status) compared to mean ovarian length or diameter, Table 2. The overall statistical models explained between 24–27% of the variability in FSH levels.
Table 2.
Magnitude (β coefficient) and reliability of associations (P value) between individual ovarian measurements and ovarian reserve (FSH levels), adjusting for parameters known to influence ovarian size (age, BMI, smoking status and a history of anovulation):
| Ovarian Parameter | (β coefficient) | P value | R² |
|---|---|---|---|
| Ovarian Width (cm) | −1.17 | 0.01* | 0.26 |
| Ovarian Length (cm) | −0.78 | 0.02* | 0.24 |
| Ovarian diameter (cm) | −0.78 | <0.01* | 0.27 |
statistically significant
Discussion
An improved ascertainment of the OR status may help optimize the planned therapeutic intervention, and thus minimize the emotional and financial strain placed upon couples seeking fertility treatment. The expanding list of tests predictive of OR reflects the ongoing quest for reliable, efficient, affordable and simple methods that can be incorporated into the day to day clinical practice. Although the ellipsoid ovarian volume as assessed by transvaginal ultrasound (32) has demonstrated prognostic value in terms of quantitative ovarian response (21), with minimal interand intra-observer variability (41), this requires an additional 3rd dimensional measurement that may not be performed in routine clinical practice. Assessment of the OR by measuring the ovarian volume is thus somewhat cumbersome (32) and even though the time spent in procuring the additional third measurement may not amount to much for an individual patient, the cumulative minutes accrued in a busy clinical practice may be meaningful.
A review of the literature has yielded two published reports addressing a simpler ovarian morphometric assessment utilizing the mean ovarian diameter as a prognosticator of OR status (11, 32). In a retrospective study by Frattarelli et al, ovarian measurements were performed by TVUS in the early follicular phase of the menstrual cycle in 278 patients. The authors demonstrated an inverse association between the ovarian diameter (computed as an average of length and width) and age, basal FSH levels and the basal FSH: LH ratio (11). Statistical methodology employed in the mentioned reports (11, 32) however has been limited to a correlation analysis without addressing the potential confounding influences.
We have attempted to explore the associations of the individual ovarian measurements (i.e. ovarian length and width) as well as the overall mean ovarian diameter (average of the length and width) independently with the OR, as reflected by the highest reported FSH value for each participant, as well as with advancing age. Our data confirm that the individual ovarian parameters (width, length or an average of the two) reliably reflect ovarian reserve in pre-menopausal infertile women. We have further attempted to adjust for potential confounders that may influence ovarian size, including a history of smoking (33, 34) and ovulatory status (i.e. larger ovarian size associated with annovulatory status, specifically polycystic ovarian syndrome (35–37), as well as the BMI (37–39). While all the three ovarian measurements demonstrate a negative association of statistical significance with increasing FSH levels (and hence declining OR), and with advancing age, the magnitude of these associations was most robust for ovarian width. Although larger mean ovarian length measurements were noted in our patients diagnosed with PCOS (5 mm) compared to those with other infertility etiologies (4.6mm), this difference was not of statistical significance (p=0.81).
Our data demonstrate that while all the three individual parameters are reliable prognosticators of OR as reflected by the FSH levels, the mean ovarian width exhibits a more robust relationship with the OR status compared to the ovarian length or the average of the two dimensions. Future clinical and research endeavors should focus on validation of these simple tools that are commonly utilized in the day to day clinical practice for the assessment of ovarian reserve status.
Acknowledgements
The authors would like to acknowledge Joshua Skorupski, MD. for his valuable contributions to this project, The work is supported in part by NIH 5K12 RR17672 (to LP) and CD 41978 (to NS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Menken J, Trussell J, Larsen U. Age and Infertility. Science. 1986;233:1389–1394. doi: 10.1126/science.3755843. [DOI] [PubMed] [Google Scholar]
- 2.Speroff L. The effect of aging on fertility. Curr Opin Obstet Gynecol. 1994;6:115–120. [PubMed] [Google Scholar]
- 3.Buyalos RP, Daneshmand S, Brzechffa PR. Basal estradiol and follicle stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction therapy. Fertil Steril. 1997;68:272–277. doi: 10.1016/s0015-0282(97)81514-2. [DOI] [PubMed] [Google Scholar]
- 4.Creus M, Penarrubia J, Fabregues F, Vidal E, Carmona F, Casamitjana R, Vanrell JA, Balasch J. Day 3 serum inhibin B and FSH and age as predictors of assisted reproduction treatment outcome. Human Reproduction. 2000;15:2341–2346. doi: 10.1093/humrep/15.11.2341. [DOI] [PubMed] [Google Scholar]
- 5.Galey-Fontaine J, Cedrin-Durnerin I, Chaibi R, Massin N, Hugues JN. Age and ovarian reserve are distinct predictive factors of cycle outcome in low responders. Reprod Biomed Online. 2005;10:94–99. doi: 10.1016/s1472-6483(10)60808-5. [DOI] [PubMed] [Google Scholar]
- 6.de Boer EJ, den Tonkelaar L, te Velde ER, Burger CW, Klip H, van Leeuwen FE OMEGA-project group. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77:978–985. doi: 10.1016/s0015-0282(02)02972-2. [DOI] [PubMed] [Google Scholar]
- 7.de Boer EJ, den Tonkelaar L, te Velde ER, Burger CW, van Leeuwen FE. OMEGA-project group Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum Reprod. 2003;18:1544–1552. doi: 10.1093/humrep/deg278. [DOI] [PubMed] [Google Scholar]
- 8.Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, Parsons J, Seed P. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod. 2003;18:527–533. doi: 10.1093/humrep/deg101. [DOI] [PubMed] [Google Scholar]
- 9.Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-Stimulating Hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51:651–654. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 10.Toner JP, Philput CB, Jones GS, Mausher SJ. Basal follicle stimulating hormone level is a better predictor of in vitro fertilization performance than age. Fertil Steril. 1991;55:784–791. doi: 10.1016/s0015-0282(16)54249-6. [DOI] [PubMed] [Google Scholar]
- 11.Frattarelli JL, Lauria-Costa DF, Miller BT, Bergh PA, Scott RT. Basal antral follicle number and mean ovarian diameter predict cycle cancellation and ovarian responsiveness in assisted reproductive technology cycles. Fertil Steril. 2000;74:512–517. doi: 10.1016/s0015-0282(00)00708-1. [DOI] [PubMed] [Google Scholar]
- 12.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77:468–471. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Welt CK, Cramer DW. Inhibin A and Inhibin B reflect ovarian function in assisted reproduction but are less useful at predicting outcome. Hum Reprod. 1999;14:409–415. doi: 10.1093/humrep/14.2.409. [DOI] [PubMed] [Google Scholar]
- 14.De Vet A, Laven J, de Jong F, Themmen A, Fauser B. Antimullerian Hormone Serum levels: a putative marker of ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 15.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP. Serum Anti-Mullerian Hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 16.Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti mullerian hormone is more strongly related to ovarian follicular status than serum inhibin, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 17.Tremellen KP, Kolo M, Gilmore A, LeKanage DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20–24. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee T, Copperman AB, Lapinski R, Sandler B, Bustillo M, Grunfeld L. An elevated day three follicle stimulating hormone: luteinizing hormone ratio (FSH: LH) in the presence of a normal day 3 FSH predicts a poor response to controlled ovarian hyperstimulation. Fertil Steril. 1996;65:588–593. doi: 10.1016/s0015-0282(16)58159-x. [DOI] [PubMed] [Google Scholar]
- 19.Ho JY, Guu HF, Yi YC, Chen MJ, Ho ES. The serum follicle-stimulating hormone-to-luteinizing hormone ratio at the start of stimulation with gonadotropins after pituitary down-regulation is inversely correlated with a mature oocyte yield and can predict "low responders". Fertil Steril. 2005;83:883–888. doi: 10.1016/j.fertnstert.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in-vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–336. doi: 10.1016/s0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- 21.Lass A, Skull J, McVeigh E, Margara R, Winston RM. Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotropin for in vitro fertilization can predict poor response. Hum Reprod. 1997;12:294–297. doi: 10.1093/humrep/12.2.294. [DOI] [PubMed] [Google Scholar]
- 22.Tomas C, Nuojua-Huttunen S, Martikainen H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotropins in in vitro fertilization. Hum Reprod. 1997;12:220–223. doi: 10.1093/humrep/12.2.220. [DOI] [PubMed] [Google Scholar]
- 23.Syrop CH, Dawson JD, Husman KJ, Sparks AF, van Voorhis J. Ovarian volume may predict assisted reproductive outcomes better than follicle stimulating hormone concentration on day 3. Hum Reprod. 1999;14:1752–1756. doi: 10.1093/humrep/14.7.1752. [DOI] [PubMed] [Google Scholar]
- 24.Loumaye E, Billion JM, Mine JM, Psalti I, Pensis M, Thomas K. Prediction of individual response to controlled ovarian hyperstimulation by means of clomiphene citrate challenge test. Fertil Steril. 1990;53:295–301. doi: 10.1016/s0015-0282(16)53284-1. [DOI] [PubMed] [Google Scholar]
- 25.Scott RT, Leonardi MR, Hofmann GE, Illions EH, Neal GS, Navot D. A prospective evaluation of clomiphene citrate challenge test screening in the general infertility population. Obstet Gynecol. 1993;82:539–544. [PubMed] [Google Scholar]
- 26.Navot D, Rosenwaks Z, Margolith E. Prognostic Assessment of female fecundity. Lancet. 1987;2:645–647. doi: 10.1016/s0140-6736(87)92439-1. [DOI] [PubMed] [Google Scholar]
- 27.Fanchin R, de Ziegler D, Olivennes F, Taieb J, Dzik A, Frydman R. Exogenous follicle stimulating hormone ovarian reserve test (EFORT): a simple and reliable screening test for detecting ‘poor responders’ in in-vitro fertilization. Hum Reprod. 1994;9:1607–1611. doi: 10.1093/oxfordjournals.humrep.a138760. [DOI] [PubMed] [Google Scholar]
- 28.Winslow KL, Toner JP, Brzyski RG, Oehninger SC, Acosta AA, Muasher SJ. The gonadotropin releasing hormone agonist stimulation test—a sensitive predictor of performance in the flare-up in vitro fertilization cycle. Fertil Steril. 1991;56:711–717. doi: 10.1016/s0015-0282(16)54604-4. [DOI] [PubMed] [Google Scholar]
- 29.Guleki B, Bulbul Y, Onvural A, Yorukoglu K, Posaci C, Demir N, Erten O. Accuracy of ovarian reserve tests. Hum Reprod. 1999;14:2822–2826. doi: 10.1093/humrep/14.11.2822. [DOI] [PubMed] [Google Scholar]
- 30.Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle Variability of OR tests: results of a prospective randomized study. Hum Reprod. 2004;19:590–595. doi: 10.1093/humrep/deh119. [DOI] [PubMed] [Google Scholar]
- 31.Abdalla H, Thum MY. Repeated testing of basal FSH levels has no predictive value for IVF outcome in women with elevated basal FSH. Hum Reprod. 2006;21:171–174. doi: 10.1093/humrep/dei288. [DOI] [PubMed] [Google Scholar]
- 32.Frattarelli JL, Levi AJ, Miller BT. A Prospective Novel Method of Determining Ovarian Size During In Vitro Fertilization Cycles. J Assist Reprod Genet. 2002;19:39–41. doi: 10.1023/A:1014062723606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharara FI, Beatse SN, Leonardi MR, Navot D, Scott RT., Jr Cigarette smoking accelerates the development of diminished ovarian reserve as evidenced by the clomiphene citrate challenge test. Fertil Steril. 1994;62:257–262. doi: 10.1016/s0015-0282(16)56875-7. [DOI] [PubMed] [Google Scholar]
- 34.Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, Syrop CH. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol. 1996;88:785–791. doi: 10.1016/0029-7844(96)00286-4. [DOI] [PubMed] [Google Scholar]
- 35.Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 36.Jonard S, Robert Y, Dewailly D. Revisiting the ovarian volume as a diagnostic criterion for polycystic ovaries. Hum Reprod. 2005;20:2893–2898. doi: 10.1093/humrep/dei159. [DOI] [PubMed] [Google Scholar]
- 37.Balen AH, Laven JSE, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definition. Hum Reprod Update. 2003;9:505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 38.Harlass FE, Plymate SR, Fariss BL, Belts RP. Weight loss is associated with correction of gonadotropin and sex steroid abnormalities in the obese anovulatory female. Fertil Steril. 1984;42:649–652. doi: 10.1016/s0015-0282(16)48154-9. [DOI] [PubMed] [Google Scholar]
- 39.Reid R, Van Vugt DA. Weight related changes in reproductive function. Fertil Steril. 1987;48:905–911. doi: 10.1016/s0015-0282(16)59581-8. [DOI] [PubMed] [Google Scholar]
- 40.Spandorfer SD, Kump L, Goldschlag D, Brodkin T, Davis OK. Obesity and in vitro fertilization; negative influences and outcome. J Reprod Med. 2004;49:973–977. [PubMed] [Google Scholar]
- 41.Higgins RV, van Nagell JR, Woods CH, Thompson EA, Kryscio RJ. Interobserver variation in ovarian measurements using transvaginal sonography. Gynecol Oncol. 1990;39:69–71. doi: 10.1016/0090-8258(90)90401-6. [DOI] [PubMed] [Google Scholar]


