Abstract
In the absence of any overt task performance, it has been shown that spontaneous, intrinsic brain activity is expressed as systemwide, resting-state networks in the adult brain. However, the route to adult patterns of resting-state activity through neuronal development in the human brain is currently unknown. Therefore, we used functional MRI to map patterns of resting-state activity in infants during sleep. We found five unique resting-states networks in the infant brain that encompassed the primary visual cortex, bilateral sensorimotor areas, bilateral auditory cortex, a network including the precuneus area, lateral parietal cortex, and the cerebellum as well as an anterior network that incorporated the medial and dorsolateral prefrontal cortex. These results suggest that resting-state networks driven by spontaneous signal fluctuations are present already in the infant brain. The potential link between the emergence of behavior and patterns of resting-state activity in the infant brain is discussed.
Keywords: development, functional MRI, spontaneous activity
Recent research on functional connectivity in the brain, in particular during resting-state conditions, has come to focus on low-frequency (<0.1 Hz), spontaneous fluctuations in the functional MRI (fMRI) signal. Discovered by Biswal et al. (1), it has been shown that systemwide networks in the resting brain are synchronized in time through intrinsic low-frequency signal fluctuations. Whereas early fMRI studies demonstrated synchronicity of intrinsic brain activity across hemispheres in primary sensory cortices (2, 3), succeeding studies have shown temporal synchronization in a resting-state network encompassing higher-order cortices (4). A systematic investigation of resting-state activity in the adult human brain was recently presented by Damoiseaux et al. (5). Using independent-component analysis (ICA), a data-driven explorative data analysis approach, they showed that there are numerous networks in the brain that are driven by spontaneous activity. Besides networks that are in part or fully described by the previously reported default mode (6) and task-positive network (7, 8), they found consistent patterns of resting-state activity in the visual cortex, sensorimotor areas, auditory areas, as well as extrastriate brain regions. These findings together with previous investigations on spontaneous activity suggest that the assumption that the brain during rest is idle and waiting to be triggered and respond to changes in the environment is not strictly valid. Rather, in addition to responding to changes in external stimuli or tasks, the brain is characterized by intrinsic dynamics in the form of coherent and spontaneous fluctuations, clustered together in networks that are credible from an anatomical and functional perspective.
Interestingly, recent studies have presented evidence that spontaneous activity is relevant for human behavior. Momentary lapses of attention, affecting goal-oriented behavior on a global/local selective attention task, were related to a failure to reduce activity in the default-mode network (9). Similarly, Fransson has shown that spontaneous, intrinsic activity in the default-mode network is markedly attenuated during a continuous verbal working memory task compared with rest (10). Moreover, spontaneous activity was found to correlate with behavioral measures such as anxiety ratings and executive-control performance (11). In a similar vein, it has recently been reported that the amplitude of spontaneous activity in pain and attention-related brain regions correlates with the degree of experienced pain (12). In addition, potential relationships between spontaneous activity and disease have been investigated (13, 14).
So far, spontaneous activity has only been studied in adults with the exception of one study performed in children that was restricted in scope to the primary visual cortex (15). To our knowledge, spontaneous brain activity at an early developmental phase has not been addressed in the literature. To understand how cognitive circuits are developed in the human brain, we studied spontaneous brain activity by a systematic investigation of resting-state networks in the infant brain by using fMRI. Twelve preterm infants were scanned at term-equivalent age during sleep for 10 min. Physiologically relevant resting-state networks across subjects were extracted by using ICA. Additionally, the possibility that a negative finding might be the result of inadequate sensitivity was addressed by performing multiple resting-state scans on a healthy adult subject on the same MR scanner with identical scanning parameters.
Results
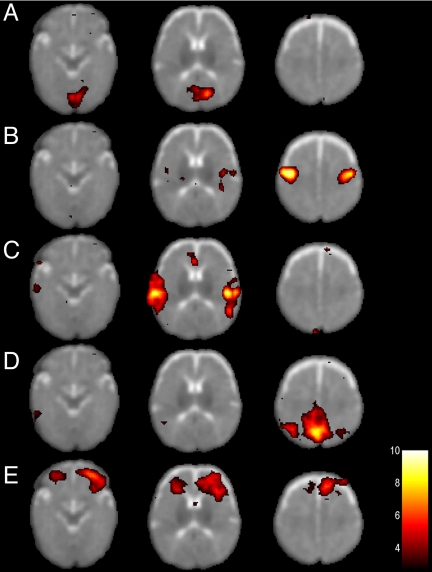
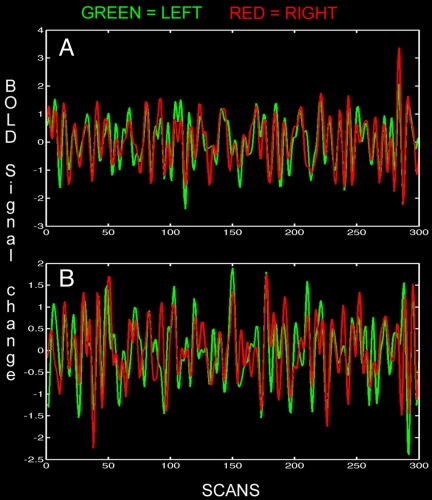
In individual subjects, anatomically coherent resting-state networks showed low-frequency BOLD fMRI signal changes <0.1 Hz, with the majority of the signal variance residing in the frequency range 0.01–0.05 Hz. In total, we could identify five resting-state networks in the infant brain. Representative spatial maps of resting-state networks in a single infant are shown in Fig. 1. Coherent patterns of spontaneous signal fluctuations at the individual level were typically observed in the medial section of the occipital lobe (Fig. 1A), bilaterally in the somatomotor cortex (Fig. 1B), bilaterally in the posterior temporal cortex (Fig. 1C), in the posterior medial and lateral parts of the parietal cortex (Fig. 1D), and in the anterior prefrontal cortex (Fig. 1E). The degree of temporal synchronicity of spontaneous BOLD signal changes across the hemispheres is shown in Fig. 2. Fig. 2A shows the temporal profile of BOLD signal oscillations in the bilateral somatomotor cortex during rest (see Fig. 1B), and the signal intensity time course in the bilateral posterior temporal cortex (see Fig. 1C) is shown in Fig. 2B.
Fig. 1.
Resting-state networks in a single infant. Five resting-state networks are shown that were consistently observed in all infants. Each row represents one resting-state network shown at three representative axial sections. Statistical maps are thresholded at P > 0.5 (alternative hypothesis threshold for activation vs. null) and shown superimposed on the T2-weighted infant image template. The left side of the image corresponds to the left side of the brain.
Fig. 2.
Bandpassed-filtered (0.012–0.1 Hz) BOLD signal intensity time courses showing coherent spontaneous oscillations across the hemispheres for the same infant shown in Fig. 1 during rest. (A) BOLD signal intensity in the left and right sensorimotor cortex (temporal correlation coefficient, 0.73) for the same subject and resting-state network as shown in Fig. 1A. (B) BOLD signal intensity time course in the left and right posterior temporal cortex (temporal correlation coefficient, 0.81) for the resting-state network shown in Fig. 1C.
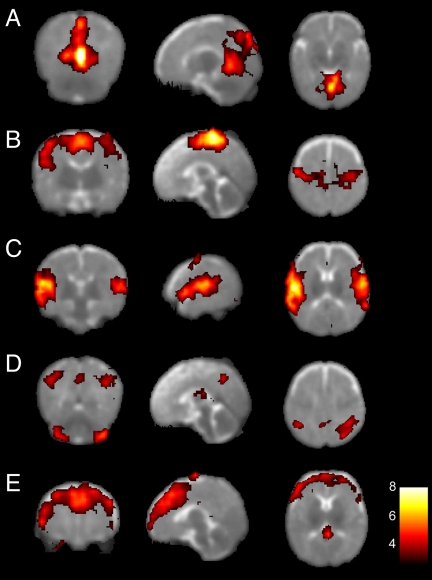
A group level analysis of resting state activity is shown in Fig. 3. Accordingly, Fig. 3A shows a resting-state network that encompasses primary visual cortex in the occipital lobe, extending into the parietal lobe, whereas the network displayed in Fig. 3B is predominantly located along the somatosensory and motor cortices bilaterally. The resting-state network shown in Fig. 3C is primarily located in the superior and posterior parts of the temporal cortex and the inferior parietal cortex, including the auditory area in the superior temporal gyrus. Fig. 3D shows a resting-state network that encloses the bilateral superior parietal cortex, precuneus as well as the lateral aspects of the cerebellum. Finally, a resting-state network was observed that consisted of the medial as well as the dorsolateral section of the prefrontal cortex (Fig. 3E). It should be noted that we could faithfully reproduce all 10 resting-state networks previously described by Damoiseaux et al. (5) using identical scanning parameters and equipment in a single adult subject [see supporting information (SI) Fig. 4].
Fig. 3.
Group resting-state networks in infants. Each row shows in a coronal, sagittal, and axial view resting-state networks thresholded at P > 0.5 (alternative-hypothesis threshold for activation vs. null) superimposed on a T2-weighted infant brain image template. Consistent resting-state patterns were found in the primary visual areas (A), somatosensory and motor cortices bilaterally (B), bilateral temporal/inferior parietal cortex encompassing the primary auditory cortex (C), posterior lateral and midline parts of the parietal cortex as well as the lateral aspects of the cerebellum (D), and medial and lateral sections of the anterior prefrontal cortex (E). The left side of the image corresponds to the left side of the brain.
Discussion
Our results suggest that resting-state networks are present already in the infant brain, which implies that long-range functional connectivity is implemented and in operation during an early phase in human brain development, in line with previous electroencephalographic investigations of neuronal activity in the infant brain (for a recent review, see ref. 16). Whereas the adult brain seems to include at least 10 networks (5), we found experimental support for five resting-state networks in the infant brain. There are both similarities and differences in terms of resting-state activity across the age span. The resting-state network located in the medial aspects of the occipital cortex (Fig. 3A) has repeatedly been found in the adult brain (2, 5, 17–19) as well as in the child brain (15). Similarly, the resting-state network predominantly residing along the somatosensory and motor cortex (Fig. 3B) has previously been reported in the adult brain (1, 5, 18). Moreover, our finding of a resting state in the temporal lobe and the inferior parietal cortex that largely included the primary auditory cortex in the superior temporal gyrus has previously been described in the adult brain (3, 5). However, the network depicted in Fig. 3D shows no direct correspondence with any previously reported resting-state networks in the adult brain. The anteriorly oriented resting-state network shown in Fig. 3E resembles the prefrontal resting-state network described previously by Beckmann et al. (see Fig. 6F in ref. 20) and Damoiseaux et al. (see Fig. 2J in ref. 5) in adults.
Notably, investigations performed in adults have presented evidence for resting-state networks that are lateralized and confined to one hemisphere. The study by Damoiseaux et al. showed that there are strongly lateralized frontotemporal and parietal resting-state networks in the adult brain (see Fig. 2 C and D in ref. 5). In contrast, our findings suggest that resting-state activity in the infant brain is predominantly nonlateralized and displays a strong functional correlation across the brain hemispheres. It is also interesting to note that the spatial configuration of all five resting-state networks in the infant brain seemingly lack a component along the posterior–anterior direction. In other words, our results suggest that resting-state activity largely is confined to correlation in functionally and anatomically homologous areas across the hemispheres. This finding is in contrast with previous observations in the adult brain where resting-state activity shows a functional correlation both across hemispheres and across brain regions that are spatially separated along the anterior–posterior direction (5, 7, 8). In the adult brain, the above-mentioned frontotemporal and parietal network as well as the default-mode network have in common a functional connectivity that stretches out in the anterior–posterior direction. This discrepancy in the spatial configuration of resting-state patterns may be related to recent findings regarding white matter (WM) fiber tracts in infants. A diffusion tensor MR imaging investigation revealed a significantly lower anisotropy index in the inferior longitudinal fasciculus, inferior frontooccipital fasciculus, and superior longitudinal fasciculus compared with the detected degree of anisotrophy in the interhemispheric callosal fibers (21), which suggests that the WM tracts that support functional connectivity in the anterior–posterior direction are less well developed in the infant brain compared with the tracts that support transcallosal functional connectivity (50).
The fact that resting-state patterns are largely confined to gray matter combined with the observation that the individual clusters are located in brain regions that collectively span systemwide, well known functional architectures in the brain suggests that coherent resting-state activity driven by low-frequency signal fluctuations is of functional relevance. It is interesting to compare our findings of intrinsic resting-state activity with results obtained from behavioral and conventional task-evoked neuroimaging studies in infants. Three of the five resting-state networks found in infants primarily involved brain areas that are known to be active for visual, auditory, and sensorimotor processing, respectively. To this end, task-evoked fMRI signal changes in response either to a reversing checkerboard (22) or to a flickering light (23) were observed in the primary visual cortex along the calcarine sulcus in sleeping infants. Moreover, somatosensory areas in sedated infants were activated bilaterally in response to a unilateral, passive extension and flexion of the hand (24). In the case of auditory perception, activity in the temporal lobe has been reported for both simple tone stimuli in sleeping infants (25) as well as for speech processing in awake infants (26, 27). Our findings regarding resting-state activity together with previous results showing task-evoked responses are in agreement with positron-emission tomography investigations that have shown the highest degree of glucose metabolism in the primary sensory regions of the infant brain (28, 29).
There are two caveats to the present work. First, all data were collected in infants that were born preterm (gestational age <28 weeks). Although all infants were scanned at term-equivalent age and we took precautions to exclude subjects who showed signs of WM abnormalities or who otherwise showed abnormal brain development, it cannot be ruled out that preterm birth or perinatal factors as such might influence the observed patterns of resting-state activity in the infant brain. For example, diffusion tensor MRIs have detected subtle regional changes in specific WM regions, including the corpus callosum and internal capsule in unselected preterm infants at term compared with term-born infants (30, 31). Such abnormalities could potentially have influenced our results (see also refs. 32 and 33). On the other hand, the resting-state networks reported here clearly indicate that functional connectivity was present across the above-mentioned WM regions.
Second, resting-state networks were observed in slightly sedated and sleeping infants. To this end, a previous investigation that used transcranial magnetic stimulation and electroencephalography has shown that the cortical electrical activity after a magnetic pulse to the cortex does not spread along the cortical surface during sleep. This observation is in contrast to the sequence of spatial waves of activity in the cortex observed during quiet wakefulness (34). These results suggest that the loss of consciousness during sleep is caused by a breakdown of cortical connectivity during certain stages of sleep. On the other hand, a recent study has shown that the resting-state activity in the form of spontaneous, low-frequency BOLD fMRI signal changes persists and even is enhanced during the early stages of sleep (35). In a similar vein, the previous study by Kiviniemi et al. (15) showed that low-frequency, spontaneous fMRI signal changes in the primary visual cortex persist during sedation in children. In addition, speech sounds have been found to be learned by sleeping newborns as indicated by electrophysiological responses (36). We could detect resting-state patterns in primary sensory regions (Figs. 1 A–C and 3 A–C) that are in good agreement with data from awake adult subjects. Hence, it seems unlikely that sedation and/or sleep per se would account for the observed differences in resting-state patterns between infants and adults. Importantly, a recent study has presented convincing evidence that spontaneous brain activity in monkeys is preserved during anesthesia (37).
We failed to detect a direct equivalent of a default-mode network in the infant brain. There could be several reasons for this negative finding. It seems unlikely that it was caused by insufficient sensitivity in terms of signal to noise because all 10 previously described resting-state networks in adults could be detected in a single adult subject (see SI Fig. 4). As stated above, one cannot rule out the possibility that an absence of the default mode is related to subtle brain pathologies in the premature brain that were not visible on anatomical MR scans. However, we suggest that the absence of the default mode might be related to the relative immaturity of the infant brain. It has been suggested that the default-mode brain regions are primarily involved in self-referential processing, social cognition, and self-projection (38; for recent reviews, see refs. 39 and 40). Speculatively, the absence of a default-mode network might indicate that the neuronal processes responsible for self-processing and social interaction as well as mental projection in time have not matured in the infant brain. Although the network depicted in Fig. 3E shows similarities with the bilateral prefrontal resting-state network previously found in adults (5, 20), it also involves the medial prefrontal cortex. If one collapses the anterior (Fig. 3E) and the posterior (Fig. 3D) into one network and considers them collectively, they will together to some degree resemble the default-mode network. To test the hypothesis that the two networks are predecessors of the default-mode network, we performed a correlation analysis of the signal intensity time courses from the two networks in all subjects. The correlation analysis was computed from individual independent components selected on the basis of a spatial-correlation analysis (18). The correlation between the networks shown in Fig. 3 D and E was not significant (Z score, −0.85, P > 0.1), which would indicate that the anterior network in Fig. 3E is indeed related to the resting-state network described previously (5, 20). Rather, we speculatively suggest that the network encompassing the posterior parts of the default mode including the bilateral parietal cortex and the precuneus in Fig. 3D can tentatively be regarded as a proto-default-mode network in the infant brain. Moreover, Dehaene-Lambertz et al. (27) recently reported a distributed pattern of task-evoked signal deactivations in the medial occipital, superior frontal cortices and the caudate nuclei in 3-month-old infants in response to auditory presentation of sentences. The detected pattern of task-evoked deactivation was largely not in spatial agreement with the default-mode network commonly observed in adults.
The value of studying resting-state brain activity has recently been debated (41, 42). Although we do agree with Buckner and Vincent (42) in that activity driven by spontaneous fluctuations is a relevant area of research that adds to our knowledge of human brain function, contributes to our understanding of clinical conditions such as Alzheimer's disease, as well as potentially facilitates the study of comparative anatomy across species, we believe that in this work we have added a developmental aspect to the study of resting-state activity. Similar to the recent surge of interest in establishing links between spontaneous brain activity and behavior, we believe that the study of spontaneous activity in the brain at different time points along the early part of the human life span will contribute to our knowledge of how cognitive abilities are gradually developed and finally reach a mature form in the human brain.
In conclusion, by recording BOLD fMRI signals in sleeping infants we found that the infant brain hosts resting-state brain activity that showed both commonalities and disparities with patterns previously reported for the adult brain. Whereas resting-state patterns in the visual, sensorimotor, and auditory regions in the infant brain showed large similarities with their adult counterparts, the default-mode network was not readily apparent in the infant brain.
Materials and Methods
Participants.
The studied infants were part of an ongoing, population-based study of infants born at an extremely low gestational age. MRI was performed at a term-equivalent age. The MR images were analyzed by a neuroradiologist experienced in pediatric MRI. Only infants without overt WM lesions such as parenchymal hemorrhagic infarction (PHI) or periventricular leukomalacia (PVL) were eligible for this work. Low-grade intraventricular hemorrhages (IVH grades 1 and 2) were accepted. In addition, all scans were evaluated according to a scoring system for WM abnormalities regarding MRI signal abnormalities, reduction in WM volume, cysts, ventriculomegaly, myelination, and thinning of the corpus callosum (43). Only infants with normal WM according to the scoring system were included in the study (44). Thus, a total of 12 infants (5 girls and 7 boys) were recruited from the four neonatal units in Stockholm. The mean gestational age of the infants was 25 weeks and 6 days (range 24 weeks and 4 days to 27 weeks and 5 days) and mean birth weight was 813 g (range 499–1,325 g). Mean postmenstrual age at time of scanning was 41 weeks and 0 days (range 39 weeks and 1 day to 44 weeks and 2 days). Six of 12 infants were delivered by cesarean section. Three of 12 infants did not need any ventilatory support and were only treated with continuous positive airway pressure. Five of 12 infants had a low grade IVH, none had PHI, posthemorrhagic hydrocephalus, or PVL. Further perinatal details are given in Table 1.
Table 1.
Perinatal parameters for infants included in the work
| Patient | Gender | BW, g | GA, weeks + days | GA at MRI | Delivery | BPD | PDA ligation | NEC | IVH grades 1 and 2 | IVH grade 3, PHI, PHHC, PVL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 755 | 26w2d | 40w5d | CS | 0 | 0 | 0 | 1 | 0 |
| 2 | F | 748 | 25w1d | 40w1d | CS | 1 | 1 | 0 | 1 | 0 |
| 3 | M | 882 | 25w4d | 40w1d | VD | 1 | 1 | 0 | 1 | 0 |
| 4 | M | 765 | 25w1d | 40w4d | VD | 0 | 0 | 0 | 0 | 0 |
| 5 | F | 948 | 25w6d | 41w6d | VD | 0 | 0 | 0 | 1 | 0 |
| 6 | M | 528 | 26w3d | 41w4d | CS | 0 | 0 | 0 | 0 | 0 |
| 7 | M | 1,325 | 27w5d | 44w2d | VD | 1 | 1 | 1 | 1 | 0 |
| 8 | F | 888 | 26w6d | 42w0d | VD | 0 | 0 | 0 | 0 | 0 |
| 9 | F | 499 | 25w1d | 39w1d | CS | 1 | 0 | 0 | 0 | 0 |
| 10 | F | 873 | 26w1d | 40w0d | CS | 0 | 0 | 0 | 0 | 0 |
| 11 | M | 660 | 24w4d | 39w6d | CS | 1 | 1 | 0 | 0 | 0 |
| 12 | M | 885 | 25w6d | 41w5d | VD | 1 | 1 | 0 | 0 | 0 |
Abbreviations: BW, birth weight; GA, gestational age; CS, cesarean section; VD, vaginal delivery; BPD, bronchopulmonary dysplasia (need for supplementary oxygen at 36 weeks postmenstrual age); PDA, patent ductus arteriosus; NEC, necrotizing eterocolitis; IVH, intraventicular hemorrhage; PHI, parenchymal hemorrhagic infarction; PHHC, posthemorrhagic hydrocephalus; PVL, periventricular leukomalacia.
The study was approved by the local ethical committee at the Karolinska University Hospital, and informed consent was obtained from all parents of the participating infants. A pediatrician stayed with the infant inside the scanner room throughout the imaging session to check on the infant's comfort. In agreement with our standard clinical protocol for neonatal MRI, infants were fed and given a low dose of the sedative agent chloral hydrate (30 mg/kg orally or rectally) with sustained spontaneous breathing, 15–30 min before MR scanning. Throughout the scanning session, arterial oxygen saturation and heart rate were continuously monitored.
MRI Acquisition.
All MRI data were acquired on an Intera 1.5 T scanner with an 8-channel receive-only head coil (Philips, Best, The Netherlands) at the Astrid Lindgren Children's Hospital in Stockholm. Anatomical high-resolution imaging included a T1-weighted turbo spin echo scan, an inversion recovery scan, as well as a three-dimensional gradient echo sequence (TR/TE/flip, 40 ms/4.6 ms/30 degrees; voxel size, 0.7 × 0.7 × 1 mm3). Further, T2-weighted turbo spin echo images were acquired in both a sagittal and a coronal slice orientation. Functional MRI data were acquired by means of a gradient echo planar imaging (EPI) sequence sensitized to T2*-weighted signal changes (TR/TE/flip, 2,000 ms/50 ms/80 degrees). Whole-brain coverage was accomplished by acquiring 20 echo-planar images in an axial-slice orientation (FOV, 180 mm; matrix size, 64 × 64; thickness, 4.5 mm, interleaved slice acquisition order) yielding a spatial resolution of 2.8 × 2.8 × 4.5 mm3. Resting-state functional connectivity was assessed by recording BOLD signal changes during 10 min of silent sleep (300 EPI image volumes). An additional four dummy scans were included to the beginning of the EPI acquisition to achieve steady-state magnetization. Because infants are more sensitive than adults to acoustic noise, particular care was taken to minimize exposure to high noise levels during MR scanning. In each infant, individually molded ear plugs (Affinis Dental Putty Soft; Forsberg Dental, Stockholm, Sweden) were used together with neonatal (MiniMuffs Natus Medical, Inc., San Carlos, CA) as well as pediatric ear muffs (Bilsom Junior; Bacou-Dalloz Nordic, Helsingborg, Sweden). To reduce the noise level inside the scanner even further, we used a tailor-made sound-dampening hood that was tightly attached to the upper half semicircle of the magnet bore, reducing the noise level with up to 24 dB (45). The total scanning time was ≈45–50 min.
Image Preprocessing.
As a first step, we used the BET algorithm included in the FSL (FMRIB, Oxford University, U.K.) software package to exclude all voxels that contained non-brain tissue from further analysis (46). Subsequent preprocessing steps were carried out within the SPM2 software package (Wellcome Trust Center for Neuroimaging, University College London, U.K.; ref. 47). Correction for subject movement was performed by realigning all EPI image volumes to the first image volume. Generally, subject movements were small both in terms of rotational and translational movement. However, in 6 of 12 subjects, the realignment procedure showed that the resting-state EPI datasets contained one, or in a few cases two, sudden and isolated “jerk-like” head movements, during which the infant's head was tilted away from its original position. Further, the realignment procedure showed that in all of these cases, within 10–20 s the infant tilted its head back into a position that was very close in space to its previous position. Because realignment correction algorithms are unable to cope with this sort of movement adequately, the affected image volumes were removed from the fMRI dataset, and the remaining image volumes were treated as one continuous dataset (mean no. of removed image volumes in affected datasets, 18; standard, 4 images). This procedure might be problematic in a stimulus-driven experimental design, but it has recently been shown that it is not detrimental in the context of analysis of low-frequency, spontaneous BOLD signal fluctuations (48). Subsequently, the realigned EPI data series were spatially normalized to an infant T2-weighted image template (26). Finally, all normalized EPI datasets were spatially smoothed by using a Gaussian isotrophic filter with a full-width, half-maximum of 6 mm.
Image Analysis.
At the individual level, spontaneous brain activity in the infant brain in the form of spatiotemporal resting-state patterns was extracted by using PICA (probabilistic independent-component analysis) as implemented in the MELODIC software within the FSL package (49). ICA is a data-driven, exploratory analysis tool that attempts to decompose the four-dimensional EPI datasets into separate components, based on the assumption that the spatial maps and their corresponding signal intensity time courses are statistically independent. Ideally, the resulting spatial maps will represent resting-state activation patterns and MR image artifacts of various kinds. Model-free approaches to data analysis such as ICA have previously been criticized because of the lack of interpretability and the possibility to do statistical inference testing. However, the PICA approach circumvents this problem by modeling the data as mixtures of spatially non-Gaussian signals and artifacts in the presence of Gaussian noise. Moreover, the overfitting problem, i.e., how many independent components that are present in the data, in PICA is handled by a dimensionality estimation procedure that is based on an objective assessment of the amount of Gaussian noise through Bayesian analysis of the data. Taken together, these methodological developments implemented in PICA allow a unique decomposition of the EPI data where each independent component is very likely to be the result of only one physical or physiological process (49).
The consistency of independent components across subjects was assessed by using a data concatenation approach that previously has been described by Beckmann et al. (20). In brief, the concatenation approach to multisubject analysis of resting-state patterns entailed as a first step a data reduction by forming a principal component analysis eigenbasis calculated from the mean data covariance matrix encompassing data from all 12 subjects. Second, the 30 most dominant eigenvectors obtained from the first step were used to project the individual dataset onto a dimensionality reduced data space. Third, an independent component analysis was performed on the temporally concatenated dataset, yielding 18 spatiotemporal patterns. The alternative hypothesis was tested at P > 0.5 for “activation” versus null, to create thresholded results for each resting-state network. A more detailed description of the concatenation approach to multisubject analysis of resting-state patterns is given in ref. 20.
Supplementary Material
Acknowledgments
We thank Johan Bengtsson and Dr. Lena Douglas for technical assistance and Dr. Christian Beckmann for statistical advice. H.L. was supported by a grant from the Torsten and Ragnar Söderberg Foundation. M.B. and S.H. were supported by grants from the Swedish Research Council (2006–6151), SLL (ALF 6076/os-226), the Jeanssons Foundation, and the Swedish Medical Society. A.N. was supported by a grant from the Frimurare Foundations. P.F. was supported by a grant from the Karolinska Institute Research Foundation.
Abbreviations
- EPI
echo-planar imaging
- fMRI
functional MRI
- ICA
independent-component analysis
- PICA
probabilistic independent-component analysis
- WM
white matter.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704380104/DC1.
References
- 1.Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Lowe MJ, Mock BJ, Sorenson JA. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 3.Cordes D, Haugton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 4.Greicius MD, Krasnow B, Reiss AL, Menon V. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fransson P. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MD, Snyder AZ, Vincent JL, Corbetta M, van Essen DC, Raichle ME. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 10.Fransson P. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Proc Natl Acad Sci USA. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greicius MD, Menon V. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonuga-Barke EJS, Castellanos FX. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kiviniemi V, Jauhianinen J, Tervonen O, Pääkkö E, Oikarinen J, Vainionpää V, Rantala H, Biswal B. Magn Reson Med. 2000;44:373–378. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 16.Vanhatalo S, Kaila K. Semin Fetal Neonatal Med. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Am J Neuroradiol. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 18.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Tian L, Jiang T, Liang M, Li X, He Y, Wang K, Cao B, Jiang T. Hum Brain Mapp. 2007;28:681–690. doi: 10.1002/hbm.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Philos Trans R Soc London B. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, et al. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Born P, Helle L, Miranda MJ, Rostrup E, Stensgaard A, Peitersen B, Larsson HBW, Lou HC. Ped Res. 1998;44:578–583. doi: 10.1203/00006450-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Morita T, Kochiyama T, Yamada H, Konischi Y, Yonekura Y, Matsumura M, Sadato N. Neurosci Res. 2000;38:63–70. doi: 10.1016/s0168-0102(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 24.Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F. Neuroimage. 2006;29:155–161. doi: 10.1016/j.neuroimage.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, Schneider KC, Gore JC, Ment LR. Magn Reson Imag. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- 26.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 27.Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. Proc Natl Acad Sci USA. 2006;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chugani HT, Phelps ME, Mazziotta JC. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 29.Chugani HT. Prev Med. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 30.Huppi PS, Maier SE, Pelad S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edvards AD, Counsell SJ. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Ment LR, Peterson BS, Vohr B, Allan W, Schneider KC, Lacadie C, Katz KH, Maller-Kesselman J, Pugh K, Duncan CC, et al. J Pediatr. 2006;149:490–498. doi: 10.1016/j.jpeds.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ment LR, Peterson BS, Meltzer JA, Vohr B, Allan W, Katz KH, Lacadie C, Schneider KC, Duncan CC, Makuch RW, Constable RT. Pediatrics. 2006;118:961–970. doi: 10.1542/peds.2005-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 35.Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RHR, Leopold DA, Duyn JH. Magn Reson Imag. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Cheour M, Martynova O, Näätänen R, Erkkola R, Sillanpää M, Kero P, Raz A, Kaipio ML, Hiltunen J, Aaltonen O, et al. Nature. 2002;415:599–600. doi: 10.1038/415599b. [DOI] [PubMed] [Google Scholar]
- 37.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 38.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin LQ, Iacoboni M, Lange C, Keenan JP. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Buckner RL, Carroll DC. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Morcom AM, Fletcher PC. Neuroimage. 2007 in press. [Google Scholar]
- 42.Buckner RL, Vincent JL. Neuroimage. 2007 doi: 10.1016/j.neuroimage.2007.01.010. in press. [DOI] [PubMed] [Google Scholar]
- 43.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 44.Horsch S, Hallberg B, Leifsdotter K, Skiöld B, Nagy Z, Mosskin M, Blennow M, Åden U. Acta Paediatr. 2007;96:979–984. doi: 10.1111/j.1651-2227.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 45.Lundh M. Stockholm, Sweden: Karolinska Institute; 2006. Thesis. [Google Scholar]
- 46.Smith SM. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friston KJ, Holmes AP, Worsley KP, Poline JB, Frith CD, Frackowiak RSJ. Hum Brain Mapp. 1995;2:189–200. doi: 10.1002/(SICI)1097-0193(1996)4:2<140::AID-HBM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beckmann CF, Smith SM. IEEE Trans Med Imag. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 50.Dubois J, Dehaene-Lambertz G, Perrin M, Mangin J-F, Contepas Y, Duchesnay E, Le Bihan D, Hertz-Pannier L. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20363. 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.