Abstract
Apparent blockage of monovalent cation currents by the permeating blocker Ca2+ is a physiologically essential phenomenon relevant to cyclic nucleotide-gated (CNG) channels. The recently determined crystal structure of a bacterial homolog of CNG channel pores, the NaK channel, revealed a Ca2+ binding site at the extracellular entrance to the selectivity filter. This site is not formed by the side-chain carboxylate groups from the conserved acidic residue, Asp-66 in NaK, conventionally thought to directly chelate Ca2+ in CNG channels, but rather by the backbone carbonyl groups of residue Gly-67. Here we present a detailed structural analysis of the NaK channel with a focus on Ca2+ permeability and blockage. Our results confirm that the Asp-66 residue, although not involved in direct chelation of Ca2+, plays an essential role in external Ca2+ binding. Furthermore, we give evidence for the presence of a second Ca2+ binding site within the NaK selectivity filter where monovalent cations also bind, providing a structural basis for Ca2+ permeation through the NaK pore. Compared with other Ca2+-binding proteins, both sites in NaK present a novel mode of Ca2+ chelation, using only backbone carbonyl oxygen atoms from residues in the selectivity filter. The external site is under indirect control by an acidic residue (Asp-66), making it Ca2+-specific. These findings give us a glimpse of the possible underlying mechanisms allowing Ca2+ to act both as a permeating ion and blocker of CNG channels and raise the possibility of a similar chemistry governing Ca2+ chelation in Ca2+ channels.
Keywords: calcium blockage, cyclic nucleotide-gated channel pore, NaK channel, nonselective cation channel
Cyclic nucleotide-gated (CNG) channels play an essential role in signal transduction pathways of visual and olfactory sensory systems, among others (1–4). Generally nonspecific for Group 1A monovalent cations, CNG channels also allow the passage of divalent cations like Ca2+ and Mg2+, albeit at much slower rates, through their pores. This, in turn, leads to an effective blockage of monovalent currents (5–14). Ca2+ blockage from the extracellular side, a property central to the physiological functioning of CNG channels (1), has been extensively studied by using electrophysiological tools and is thought to arise primarily from the involvement of a conserved acidic residue, usually glutamate, in the selectivity filter of the CNG channel (14–17). Mutating this residue to a neutral amino acid has been shown to drastically decrease external Ca2+ blockage, whereas substituting glutamate with aspartate preserved it. In the absence of structural information, it was previously speculated that the four conserved acidic residues of the channel tetramer, one from each subunit, point their side chains toward the ion conduction pathway and directly chelate a Ca2+ ion with their carboxylate oxygen atoms.
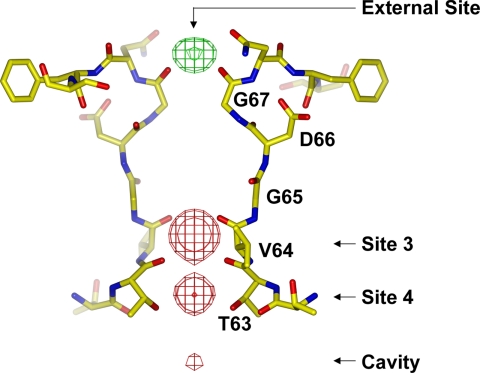
The recently discovered NaK channel from Bacillus cereus provides a viable structural model for analyzing Ca2+ permeation in CNG channels. NaK has a selectivity filter sequence of 63TVGDG67 similar to that of CNG channels [supporting information (SI) Fig. 7], and it exhibits similar ion conduction properties. NaK conducts various monovalent cations (18), and monovalent cation conduction can be reduced by divalent cations (SI Fig. 8). A discrete Ca2+ binding site at the extracellular entrance to the pore was observed in the crystal structure of NaK, revealing a possible structural basis for external divalent cation blockage in CNG channels (Fig. 1). Contrary to expectations based on the mutagenesis of CNG channels, the conserved acidic residue in the NaK filter (Asp-66) does not directly bind Ca2+. Instead, the four backbone carbonyl oxygen atoms of Gly-67, one from each subunit, chelate the Ca2+ ion. The side chain of Asp-66 actually points away from the ion conduction pathway with its carboxylate group positioned at the opposite side of the Ca2+-binding carbonyl group of Gly-67 and very close to the amide nitrogen of the peptide bond between Gly-67 and Gln-68. Here we performed an extensive crystallographic study of the NaK channel and its mutants to analyze their Ca2+ permeability and blockage properties, with a particular focus on the role of Asp-66 in specific Ca2+ binding at the extracellular entrance. Our results reveal a mechanism underlying specific Ca2+ binding in NaK, which most likely also applies to CNG channels.
Fig. 1.
Structure of the NaK selectivity filter (PDB ID code 2AHY) with an Fo − Fc ion omit map contoured at 8 σ indicating the bound ions (green mesh for Ca2+ and red for Na+).
Results
Extracellular Ca2+ Binding in D66E and D66N Mutants.
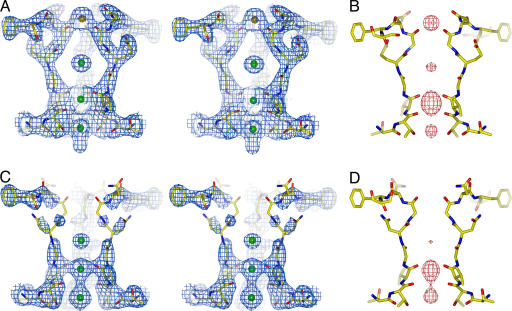
To test whether the conserved acidic residue (Asp-66) in the NaK filter is important for Ca2+ binding, similar to what has been observed in CNG channels, mutant NaK channels were generated in which Asp-66 was substituted with glutamate (D66E) and asparagine (D66N). By using 86Rb flux assays, these mutants were shown to conduct monovalent cations like the wild-type channel, indicating that the respective mutations did not affect the ion conduction properties of NaK (data not shown). Both the D66E and D66N mutants were crystallized in the presence of 200 mM CaCl2 and their structures were determined at 2.6 Å and 2.4 Å, respectively (Materials and Methods and SI Table 1) and shown to be virtually identical to wild type. However, strong electron density of a bound Ca2+ ion at the external entrance of the filter was present in the D66E mutant, as in wild type (Fig. 2 A and B) but absent in the D66N structure (Fig. 2 C and D), indicating a loss of external Ca2+ binding. The bound ion at the external entrance of the D66E filter was confirmed to be Ca2+ through soaking experiments which showed it is replaceable by Ba2+ but not monovalent cations (Rb+ or Cs+) (SI Fig. 9). Ca2+ binding at the external site therefore seems to be mediated primarily by the preservation of charge rather than side chain size. We also note that, in contrast to wild-type and D66E mutant channels, residues forming the entrance to the pore region (residues 65–68) in the D66N mutant appear to have become more disordered (Fig. 2C), suggesting that Ca2+ binding to the entryway might rigidify the local structure.
Fig. 2.
Structural analysis of NaK D66E and D66N mutants. (A) Stereoview of a 2Fo − Fc map (1.5 σ) of D66E at the filter region. The bound Ca2+ ion is modeled as an orange sphere. (B) An Fo − Fc ion omit map (8 σ) at the D66E filter. (C) Stereoview of a 2Fo − Fc map (1.5 σ) of D66N at the filter region. (D) An Fo − Fc ion omit map (8 σ) at the D66N filter.
Extracellular Ca2+ Binding in D66AS70E and D66A.
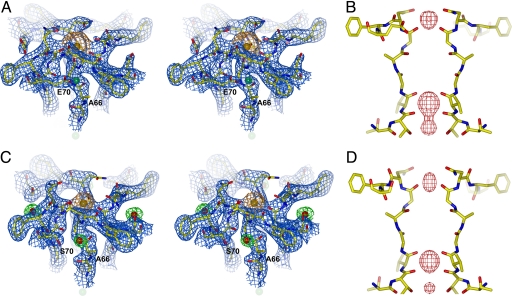
The D66E and D66N mutant structural data suggests that the presence of a negative charge in close proximity to the peptide bond between Gly-67 and Asn-68 is essential to mediate Ca2+ binding at the external site. To further test this hypothesis we designed a double mutant channel, D66AS70E, in which Asp-66 was replaced by alanine and Ser-70 was replaced by glutamate. The double mutant structure was determined at 2.5 Å (SI Table 1) and revealed the carboxylate group from the original Asp-66 residue replaced by one from Glu-70 of a neighboring subunit (Fig. 3A). Consistent with our hypothesis, strong Ca2+ ion density was observed at the pore entrance in both 2Fo − Fc and Fo − Fc ion omit maps (Fig. 3 A and B). The presence of a bound Ca2+ ion at this site was further confirmed by soaking experiments performed with other monovalent (Rb+ and Cs+) and divalent (Ba2+) cations (SI Fig. 10). As a control, the structure of an D66A mutant channel also was determined at 2.3 Å (SI Table 1). Interestingly, Ca2+ binding was still observed in this structure (Fig. 3 C and D) which, as discussed below, turned out to be much weaker than that observed in the wild-type, D66E, and D66AS70E channels. A closer inspection of the D66A structure revealed an ordered water molecule in the exact location of the missing Asp-66 carboxylate group (Fig. 3C). A partial negative charge on this water molecule could weakly compensate for the missing negative charge contributed by Asp-66 and, combined with the high concentration of Ca2+ in the crystallization conditions, likely accounts for the observed Ca2+ binding in the D66A mutant.
Fig. 3.
Structural analysis of NaK D66AS70E and D66A mutants. (A) A 2Fo − Fc map (1.5 σ) of the D66AS70E mutant around the external Ca2+ binding site. The density and sphere model of the Ca2+ ion are colored orange. (B) An Fo − Fc ion omit map (8 σ) at the D66AS70E filter. (C) A 2Fo − Fc map (1.5 σ) of the D66A mutant around the Ca2+ binding site. The density and sphere model of the Ca2+ ion are colored orange. Density for water molecules (red spheres) is colored green. (D) An Fo − Fc ion omit map (8 σ) at the D66A filter. Also see Fig. 6A for a close-up view of mutant NaK channels focused on the region surrounding residue 66.
Relative Ca2+ Binding Affinities in Wild-Type and Mutant NaK Channels.
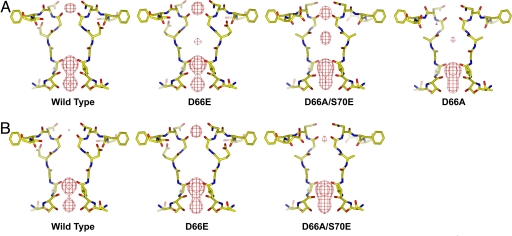
For a comparison of relative Ca2+ binding affinities in the wild-type and different mutant channels, we soaked the respective protein crystals in stabilization solutions containing lower concentrations of Ca2+ (10 mM and 1 mM; see Materials and Methods). Fig. 4 A and B presents Fo − Fc ion omit maps generated from crystals subjected to these titration experiments. At 10 mM Ca2+, density of externally bound Ca2+ was present in wild type and in the D66E and D66AS70E mutants but not observed in the D66A mutant (Fig. 4A). At 1 mM Ca2+, Ca2+ density became weak in wild type and in the D66AS70E mutant but remained strong in the D66E mutant, indicating higher Ca2+ binding affinity in D66E (Fig. 4B). The density of bound Ca2+ in D66E was shown to be abolished by soaking the crystals in a solution containing no Ca2+ (with 5 mM EGTA; data not shown). A number of important conclusions can be drawn from this crystallographic titration assay. First, the loss of Ca2+ binding at 10 mM Ca2+ concentration confirms that Ca2+ binding in the D66A mutant is very weak. Second, the results not only provide a qualitative picture of the relative Ca2+ affinities among wild-type and mutant channels, which follows the sequence D66E > wild type ≈ D66AS70E > D66A > D66N, but they also provide further support for the hypothesis that the presence of negative charge in the vicinity of Gly-67 plays the determinant role in Ca2+ binding. Finally, the reduction and eventual loss of electron density at lower Ca2+ concentrations further confirms the specificity of the external site for divalent cations.
Fig. 4.
Titration analysis of Ca2+ affinity in NaK and its mutants. Shown are the Fo − Fc ion omit maps (6 σ) of wild-type and mutant channel crystals soaked in stabilization solution containing 10 mM (A) and 1 mM (B) Ca2+.
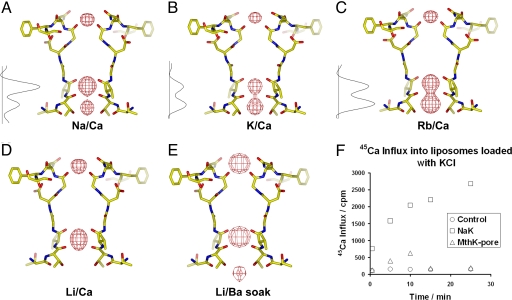
Second Ca2+ Binding Site Within the NaK Selectivity Filter.
An interesting and physiologically significant property of CNG channels is their ability to conduct Ca2+ in addition to monovalent cations. Functional studies of Ca2+ permeation and blockage in CNG channels have pointed to the presence of multiple Ca2+ binding sites (7, 15, 19, 20). This prompted us to look for evidence of Ca2+ binding elsewhere in the NaK filter in addition to the external site. Site 3 in the NaK channel was previously shown by soaking experiments to bind Ba2+ in addition to monovalent cations (19), suggesting that Ca2+ might also bind here. A second indication of Ca2+ binding at site 3 arose from a closer analysis of the Na+ and K+ complex structures of NaK (SI Table 2). The Na+ complex structure revealed much stronger electron density of bound ions at site 3 than at site 4 (Fig. 5A), whereas, in the K+ complex, structure electron density at both sites was roughly equivalent, with the density at site 4 being marginally stronger (Fig. 5B). Furthermore, we also determined the Rb+ complex structure of NaK at 2.6 Å, which revealed noticeably stronger electron density at site 4 than at site 3 (Fig. 5C). Taking into account the number of electrons in these various cations (Na+ = 10e, K+ = Ca2+ = 18e, and Rb+ = 36e) and the fact that monovalent cations bind at both sites 3 and 4 (based on previous soaking experiments), we predict that the observed differences in relative intensity of the electron density at sites 3 and 4 could arise from Ca2+ (the only other cation present in crystallization conditions) binding at site 3 in addition to monovalent cations. To confirm this, we purified the NaK channel in LiCl, crystallized it in the presence of 200 mM CaCl2, and determined its structure at 2.5 Å (SI Table 2). As shown in the Fo − Fc ion omit map, in addition to the external entrance of the pore, strong electron density also was observed at site 3 (Fig. 5D). Because the Li+ ion has only two electrons, this strong density must come from a bound Ca2+ ion. Site 4 did not reveal any such density, indicating that Ca2+ binds preferably to site 3 within the NaK filter. Difference maps calculated from the Li+ complex crystals soaked in a solution containing BaCl2 gave rise to strong density at the external entrance and site 3, indicating that both Ca2+ ions can be replaced by Ba2+ (Fig. 5E). Although not a direct measurement of Ca2+ permeation in NaK, the structural observation that Ca2+ binds deep in the selectivity filter in addition to the external site raises the possibility of the NaK channel, like CNG channels, being permeable to Ca2+.
Fig. 5.
Structural study of NaK channels crystallized in the presence of different monovalent cations. All crystallization conditions contain 200 mM CaCl2. Shown are Fo − Fc ion omit maps (8 σ) at the filter region calculated from crystals grown in the presence of 100 mM concentrations of NaCl (2.4 Å) (A), KCl (2.8 Å) (B), RbCl (2.6 Å) (C), and LiCl (2.5 Å) (D). The maps of Na+ and K+ complexes were calculated from previously determined structures with the PDB ID codes 2AHY and 2AHZ, respectively. One-dimensional electron density profiles of bound ions at sites 3 and 4 in Na+, K+, and Rb+ complexes were obtained by sampling the Fo − Fc ion omit maps along the central axis of the filter. (E) An Fsoak − Freference difference map (8σ) at 3.2 Å between crystal grown in LiCl/CaCl2 (reference) and crystal soaked in LiCl/BaCl2 (soak). (F) Time-dependent 45Ca influx into liposomes loaded with 400 mM KCl and reconstituted with NaKNΔ19, MthK pore, and no protein (as control).
Monitoring Ca2+ Permeation in NaK by Using 45Ca Flux Assays.
NaK has so far proven to be averse to electrophysiological analysis, possibly because of a low open probability and/or conductance. Additionally, the 86Rb flux assay used for initial analysis of ion conduction in NaK is not sensitive enough to measure Ca2+ permeation in NaK, nor can this assay distinguish between internal and external blockage of NaK by Ca2+. We therefore measured the uptake of Ca2+ directly in liposomes loaded with 400 mM KCl and reconstituted with NaKNΔ19, a truncated form of NaK lacking the N-terminal 19 residues. The method is similar to the 86Rb flux assays except that 45Ca is used as the radioactive tracer (see Materials and Methods). Results from these 45Ca flux assays show time-dependent accumulation of 45Ca in liposomes containing NaK but not in control liposomes containing either no reconstituted protein or the K+ conducting MthK pore (21), indicating that NaK likely conducts Ca2+ (Fig. 5F). The activity of the reconstituted proteins also was analyzed by 86Rb flux assays, which showed strong, time-dependent accumulation of 86Rb in liposomes reconstituted with NaKNΔ19 or the MthK pore (data not shown), confirming that both had the ability to conduct K+ and build up the required membrane potential for influx of 86Rb or 45Ca. These data, along with our structural studies, which were guided by functional and mutational analyses of Ca2+ permeation and blockage in CNG channels, offer a plausible structural basis for the so called “permeating block” associated with Ca2+ permeability.
Discussion
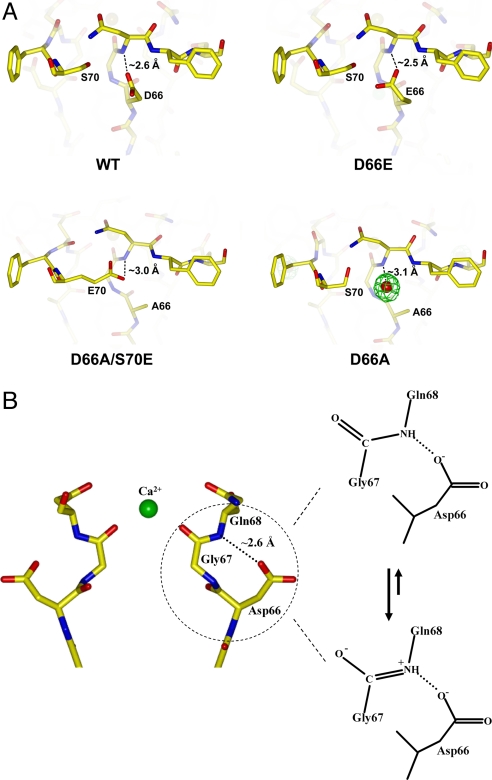
Ca2+ binding motifs in most known Ca2+ binding proteins, although diverse, use oxygen atoms contributed by carboxylate, carbonyl, and hydroxyl groups, as well as water molecules, as ligands (22, 23). Additionally, side chain carboxylate groups are always seen to act as ligands and most likely play a part in balancing the Ca2+ ion's positive charge. The mode of Ca2+ chelation in NaK, however, appears to be unique. The ion is chelated at both sites in the NaK pore exclusively by backbone carbonyl oxygen atoms rather than the carboxylate oxygen atoms of acidic residues. Only the external site is Ca2+-specific, and our data suggest that this specificity arises from a through-space interaction between the Asp-66 side chain and Gly-67 backbone carbonyl group, most likely electrostatic in nature, which helps generate a highly favorable environment for Ca2+ chelation by the latter. A close-up view of the region immediately surrounding residue 66 in NaK and its mutants (D66E, D66AS70E, and D66A) provides a plausible explanation for the specificity of external Ca2+ binding in NaK that most likely applies to CNG channels also (Fig. 6A). A common feature among these NaK channels is the close proximity of the amide nitrogen atom of the Gly-67/Asn-68 peptide bond and the negatively charged oxygen atom from the side chain carboxylate group (Asp-66 in wild type, Glu-66 in the D66E mutant, and Glu-70 in the D66AS70E mutant) or partially charged oxygen atom from the water molecule in the D66A mutant. In our view, using the wild-type channel as an example, the most likely scenario places the negatively charged carboxylate oxygen atom of Asp-66 close enough to the amide nitrogen of the Gly-67/Asn-68 peptide bond to stabilize its double bonded resonance form through a salt-bridge-like interaction (Fig. 6B). This, in turn, places a negative charge on the backbone carbonyl oxygen atom of Gly-67 from each channel monomer, making it a more suitable ligand for Ca2+. Our data also suggest that Ca2+ likely permeates the channel by subsequently binding to another intrapore binding site, site 3, which lacks the involvement of any acidic residues and is therefore nonspecific and able to bind monovalent cations.
Fig. 6.
Mechanism for the specificity of external Ca2+ binding in NaK. (A) Close-up view of wild-type and mutant NaK channels at the region surrounding residue 66. In wild type and in the D66E mutant, the carboxylate oxygen atoms from D66 or E66 form hydrogen bond interactions with the amide nitrogen of the Gly-67/Asn-68 peptide bond. In the D66A/S70E double mutant, the carboxylate oxygen of E70 from the neighboring subunit forms a similar hydrogen bond interaction with the same amide nitrogen. In D66A, a water molecule (red sphere with green mesh representing its electron density) sits at the same position as the carboxylate group in the wild-type channel and interacts with the amide nitrogen. Distances between the amide nitrogen of the Gly-67/Asn-68 peptide bond and the oxygen atoms involved in the hydrogen bond interaction are indicated. (B) Proposed mechanism underlying specific Ca2+ binding at the external entrance of the NaK filter mediated by the nearby acidic residue Asp-66.
The mechanism discussed above, whereby the properly positioned carboxylate group of Asp-66, instead of binding Ca2+ directly, appears to confer Ca2+ specificity to the external Ca2+ binding site, has intriguing implications for Ca2+ selectivity in Ca2+ channels, suggested to occur through similar mechanisms as those in CNG channels (2, 4). It is likely that Ca2+ channels also use backbone carbonyl groups to bind Ca2+ whereas the four acidic residues of the EEEE locus, shown by mutagenesis to be the sole element determining Ca2+ selectivity (24–26), confer Ca2+ specificity through similar mechanisms as seen here in NaK. This model is more readily reconciled with the plethora of evidence suggesting that Ca2+ channels have multi-ion pores when compared with concurrent views of a single, flexible, and rapidly rearranging Ca2+ binding site formed by the EEEE locus (27).
Materials and Methods
Protein Preparation and Crystallization.
All genes encoding wild-type and mutant NaK channel proteins were cloned into the pQE60 expression vector and expressed in Escherichia coli XL1-Blue cells. NaK mutants were generated by QuikChange site-directed mutagenesis (Strategene, La Jolla, CA). Proteins were purified as tetramers in n-decyl-β-d-maltoside (DM) with NaCl, RbCl, or LiCl present as the monovalent salt. Crystals of wild-type and mutant channels were grown by using the sitting drop vapor diffusion method at 20°C by mixing equal volumes of protein (30–35 mg/ml) and reservoir solution containing 200 mM CaCl2, 100 mM Tris·HCl (pH 8.0), 37–42% PEG 400, and 4% tert-butanol. All crystals were of space group C2221 with similar unit cell dimensions (SI Tables 1 and 2).
Crystal Soaking Experiments.
Mutant NaK crystals were soaked in stabilization solutions containing 40% PEG 400, 100 mM Tris·HCl (pH 8.0), 20 mM DM, 4% tert-butanol, 100 mM XCl, and 200 mM YCl2, where X and Y represent a monovalent cation (Na+, Rb+, or Cs+) and a divalent cation (Ca2+ or Ba2+), respectively. To minimize nonisomorphism, data from the respective mutant crystals soaked in a stabilization solution containing 100 mM NaCl and 200 mM CaCl2 was used as reference data, against which all data from other soaked crystals were merged and scaled. For the titration analysis of Ca2+ affinity, the respective crystals were soaked in stabilization solutions containing 40% PEG 400, 100 mM Tris·HCl (pH 8.0), 20 mM DM, 4% tert-butanol, 500 mM NaCl, and 1 or 10 mM CaCl2. The structures of those crystals used in the titration experiments were refined to resolutions between 2.8 Å and 3.1 Å.
Data Collection and Structure Determination.
All data were collected at the Advanced Photon Source and processed with HKL2000 (28). Structures were determined by rigid-body refinement using the Na+ complex structure of NaK as the initial model followed by iterative cycles of simulated annealing with torsion-angle dynamics in CNS (29) and model rebuilding in O (30). Detailed statistics of data collection and refinement are listed in SI Tables 1 and 2. All ion omit difference maps using Fourier coefficients (Fo − Fc) were calculated with phases from refined models that contained no ions in the selectivity filters.
Protein Reconstitution and Flux Assays.
The MthK pore and NaKNΔ19 constructs were purified as described previously (18, 21). The proteins were reconstituted in lipid vesicles composed of a 3:1 ratio of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine and 1-palmitoyl-2-oleoyl-phosphatidylglycerol at a protein/lipid ratio of 6 and 10 μg/mg, respectively, as described previously (31), with the following modifications: 10 mM DM was used to solubilize the lipid and dialysis [against a reconstitution buffer of 10 mM Hepes (pH 7.4), 400 mM KCl, and 4 mM N-methyl-d-glucamine] was used to slowly remove the detergent from the detergent/lipid/protein mixture. The reconstituted liposome samples were kept at −80°C in 100-μl aliquots.
86Rb flux assays were performed as described previously (18, 32) except that gel filtration through a prespun Sephadex G-50 fine resin soaked in 400 mM sorbitol/4 mM N-methyl-d-glucamine/10 mM Hepes, pH 7.4 (buffer A), was used to remove extraliposomal 86Rb before measuring radioactivity levels in liposomes. 45Ca flux assays were performed similarly to the 86Rb flux assays except that 45Ca was used as the radioactive tracer and buffer A was supplemented with 5 mM CaCl2.
Supplementary Material
Acknowledgments
Use of the Argonne National Laboratory Structural Biology Center beamlines at the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Energy Research. We thank the beamline staff for assistance in data collection. This work was supported by grants from the David and Lucile Packard Foundation and the McKnight Foundation. A.A. was supported by National Institutes of Health Training Grant T32 GM008297.
Abbreviation
- CNG
cyclic nucleotide-gated.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 2Q67 (D66A), 2Q68 (D66AS70E), 2Q69 (D66N0), and 2Q6A (D66E)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0707324104/DC1.
References
- 1.Yau KW, Baylor DA. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 2.Kaupp UB, Seifert R. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 3.Matulef K, Zagotta WN. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- 4.Zagotta WN, Siegelbaum SA. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 5.Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn JT, Solessio EC, Yau KW. Nature. 1997;385:815–819. doi: 10.1038/385815a0. [DOI] [PubMed] [Google Scholar]
- 7.Frings S, Seifert R, Godde M, Kaupp UB. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 8.Hackos DH, Korenbrot JI. J Gen Physiol. 1999;113:799–818. doi: 10.1085/jgp.113.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picones A, Korenbrot JI. Biophys J. 1995;69:120–127. doi: 10.1016/S0006-3495(95)79881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes LW, Kay AR, Yau KW. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- 11.Stern JH, Knutsson H, MacLeish PR. Science. 1987;236:1674–1678. doi: 10.1126/science.3037695. [DOI] [PubMed] [Google Scholar]
- 12.Colamartino G, Menini A, Torre V. J Physiol. 1991;440:189–206. doi: 10.1113/jphysiol.1991.sp018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman AL, Baylor DA. J Physiol. 1992;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert R, Eismann E, Ludwig J, Baumann A, Kaupp UB. EMBO J. 1999;18:119–130. doi: 10.1093/emboj/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Root MJ, MacKinnon R. Neuron. 1993;11:459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- 16.Eismann E, Muller F, Heinemann SH, Kaupp UB. Proc Natl Acad Sci USA. 1994;91:1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavazzo P, Picco C, Eismann E, Kaupp UB, Menini A. J Gen Physiol. 2000;116:311–326. doi: 10.1085/jgp.116.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi N, Ye S, Alam A, Chen L, Jiang Y. Nature. 2006;440:570–574. doi: 10.1038/nature04508. [DOI] [PubMed] [Google Scholar]
- 19.Karpen JW, Brown RL, Stryer L, Baylor DA. J Gen Physiol. 1993;101:1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzeja C, Hagen V, Kaupp UB, Frings S. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Berke I, Chen L, Jiang Y. J Gen Physiol. 2007;129:109–120. doi: 10.1085/jgp.200609655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz AK, Glusker JP, Beebe SA, Bock CW. J Am Chem Soc. 1996;118:5752–5763. [Google Scholar]
- 23.Pidcock E, Moore GR. J Biol Inorg Chem. 2001;6:479–489. doi: 10.1007/s007750100214. [DOI] [PubMed] [Google Scholar]
- 24.Tang S, Mikala G, Bahinski A, Yatani A, Varadi G, Schwartz A. J Biol Chem. 1993;268:13026–13029. [PubMed] [Google Scholar]
- 25.Ellinor PT, Yang J, Sather WA, Zhang JF, Tsien RW. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Ellinor PT, Sather WA, Zhang JF, Tsien RW. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- 27.Sather WA, McCleskey EW. Annu Rev Physiol. 2003;65:133–159. doi: 10.1146/annurev.physiol.65.092101.142345. [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 30.Jones TA, Zou JY, Cowan SW, Kjeldgaard Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 31.Heginbotham L, LeMasurier M, Kolmakova-Partensky L, Miller C. J Gen Physiol. 1999;114:551–560. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heginbotham L, Kolmakova-Partensky L, Miller C. J Gen Physiol. 1998;111:741–749. doi: 10.1085/jgp.111.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.