Abstract
Notch signaling regulates B and T lymphocyte development and T cell effector class decision. In this work, we tested whether Notch activity affects mature B cell activation and differentiation to antibody-secreting cells (ASC). We show increased frequency of ASC in cultures of splenic B cells activated with LPS or anti-CD40 when provided exogenous Notch ligand Delta-like-1 (Dll1). Our results indicate that Notch–Dll1 interaction releases a default pathway that otherwise inhibits Ig secretion upon B cell activation. Thus, Dll1 enhanced spontaneous Ig secretion by naturally activated marginal zone B and B1 cells and reversed the inhibition of ASC differentiation mediated by B cell receptor crosslinking during LPS. Moreover, suppression of Notch signaling in B cell expression of either a dominant-negative mutant form of Mastermind-like 1 or a null mutation of Notch1 not only prevented Dll1-mediated enhancement of ASC differentiation but also reduced dramatically LPS-induced Ig secretion. Finally, we show that Dll1 and Jagged-1 are differentially expressed in discrete areas of the spleen, and that the effect of Notch engagement on Ig secretion is ligand-specific. These results indicate that Notch ligands participate in the definition of the mature B cell microenvironment that influences their terminal differentiation.
Keywords: activation, B cells, immunoglobulin, commitment, LPS
Notch receptors and their ligands are a family of conserved transmembrane proteins that regulate cell fate decisions in diverse tissues and organisms (1), including the lymphocyte lineage. The fundamental finding that Notch1–Delta-like-1 (Dll1) interactions condition hematopoietic progenitor differentiation by promoting T lineage and preventing B cell development opened a new field for the understanding of lymphocyte differentiation. These findings were born out of in vitro studies based on the usage of stromal cells transduced to express specific Notch ligands (2–4) and in vivo studies assessing loss- and gain-of-function mutant phenotypes (5, 6). Notch signaling also affects later stages of lymphocyte maturation; it participates in alternative helper T cell differentiation (reviewed in ref. 7) and in transitional B cell progression to a marginal zone (MZ) B cell phenotype. In this latter case, Notch2–Dll1 interactions and the transcription factor CBF1/Supressor of Hairless/Lag1(CSL) induced by Notch signaling have been shown to be specifically and strictly required (8–10). In contrast, the contribution of Notch signaling to B cell activation has not yet been systematically investigated.
B cell activation and subsequent differentiation to effector stages are tightly regulated, whether T cell-dependent or independent, during “natural” activation or upon immunization and infection. Which molecular components instruct or select a given clonal progeny to differentiate either to an antibody-secreting cell (ASC) or a memory cell, or to remain a nonsecreting blast, is unsolved.
Terminal differentiation to ASC is irreversible and appears to result from an all-or-nothing decision, because activated B cells either secrete high levels of Ig or retain the splice variant encoding a membrane-bound Ig. The “commitment choice” an activated B cell appears to undertake prompted us to assess whether Notch engagement participates in cell fate decision during B cell activation. We used a stromal cell line expressing the Notch ligands Dll1 or Jagged1 (Jg1), as well as purified recombinant ligands, to evidence that B cell differentiation to ASC is regulated by Notch signaling in a ligand- and receptor-specific manner.
Results
Dll1 Enhances LPS-Induced ASC Differentiation.
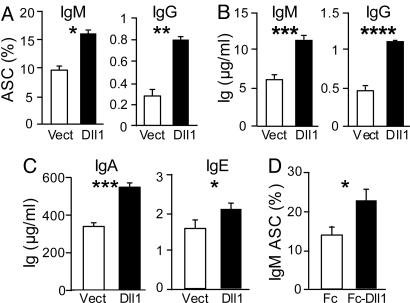
To assess the effect of Notch signaling on B cell differentiation to effector stages, resting splenic B cells were stimulated with LPS in the presence of S17 stromal cells transduced with an expression vector encoding the Notch ligand Dll1 (S17-Dll1) or GFP only (S17-vector) (2). The number of B cells recovered at day 4 in control and experimental cultures was not significantly different. In contrast, although 9–10% of the recovered B cells had differentiated to IgM-secreting cells in control conditions, the presence of S17-Dll1 increased this frequency to 16–19% (Fig. 1A). B cell differentiation to IgG-secreting cells in the presence of LPS was marginal, yet this was significantly enhanced by Dll1 (Fig. 1A). The titer of secreted IgM and IgG in the culture supernatants was directly proportional to the number of Ig-secreting cells, indicating that Dll1 controls the frequency of B cells that differentiate to Ig-secreting cells but not their secretion rate (Fig. 1B). In cultures supplemented with TGF-β or IL-4 to induce class switch, Dll1 also increased IgA or IgE secretion, respectively (Fig. 1C). Finally, immobilized recombinant Dll1 (Delta1ext-IgG; ref. 11) enhanced the differentiation of resting B cells to ASC upon LPS stimulation to a similar extent as S17-Dll1. Taken together, these results demonstrate that Dll1 directly promotes B cell terminal differentiation upon LPS stimulation.
Fig. 1.
Dll1 enhances B cell differentiation to ASCs. (A–C) Resting B cells were cultured with S17 cells retrotransduced with either the vector alone (Vect) or Dll1 construct (Dll1) in the presence of LPS (10 μg/ml) for 4 days. (A) Frequency of IgM and IgG ASC determined by Spot-Elisa. (B) Concentration of IgM and IgG in the culture supernatant. (C) IgA and IgE concentration in the supernatant of cultures supplemented in addition with TGF-β or IL-4. (D) Similar to A, except that S17 cells were replaced by coated Fc-Dll1ext-IgG (Fc-Dll1) or the Fc portion of human IgG (Fc). Shown is one representative experiment of six (A) or two (B–D). *, P ≤ 0.05; **, P ≤ 0.01.
Dll1 Operates on Late Stages of B Cell Activation to Promote Antibody Secretion.
We next investigated at which step of the activation process Dll1 would modify B cells response. Dll1 did not affect B cell survival and did not induce activation in the absence of LPS [supporting information (SI) Fig. 8]. Moreover, it increased neither the expression of activation markers (SI Fig. 9) nor the proliferative responses (Fig. 2A and SI Fig. 10) that follows exposure to LPS. Syndecan-1 (CD138) is the surface marker available to identify B cells at late stages of activation, although not exclusively at the Ig secreting stage (12). Analysis of CD138 expression on B cells stimulated for 2–4 days with LPS in the presence or not of Dll1 did not reveal significant differences (not shown). To directly test whether Dll1 influences late stages of B cell activation, cells were first activated with LPS for 3 days in the absence of stromal cells, purified according to CD138 expression, and seeded in secondary cultures for 24 h in the presence of stroma cells (Fig. 2B). Exposure of CD138+ cells to S17-Dll1 instead of S17-Vect resulted in an ≈2- to 2.5-fold increase in the number of recovered Ig-secreting cells (Fig. 2C). This effect was not due to differential B cell survival, as determined by TUNEL assays (SI Fig. 11). Dll1 also increased the number of IgM-secreting cells recovered from the CD138− cell cultures but did not modify the proportion of cells that acquired CD138 expression (Fig. 2D). These data indicate that Dll1 influences late stages of ASC differentiation, later than acquisition of the Syndecan marker.
Fig. 2.
Dll1 operates on late stages of ASC differentiation. (A) Resting B cells were cultured as in Fig. 1A. Proliferation was monitored by [3H]thymidine incorporation at day 3. (B–D) Splenic CD19+ cells were cultured alone for 3 days in medium containing 10 μg/ml LPS, purified according to CD138 expression (B), and seeded in secondary cultures together with S17-Vector (Vect) or S17-Dll1 (Dll1) and LPS (10 μg/ml) for 24 h. The frequency of IgM-secreting cells obtained from the two sorted populations (C) and CD138 expression on originally CD138− cells (D) was determined. (E) B1 and MZ B cells were maintained in cultures containing S17-Vect or S17-Dll1 in medium alone for 48 h before measuring the frequency of IgM-secreting cells. (F) Resting B cells were seeded on transduced S17 cells and treated with various amounts of antimicron chain mAb 2 h before the addition of 10 μg/ml LPS. On day 4, the number of IgM-secreting cells was scored by ESA. Shown are the percentages of IgM-secreting cells obtained in the respective control cultures (no antimicron mAb) from the means of triplicate cultures. The 50% inhibition is indicated by the dotted line. One experiment representative of three (A) and of two (B–F) independent repeats is shown. *, P ≤0.05.
In unimmunized mice, MZB and peritoneal B1 cells display a phenotype of nonsecreting activated cells. Their conversion to ASC in vivo requires migration out of the MZ and peritoneal cavity, respectively (13), a phenomenon proposed to result from release of local inhibition (14). Culturing MZB and B1 cells in medium without additional stimuli partially mimics this phenomenon, because a significant fraction of these cells convert to ASC. This conversion increased ≈3-fold in the presence of S17-Dll1 as compared with controls (Fig. 2E). These results suggest that activation of Notch signaling by Dll1 releases an inhibitory signal that otherwise maintains B cells in a nonsecreting blast state. An in vitro assay that reveals late inhibition of ASC differentiation is based on BCR cross-linking concomitant with LPS-mediated stimulation (15). In this assay, ASC inhibition is an “all-or-nothing” phenomenon at the single-cell level, dependent on BCR signal strength and BCR ligand concentration and independent of proliferation and Ig secretion rate (16). Dll1 induced ≈1-log decrease in the sensitivity of B cells to antimicron-mediated inhibition of ASC differentiation (Fig. 2F). This result strengthens our hypothesis that Dll1-Notch activation promotes ASC differentiation by reversion of inhibitory signals otherwise preventing Ig secretion.
LPS-Induced Ig Secretion Is Suppressed in the Absence of Notch1 and CSL Activity.
Dll1 appeared to trigger Notch signaling on activated B cells, because it led to the transcriptional up-regulation of the Notch target genes Hes-1 (17) and Deltex-1 (18) as revealed by semiquantitative RT-PCR analyses of day 4 LPS cultures (Fig. 3A). The canonical Notch pathway operates through the formation of a complex involving the cleaved intracellular portion of Notch (ICN), the CSL transcription factor, and the Mastermind-like proteins (MAMLs) (19). Expression of a dominant-negative form of MAML1 (DNMAML) inactivates this complex (20, 21). Follicular (FO) CD23+ B (FOB) cells expressing DNMAML were prepared from either DNMAMLf or poly(I:C)-treated Mx-Cre+ DNMAMLf mice (20). In the former case, DNMAML expression was induced in DNMAMLf B cells by Tat-Cre transduction, and cells expressing the DNMAML-GFP fusion protein (GFP+) were sort-purified. DNMAML-expressing and control cells proliferated equally upon LPS stimulation, irrespectively of whether they were plated on S17-Dll1 or S17-vect (not shown). However, DNMAML-expressing B cells poorly differentiated to ASC, and exogenous Dll1 failed to enhance this response (Fig. 3 B and C). These results confirm that the enhancement of ASC differentiation by Dll1 operates through the canonical Notch pathway. More importantly, these experiments revealed that plasma cell induction by LPS requires intact Notch/CSL/MAML pathway irrespective of the presence of exogenous ligand. In turn, this finding predicts that LPS-activated B cells express both the Notch receptor(s) and Notch ligand(s) necessary to allow Ig secretion. Real-time RT-PCR analysis confirmed that resting B cells express Notch2 but little Notch1 and Delta-1 (ref. 9 and data not shown). Western blot analysis revealed that resting and activated B cells express Dll1 protein to a level comparable to that of the S17 vector (Fig. 4A Right). As described by the supplier, Dll1-specific Ab also cross-react with Delta-4. The additional specific band detected by Western blot analysis may correspond to either Delta-4 or posttranscription modification of Dll1. Strikingly, upon LPS-mediated activation, B cells undergo a marked increase in Notch1 (N1) protein expression (Fig. 4A). To directly test whether N1 is required for ASC differentiation in the absence of exogenous ligand, splenic FO cells were isolated from poly (I:C)-treated Mx-cre+ Notch1f/f (22) or Mx-cre+ DNMAMLf/+ mice and respective controls and stimulated with LPS in the absence of stromal cells. Proliferation monitored at day 3 and cell counts at day 4 confirmed similar expansion between mutant and control cells (not shown). Both DNMAML expression and N1 disruption reduced by ≈3-fold the frequency of ASC recovered at day 4 of culture, when compared with controls (Fig. 4B). Overall, we interpret these results as indicating that LPS-induced ASC differentiation relies on N1 signaling through the CSL transcription factor. In vitro, in the absence of the exogenous Notch ligand, this signal may be triggered by presentation of the Notch ligand Dll1 by neighboring B cells. In vivo, several other cell types expressing higher amounts of Dll1 may be responsible for local triggering of Notch1 on activated B cells.
Fig. 3.
Inhibition of Dll1/Notch/CSL signaling abrogates ASC differentiation. (A) Expression of Hes1 and Deltex1 was assessed by conventional RT-PCR on cultures set as in Fig. 1A. Representative of at least two independent samples. (B) CD23+ splenic B cells purified from poly (I:C)-induced DNMAMLf Mx-Cre- (Cont) or DNMAMLf Mx-Cre+ (DN) BM chimeras were cultured and monitored as in Fig. 1A. (C) DNMAMLf/+splenic B cells were transduced with Tat-Cre and sorted 24 h later according to GFP expression. GFP− and GFP+ cells were plated separately in secondary cultures as in B (one experiment representative of two). *, P ≤0.05; ***, P ≤ 0.005.
Fig. 4.
LPS-induced ASC differentiation is suppressed in the absence of Notch1 and CSL activity. (A) Expression of Dll1 (Left) and N1 (Right) proteins in resting (−) and LPS-activated B cells isolated from WT splenocytes revealed by Western blot. Resting B cells were purified by Percoll gradient. Protein lysates from S17-vector and S17-Dll1 (Upper) or 3T3-vector and 3T3-N1ΔE (Lower) served as controls. The white lines indicate that intervening lanes have been spliced out. The additional frame in Upper shows a lighter exposure of the S17-Dll1 extract for Dll1 detection. (B and C) CD23+ splenic B cells were purified from poly(I:C)-induced Mx-Cre × DNMAMLf/+ (DN) and Mx-Cre × Notch1f/f (N1KO) mice and from the respective control mice (Cont). B cells were cultured and monitored as in Fig. 1A (one experiment representative of three).
Discrete Expression of Delta-1 in Specific Areas of the Spleen.
B cell activation and differentiation to plasma cells take place in defined areas of the spleen and lymph nodes. We assessed the location of Dll1 in spleen by immunostaining on serial sections (Fig. 5). Dll1 expressed by FOB cells or S17-vec was undetectable by immunostaining, whereas S17-Dll1 tested strongly positive (not shown). Dll1 expression, however, was readily detectable in the MZ and the FO dendritic cell (FDC) area of primary and secondary follicles (Fig. 5A). In contrast, expression of the Notch ligand Jg1 was strictly restricted to the MZ (Fig. 5B and data not shown). In the MZ, few Dll1/Jg1 double-expressing cells were detected (Fig. 5B). Similar data were obtained when analyzing mice immunized with OVA-Alum (not shown). In conclusion, restricted areas of the spleen present either Dll1 alone or Dll1 and Jg1 together, and the two ligands appear mostly differentially expressed.
Fig. 5.
Differential localization of Dll1 and Jg1 in the spleen. Serial sections of spleen prepared from nonimmunized C57BL/6 mice were submitted to immunostainings. (A) PNA staining alone revealed primary follicles (pFO) and germinal centers (GC), whereas anti-Dll1 was combined with FDCM2 Ab on immediate adjacent sections. (B) Representative costainings for Dll1 and Jg1. Solid and open arrows indicate FO and MZ areas, respectively. (Original magnification, ×200.) The additional frames show higher magnification (×800) of the corresponding boxed areas.
Dll1 Enhances B Cell Responses to T Cell-Dependent-Like Activation Signals.
The specific expression of Dll1 by FDCs in primary follicles and germinal centers prompted us to test whether Dll1 affects plasma cell differentiation after T cell-dependent activation. Stimulation of FOB cells by anti-CD40 mAb in vitro mimics partially this path. Dll1 enhanced B cell proliferative response induced by anti-CD40 Ab treatment (Fig. 6A) and consequently the number of cells recovered (not shown). In addition, the frequencies of cells secreting IgM or IgG (Fig. 6B) were increased. Taken together, these results indicated that Dll1-N1 signaling affects differently CD40L/CD40 and LPS/Toll-like-Receptor-4-mediated B cell proliferation, whereas it may still operate through the same pathway to regulate ASC differentiation.
Fig. 6.
Dll1 increases the frequency of B cells differentiating to ASC in response to T-dependent stimulus. Splenic resting B cells were cultured on S17-Dll1 (Dll1) or S17-Vector (Vect) stromal cells and supplemented with either 20 μg/ml or the indicated concentrations of anti-CD40 mAb. Incorporation of [3H]thymidine was measured at day 3 (A), and IgM- and IgG-secreting cell frequency was determined by Spot-ELISA at day 4 (B). Each assay has been performed at least twice in triplicate on independent cell samples, and similar results were obtained. *, P ≤ 0.05; **, P ≤ 0.01.
The Notch Ligand Jg1 Inhibits Ig Secretion by LPS-Activated B Cells.
The colocalization in the MZ of cells expressing either Jg1 or Dll1 prompted us to investigate whether Notch-mediated enhancement of plasma cell differentiation is ligand-specific. Surprisingly, S17-Jg1 cells inhibited both IgM (Fig. 7A) and IgG (not shown) secretion upon LPS activation. The effect seemed marginal but reproducible (1.5- to 2-fold in seven independent experiments). Similar inhibition was obtained when using coated Fc-Jg1 fusion protein (Fig. 7A). A previous report indicated that variation in ligand density could determine the outcome of Notch signaling during early hematopoiesis (4). To test whether, in our system, variation in ligand density could reproduce the differential effect of Jg1 and Dll1, we sort-purified S17-Jg1 and S17-Dll1 according to their GFP fluorescence and used each cell subset as feeders in cultures of resting B cells stimulated with LPS (Fig. 7C). The numbers of recovered B cells upon LPS cultures were similar in all conditions (not shown). In cultures seeded with S17-Dll1, the higher the GFP content in the stromal cells, the higher the frequency of IgM-secreting B cells. In sharp contrast, in cultures seeded with S17-Jg1, the higher the GFP content in the stromal cells, the lower the frequency of IgM-secreting B cells. These results demonstrate that Notch-mediated regulation of Ig secretion upon LPS activation is ligand-specific, and that ligand titration associates with a quantitative and not a qualitative alteration of the response.
Fig. 7.
Quantitatively but not qualitatively dose-dependent effect of Dll1 and Jg1 on ASC differentiation. (A) Resting B cells were cultured for 4 days in the presence of LPS in plates coated at 10 μg/ml with Fc-Dll1ext-IgG (Fc-Dll1) or Fc-Jg1ext-IgG (Fc-Jg) or the Fc portion of human IgG (Fc). (B) S17 cells transduced with Dll1 or Jd1 constructs were sort-purified according to GFP intensity. Full gray histogram, before sorting; open histograms, GFP distribution of the sorted populations. (C) Splenic resting B cells were cocultured on sorted S17-Dll1 (Left) or S17-Jg1 (Right) stromal cells and supplemented with 10 μg/ml LPS. (A and C) The numbers of IgM-secreting cells were determined at day 4 as in Fig. 1. Each assay has been performed twice on independent cell samples and yields similar results. *, P ≤ 0.05; **, P ≤ 0.01.
Discussion
In this work, we reveal that Notch signaling plays an essential role in mature B cell terminal differentiation to Ig-secreting plasma blast. Our study demonstrates that the Notch ligand Dll1 promotes ASC differentiation by a mechanism requiring Notch1 and CSL. Analysis of loss-of-function mutants for either CSL (8), Notch2 (9), or Delta-1 (10) has previously focused on addressing MZB cell differentiation. The first study in addition tested and failed to evidence a clear role for CSL in either T-dependent or independent immune responses. Whether the normal Ab responses reported in these MZB-less animals relate to additional modifications of B cell physiology associated with altered splenic architecture or compensatory mechanisms remains to be elucidated. In addition, in CSL-deficient cells but not in DNMAML-expressing cells, the potential repressor activity of CSL (discussed in ref. 7) would be absent and the expression of some Notch target genes derepressed, independently of Notch receptor–ligand interactions. During the preparation of the present manuscript, a report was made available indicating that Notch activity through CSL synergizes with a B cell antigen receptor and CD40 signals responsible for B cell proliferation and production of IgG1 (23). This study provides further support to the idea that previous CSL loss-of-function mutants did not allow direct assessment of Notch function during B cell activation. Finally, we document discrete expression of Dll1 in MZ and FDC areas of the spleen consistent with a role for this ligand in the local regulation of activated B cells during immune responses. The extension of our ex vivo study to physiological conditions and its articulation with the role of Notch in MZB differentiation will await the analysis of chimeric animals presenting normal MZB cells and specific mutation in the Notch pathway restricted to FOB cells.
We further demonstrate that Notch signaling is more than a modulator of activated B cells, because it is required for ASC differentiation. Thus, both Notch1-deficient and DNMAML-expressing B cells seeded in the absence of the exogenous ligand respond to LPS by vigorous proliferation but poor differentiation to Ig-secreting cells. These findings support the idea that B cell differentiation to Ig-secreting plasmablast upon LPS activation is not a default pathway. Transcriptional repressors and activators are regulated late during the B cell activation process that leads to Ig secretion (24). Which of these master genes are turned on or off upon Notch signal remains to be functionally assessed, because many display several CSL prototypical binding sites in their regulatory regions (unpublished data). Cell autonomous constitutive activation of the Notch pathway has never been reported. We therefore propose that B cells present to each other a Notch ligand allowing lateral Notch signaling induction and progression to terminal differentiation. This proposition is further supported by evidence that B cells express constitutively detectable levels of Delta1 and, only upon activation, high levels of Notch1 (Fig. 4). Whether this process of B cell lateral induction is restricted to culture conditions or also participates in B cell homeostasis in vivo remains to be addressed.
Our study reveals that Notch1 is involved in the regulation of B cell differentiation, namely terminal differentiation to Ig-secreting cells. Previous work assessing the role of Notch signaling in immature B cell commitment to a MZ B cell stage clearly evidenced a role for Notch2 (9). Notch2 appears constitutively expressed by cells of the B lineage (9), whereas Notch1 expression is inducible upon activation (Fig. 4). The differential expression of Notch receptor at a particular stage of B cell differentiation/activation must allow the use of common environmental clues (Notch ligand) for stage-specific regulation. A recent study revealed that Dll1 modulates the proliferative responses of B cells upon BCR and CD40 triggering, through the regulation of the MAPK signaling pathway (23). We here confirmed that Dll1 enhances B cell expansion that follows CD40 ligation and reveal that its effect on plasma cell differentiation is independent of its role in B cell proliferation and most probably of the MAPK pathway. It is conceivable that cell cycle regulation in B cells is under Notch2 control, whereas later regulation of genes involved in plasma cell differentiation is under Notch1 control.
In contrast to the B cell lineage, Notch1 had already been implicated in T cell differentiation to the effector stage. Thus, both Notch1 and Notch3 are expressed in peripheral CD4 T cells, and several conflicting studies report that the Notch signal affects, either positively or negatively, T cell differentiation to Th1 and/or Th2 (reviewed in ref. 25). Nevertheless, the genetic program that leads an activated B cell to differentiate into a plasma cell in which most metabolic resources serve the production of secreted Ig probably has little equivalent in T cell differentiation to helper cells. Moreover, our findings indicate that Notch1 signal interferes with the pathway that limits plasma cell differentiation. The genes involved in the progression of activated cells to Ig secretion are unlikely to be essential for T cell differentiation.
Our comparison of Dll1 with another Notch ligand, Jg1, revealed opposite effects on B cell terminal differentiation. We interpret these results as indicating that Jg1 does not induce the N1 signal required for ASC differentiation and competes with Dll1 for N1 binding, hence preventing B-B lateral induction of Notch signaling. Inhibitory binding of Jg1 to N1 has been reported (26) and described to result from interactions of higher affinity than those involved in the activation signal ensued by binding of Dll1 to N1. We show expression of both Jg1 and Dll1 in the MZ area, suggesting that competition may take place in situ. Moreover, because Jg1 is essential for discriminating Notch signals for tissue boundary formation and patterning during early embryogenesis (1), it may also participate in structuring the splenic B cell zone.
In conclusion, our findings that Dll1–Notch1 interactions release the inhibition of Ig secretion from LPS- and naturally activated B cells through canonical Notch signaling opens avenues for the positioning of Notch receptors, Notch ligands, and Notch signaling modulators in effector B cell regulation. In turn, it is expected that manipulation of these molecular components will help design alternative therapeutic strategies for the management of autoimmune diseases, multiple myeloma, and poor humoral protective immune responses.
Materials and Methods
Mice.
C57BL/6 (B6) mice were originally obtained from The Jackson Laboratory (Bar Harbor, ME). DNMAMLf (20) and Notch1f (22) mice have been described. All mice were bred under specific pathogen-free conditions at the Instituto Gulbenkian de Ciência facility, the University of Pennsylvania, and the Swiss Institute for Experimental Cancer Research, respectively, and used at 8–12 weeks of age. Mx-cre transgenic mice (27) were originally obtained from Taconic Farms (Gaithersburg, MD). Mouse experiments have been approved by the respective institute ethical committee and national authorities.
Induction of Cre-Mediated Floxed DNA Deletion.
DNMAMLf and Notch1f mice received polyIC (Sigma, St. Louis, MO) as described (27). Deletion was confirmed by GFP detection in blood Gr-1+CD11b+ cells (routinely >98%) and PCR on purified B cells, respectively. For DNMAML mice, after induction, 5 × 105 to 106 bone marrow cells were transplanted into lethally irradiated (900 rads) B6 mice, used at least 12 weeks after transplantation. In other experiments, DNMAML expression was induced in vitro by transduction of purified TAT-Cre (28) to CD19+ DNMAMLf splenic B cells stimulated for 24 h with LPS.
Antibodies, Flow Cytometry, and Cell Purification.
APC-conjugated anti-CD19 (1D3), phycoerythrin (PE)-conjugated anti-IgM (AFG-78), biotinylated anti-Mac-1 (M1/70), CD138 (Syndecan), CD19 (1D3), and CD23 (B3B4) were all purchased from BD Biosciences (Franklin Lakes, NJ). Biotinylated mAbs were revealed with Streptavidin-PE. Flow cytometry analysis was performed by using FACSCalibur and CellQuest (Becton Dickinson, Franklin Lakes, NJ) on gated lymph lymphocytes. Splenic resting and naturally activated B cells were separated by Percoll gradients as described (29), a preparation that yielded on average 98% CD19+CD23+ FO cells, and 80% CD21+.MZ B cells, respectively. B1 cells were purified from peritoneal lavages according to their CD19+IgM+Mac1high phenotype on a MoFlo sorter (Cytomation, Fort Collins, CO). Splenic FOB cells were prepared by magnetic purification on LS+ columns (Miltenyi Biotec, Auburn, CA) by using biotinylated anti-CD23 mAb and streptavidin microbeads. Purity was routinely >97% in all experiments.
Cell Culture Conditions and Reagents.
Dll1, Jg1, and control retrovirus, their production, and the transduction of S17 cells have been described (2). Dll1 and Jg1 expression was confirmed by immunostaining and inhibition of myoblast differentiation (2) (data not shown). LPS and anti-CD40 induced proliferation and plasma cell differentiation of B cells cultured on a monolayer of S17 cells, as well as BCR ligation and anti-CD40 stimulation assays, have been described (29). LPS from Salmonella typhimurium was purchased from Sigma; anti-μb (MB86) and anti-CD40 (FGK) mAbs were produced in-house. Culture supernatant from the transfected 3T3-IL4 cell line was used at a dilution 1/100. TGF-β was purchased from PreProTech (Rocky Hill, NJ) and used at 10 ng/ml. Delta1ext-IgG (11) was a gift from I. Bernstein (Fred Hutchinson Research Center, Seattle, WA), Jaggedext-IgG was purchased from R&D Systems (Minneapolis, MN), and reagent grade purified human Fc-IgG1 was from Sigma-Aldrich. Proteins (10 μg/ml) were immobilized as described (11). For proliferation assays, [3H]thymidine (ICN, Aurora, OH; sp. Act. 5 Ci/mol) was added for the last 6 h of 3 days of culture.
Elisa and Spot-ELISA.
IgM- and IgG-secreting cells were revealed by Spot-ELISA (29), and the number of spots was expressed as percentage of the recovered B cells in the respective cultures. IgM, IgG, IgA, and IgE concentration in culture supernatants was defined by ELISA.
PCR.
RNA was extracted with TRIzol and reverse-transcribed by using SuperScript II RT and oligo(dT)12–18 primer (Life Technologies, Gaithersburg, MD). Primer sequences are listed in SI Table 1. For standard PCR, the reaction was run for 30 cycles in a PTC-100 cycler (MJ Research, Waltham, MA).
Immunoblot Analyses.
Immunoblotting was performed as described (30). Gel loading was normalized to protein concentration and confirmed by β-actin probing. Antibodies specific for Delta-1 (C-20) and β-actin (I-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Intracytoplasmic Notch1 antibody was kindly provided by J. C. Aster (Harvard Medical School, Boston, MA). Membrane-tethered Notch1 ΔE transduced 3T3 cells were used as control (30).
Immunohistology.
Tissues were embedded in Tissue-Tek OCT (Sakura Finetek, Zoeterwoude, The Netherlands), and snap-frozen 8-μm-thick sections [Leica (Wetzlan, Germany) CM 1850] were collected onto 0.01% poly-l-lysine (Sigma–Aldrich)-coated slides, fixed in cold acetone, and blocked in PBS 3% BSA (Calbiochem, Darmstadt, Germany). Biotinylated anti-mouse FDCM2 (Immunokontact, Abingdon, U.K.) or PNA (Vector Laboratories, Peterborough, U.K.), goat anti-mouse Dll1, or rabbit anti-mouse Jg1 (both from Santa Cruz Biotechnology) were revealed by Avidin-D-Rhodamin (Vector Laboratories); anti-rabbit FITC (Jackson ImmunoResearch, West Grove, PA); anti-goat Alexa-488 and/or anti-goat Alexa-594 (both from Molecular Probes, Eugene, OR). Sections were mounted in Fluoromount-G (Southern Biotechnology, Birmingham, AL). Digital images were captured by using a Photometrics CoolSnap HQ camera and Metamorph software from a fluorescence microscope (Leica DMRA2). Postacquisition analysis used Image-J software.
Statistical Analysis.
Statistical analysis was performed by using the unpaired Student's t test.
Supplementary Material
Acknowledgments
We thank Andreia Gomes for help with the real-time PCR, Rosa Maria Santos for antibody production, and Alexis Perez for cell sorting. We thank Irwin Bernstein for the mouse FC-Dll1 fusion protein and Jan Andersson, António Coutinho, José Faro, and Thiago Lopes-Carvalho for critical evaluation of this work. This work was supported by the Fundação para a Ciência e Tecnologia, Portugal, with the coparticipation of Fundo Europeu de Desenvovimento Regional [Grants POCTI/MGI/44111/02 and BCI/37953/01 and fellowships SFRH/BD/6222/2001, SFRH/BPD/14978/2005, and SFRH/BD/2733/2000 (to M.A.S., L.M.S., and M.R. respectively)]. M.A.S. received a European Federation of Immunology Societies short-term fellowship. I.M. is supported by the Damon Runyon Cancer Research Foundation (DRG-102-05), W.S.P. by the National Institutes of Health and a Leukemia and Lymphoma Society SCOR Award, and F.R by the Swiss National Science Foundation.
Abbreviations
- ASC
antibody-secreting cell
- MZ
marginal zone
- FDC
follicular dendritic cell
- FO
follicular
- FOB
follicular B cell.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702891104/DC1.
References
- 1.Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Jaleco AC, Neves H, Hooijberg E, Gameiro P, Clode N, Haury M, Henrique D, Parreira L. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt TM, Zuniga-Pflucker JC. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 4.Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID. J Exp Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 6.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 7.Maillard I, Fang T, Pear WS. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 8.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, et al. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 10.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 11.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. J Cell Sci. 2000;113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 12.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. J Exp Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes-Carvalho T, Kearney JF. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 15.Andersson J, Bullock WW, Melchers F. Eur J Immunol. 1974;4:715–722. doi: 10.1002/eji.1830041103. [DOI] [PubMed] [Google Scholar]
- 16.Modigliani Y, Demengeot J, Vasconcellos R, Andersson J, Coutinho A, Grandien A. Int Immunol. 1997;9:755–762. doi: 10.1093/intimm/9.5.755. [DOI] [PubMed] [Google Scholar]
- 17.Davis RL, Turner DL. Oncogene. 2001;20:8342–8357. doi: 10.1038/sj.onc.1205094. [DOI] [PubMed] [Google Scholar]
- 18.Deftos ML, Huang E, Ojala EW, Forbush KA, Bevan MJ. Immunity. 2000;13:73–84. doi: 10.1016/s1074-7613(00)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 20.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, Allman D, Aster JC, Pear WS. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 22.Wolfer A, Wilson A, Nemir M, MacDonald HR, Radtke F. Immunity. 2002;16:869–879. doi: 10.1016/s1074-7613(02)00330-8. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 24.Calame KL, Lin KI, Tunyaplin C. Annu Rev Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 25.Osborne BA, Minter LM. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 26.Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. Nat Cell Biol. 2000;2:515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 28.Wadia JS, Stan RV, Dowdy SF. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 29.Demengeot J, Vasconcellos R, Modigliani Y, Grandien A, Coutinho A. Int Immunol. 1997;9:1677–1685. doi: 10.1093/intimm/9.11.1677. [DOI] [PubMed] [Google Scholar]
- 30.Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, Miele L, Cardoso AA, Classon M, Carlesso N. J Exp Med. 2005;202:157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.