Abstract
Hantaviruses are zoonotic pathogens that maintain a persistent infection in their reservoir hosts, yet the mechanisms mediating persistence remain unknown. Regulatory T cell responses cause persistent infection by suppressing proinflammatory and effector T cell activity; hantaviruses may exploit these responses to cause persistence. To test this hypothesis, male Norway rats were inoculated with Seoul virus and regulatory T cells were monitored during infection. Increased numbers of CD4+CD25+Forkhead box P3+ T cells and expression of Forkhead box P3 and TGF-β were observed in the lungs of male rats during persistent Seoul virus infection. To determine whether regulatory T cells modulate Seoul virus persistence, regulatory T cells were inactivated in male rats by using an anti-rat CD25 monoclonal antibody (NDS-63). Inactivation of regulatory T cells reduced the amount of Seoul virus RNA present in the lungs and the proportion of animals shedding viral RNA in saliva. Because regulatory T cells suppress proinflammatory-induced pathogenesis, pathologic observations in the lungs were evaluated during infection. Subclinical acute multifocal areas of hemorrhage and edema were noted in the lungs during infection; inactivation of regulatory T cells reduced the amount of pathologic foci. Expression of TNF was suppressed during the persistent phase of infection; inactivation of regulatory T cells eliminated the suppression of TNF. Taken together, these data suggest that regulatory T cells mediate Seoul virus persistence, possibly through elevated transcription and synthesis of TGF-β and suppression of TNF. These data provide evidence of regulatory T cell involvement in the persistence of a zoonotic pathogen in its natural reservoir host.
Keywords: CD25, emerging infectious diseases, Forkhead box P3, hantavirus, TGF-β
Infectious diseases contribute significantly to morbidity and mortality worldwide. Of the major global outbreaks of infectious diseases, 11 of the past 12 have been zoonotic in origin and include diseases caused by HIV, SARS, flaviviruses, arenaviruses, hantaviruses, and avian influenza (1). Zoonotic pathogens often are maintained in the environment by causing persistent infection in their vector hosts. For example, hemorrhagic fever viruses, such as arenaviruses (family, Arenaviridae) and hantaviruses (family, Bunyaviridae) both persistently infect rodent reservoirs. Human contact with zoonotic pathogens is on the rise because of changes in environmental and behavioral factors that increase exposure to reservoir hosts, including rodents (2). Globally, hantaviruses are one of the most widely distributed zoonotic pathogens. Spillover of hantaviruses from rodents to humans causes hantavirus cardiopulmonary syndrome or hemorrhagic fever with renal syndrome, depending on the species of virus (3). The mechanisms mediating persistence of zoonotic pathogens, such as hantaviruses, in their natural hosts remain unknown.
Hantaviruses are negative sense RNA viruses that comprise three segments that encode the viral nucleocapsid (N), envelope glycoproteins (GN and GC), and an RNA polymerase (L). Hantaviruses are horizontally transmitted between rodents, and each hantavirus has coevolved with an individual rodent host species (4). Old World hantaviruses that cause hemorrhagic fever with renal syndrome in humans are found worldwide and include Seoul virus, which is maintained in Norway rats (Rattus norvegicus) (5). Rodents infected with their species-specific hantavirus remain persistently infected and shed virus in saliva, urine, and feces (6, 7). Hantaviruses are noncytopathic and do not cause observable disease in their reservoir hosts (8, 9). Human pathology caused by either hantavirus cardiopulmonary syndrome or hemorrhagic fever with renal syndrome is hypothesized to be caused by elevated CD8+ T cell responses and excessively high levels of proinflammatory cytokines (3). The precise mechanisms mediating viral persistence in rodents remain unclear, but one prevailing hypothesis is that hantaviruses may suppress rodent host immune responses that are necessary to resolve infection (10). Alternatively, hantaviruses may exploit host regulatory systems to subvert protective immune responses in these highly coevolved systems.
Elevated numbers of regulatory T cells contribute to the persistence of several pathogens in rodents and humans (11, 12). Regulatory T cells suppress proinflammatory responses locally at the site of infection to allow pathogen persistence, as well as suppression of proinflammatory-mediated pathogenesis (12, 13). Activity of regulatory T cells is primarily through cell–cell contact or local secretion of antiinflammatory mediators, including IL-10 and TGF-β (11). The balance between effector and regulatory T cells may reflect an evolutionary compromise between survival of the host and survival of the pathogen. Whether regulatory T cells influence host responses to zoonotic pathogens in their reservoirs to cause persistence has not been documented and was the primary aim of this study.
Consistent with our hypothesis that regulatory T cells contribute to the persistence of hantaviruses in rodents, we observed increased percentages of CD4+CD25+Forkhead box (Fox)P3+ regulatory T cells, as well as elevated transcription of FoxP3 and TGF-β mRNA during the persistent phase of Seoul virus infection in male Norway rats. Furthermore, inactivation of regulatory T cells caused a reduction in genomic Seoul virus RNA in target organs and decreased the proportion of male rats shedding viral RNA in saliva. As a result of regulatory T cell inactivation, we expected a concurrent increase of proinflammatory responses and immune-mediated pathogenesis but, instead, observed a reduction in lung pathology during the acute and persistent phases of Seoul virus infection. These data have critical implications for understanding the role of host regulatory responses in the emergence and reemergence of zoonotic pathogens.
Results
Seoul Virus RNA Copies Are Elevated in the Lungs During Seoul Virus Infection.
Previous studies indicate that Seoul virus RNA is detectable in several organs, including the lungs and spleen and that viral RNA persists for at least 60 days postinoculation (p.i.) in male rats (ref. 14; J.D.E. and S.L.K., unpublished data). To reestablish that Seoul virus persists in the lungs, male Norway rats were inoculated with Seoul virus and viral RNA was measured 3, 15, 30, and 40 days p.i. Seoul virus RNA was detectable in both the lungs and spleen at day 3 p.i. and was significantly elevated in the lungs 15, 30, and 40 days p.i. (Fig. 1a; P = 0.002). More Seoul virus RNA was present in the lungs as compared with the spleen at all time points measured during infection (Fig. 1a; P = 0.032). Because Seoul virus persisted for at least 40 days p.i., day 30 p.i. was selected as a time point during persistent infection.
Fig. 1.
Seoul virus RNA copies and regulatory T cell responses are elevated during infection. (a) Male rats were inoculated with 104 pfu of Seoul virus (SR-11), and viral RNA was measured in the lungs and spleen at days 3, 15, 30, and 40 p.i. by real-time RT-PCR. At days 0, 15, and 30 p.i., spleen and lung cells were stained with FITC–anti-CD4, phycoerythrin–anti-CD25, and allophycocyanin–anti-FoxP3. (b) For FACS analyses, cells were gated on lymphocytes, further gated for CD4+, and are shown as the percentage of CD4+ cells that are CD25+FoxP3+ at days 0, 15, and 30 p.i. (c) Transcription of FoxP3 mRNA also was measured in the lungs and spleen at days 0, 15, and 30 p.i. by real-time RT-PCR. For all dependent measures, samples were collected from uninfected rats at the same time as infected rats and are designated as day 0 p.i. The vertical bars indicate means ± SEM, and an asterisk indicates statistically significant differences from uninfected counterparts (*, P < 0.05).
Regulatory T Cells Are Increased During Persistent Seoul Virus Infection.
To determine whether regulatory T cells are affected by Seoul virus infection, we measured the numbers of CD4+CD25+FoxP3+ lymphocytes in spleen and lung tissue before (i.e., uninfected male rats; designated as day 0 p.i.) and during the acute (i.e., day 15 p.i.) and persistent (i.e., day 30 p.i.) phases of infection. On day 30 p.i., percentages of CD4+CD25+FoxP3+ regulatory T cells were elevated in both the lungs and spleen (Fig. 1b; lungs, P = 0.04; spleen, P = 0.005). There was no effect of infection on numbers of “activated” CD4+CD25+FoxP3− T cells (data not shown; P > 0.05). Expression of the regulatory T cell transcription factor FoxP3 was increased in the lungs during persistent infection at day 30 p.i. (Fig. 1c; P = 0.017), whereas expression was not significantly altered in the spleen during infection. Although regulatory T cell numbers increased in both the lungs and spleen, significant regulatory T cell activity, as indicated by elevated FoxP3 expression, was observed in only the lungs during Seoul virus infection; therefore, the primary focus of these studies was on responses to Seoul virus in the lungs because this was the site of elevated viral persistence and regulatory T cell activity.
Regulatory T Cells Are Functionally Inactivated by Using an Anti-Rat CD25 Monoclonal Antibody.
CD25+ regulatory T cells were inactivated by using an anti-rat CD25 mAb (clone NDS-63). The efficacy of this antibody to functionally inactivate CD25+ cells had not been reported, but previous studies using NDS-63 have demonstrated successful inhibition of IL-2-induced proliferation and heightened in vivo activity as compared with the commercially available clone OX-39 (15, 16). Additionally, studies using NDS-63, as well as a more recent report using a mouse anti-CD25 mAb (7D4), suggest that administration of anti-CD25 mAb causes functional inactivation rather than depletion of CD4+CD25+ regulatory T cells by down-regulating expression or inducing shedding of CD25 from the cell surface (16, 17). Numbers of regulatory T cells (CD4+CD25+FoxP3+) cells were significantly reduced in the lungs, spleen, and lymph nodes for 7 and 30 days after administration of the anti-CD25 mAb (NDS-63) (Fig. 2; P < 0.001). Rats were not infected, but time points were selected to mimic our protocol for Seoul virus infection and to represent time points during the acute (day 7) and persistent (day 30) phases of infection.
Fig. 2.
CD4+CD25+FoxP3+ regulatory T cells are reduced after administration of an anti-CD25 mAb. Uninfected male rats were administered 2 mg of anti-CD25 mAb (NDS-63) per rat or administered vehicle alone on days −1, 1, 10, and 20 during the experiment, and at days 7 and 30, cells from lungs, spleen, and lymph nodes were collected from rats and stained with FITC–anti-CD4, phycoerythrin–anti-CD25, and allophycocyanin–anti-FoxP3. Viable cells were gated on lymphocytes and are expressed as the percentage of lymphocytes that are CD4+CD25+FoxP3+. The vertical bars indicate means ± SEM, and a significant reduction in proportion of cells is indicated (*, P < 0.05). Representative FACS analyses of spleens from rats treated with vehicle alone (left) or anti-CD25 mAb (right) for 30 days are shown as a density dot plot of CD4+CD25+ viable lymphocytes; of the CD4+CD25+ lymphocytes, ≈90% are FoxP3+ for both treatment groups.
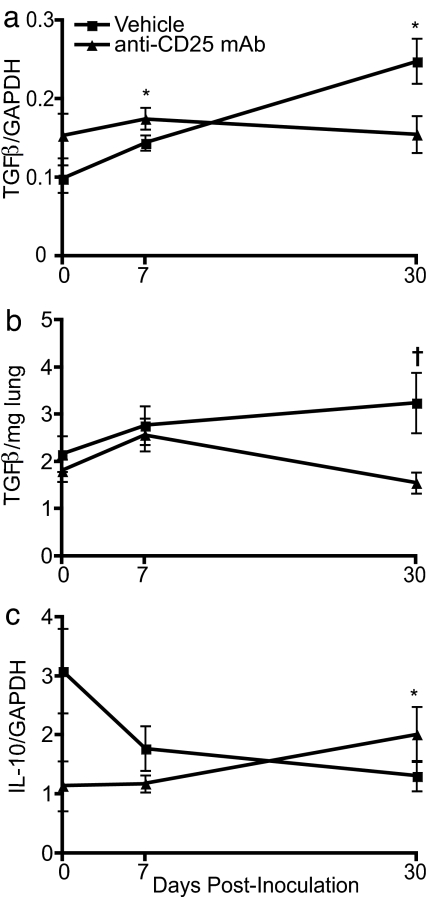
Transcription of TGF-β Is Elevated During Persistent Seoul Virus Infection and Requires Functional Regulatory T Cells.
Cytokines produced by regulatory T cells, including TGF-β and IL-10, mediate regulatory T cell activity (11, 13). Expression of TGF-β in the lungs was elevated during Seoul virus infection, with the highest levels observed at day 30 p.i., as compared with baseline expression in uninfected rats (Fig. 3a; P < 0.001). Administration of anti-CD25 mAb quenched expression of TGF-β, as well as concentrations of active TGF-β protein, suggesting that functional regulatory T cells were necessary for elevated TGF-β during Seoul virus infection (Fig. 3 a and b; mRNA, P = 0.020; protein, P = 0.015). In contrast, expression of IL-10 was reduced in the lungs during persistent Seoul virus infection (P = 0.042), and inactivation of regulatory T cells only dampened IL-10 expression before infection (Fig. 3c). Expression of TGF-β was positively correlated with Seoul virus RNA copies in the lungs (R = 0.41; P = 0.002), whereas no correlation was observed between IL-10 and Seoul virus copy number. In the spleen, neither Seoul virus infection nor functional inactivation of regulatory T cells affected the expression of TGF-β or IL-10 [supporting information (SI) Fig. 7 a and b]. Synthesis of splenic TGF-β was reduced by functional inactivation of regulatory T cells at day 30 p.i. (SI Fig. 7e).
Fig. 3.
Regulatory T cells contribute to increased amounts of TGF-β mRNA and protein in the lungs during persistent Seoul virus infection. At days 0, 7, and 30 p.i. with Seoul virus, expression of TGF-β (a) and IL-10 (c) was measured in the lungs by real-time RT-PCR in vehicle-treated and anti-CD25 mAb-treated male rats. Expression of each cytokine was normalized to the expression of the endogenous control, GAPDH, for each sample; GAPDH expression was not affected by Seoul virus infection or antibody treatment. Synthesis of TGF-β protein was measured by ELISA (b). Lung samples were collected from uninfected rats at the same time as infected rats, designated as day 0 p.i. The vertical bars indicate means ± SEM, and statistically significant differences between infected and uninfected rats within each treatment group (*) and between treatment groups (†) are indicated (P < 0.05).
Regulatory T Cells Suppress TNF During Persistent Seoul Virus Infection.
Regulatory T cells suppress proinflammatory responses in several model systems (11, 12). To determine whether regulatory T cells suppress proinflammatory responses during Seoul virus infection, the expression of IL-1β, IL-6, and TNF was measured in the lungs and spleen 7 and 30 days after inoculation with Seoul virus. Expression of IL-6 and IL-1β in the lungs was not altered by Seoul virus infection or inactivation of regulatory T cells (Fig. 4b and data not shown). Expression of TNF, however, was reduced during the persistent phase of Seoul virus infection as compared with uninfected rats (Fig. 4a; P = 0.033). Functional inactivation of regulatory T cells eliminated the suppression of TNF mRNA, indicating that regulatory T cells were necessary for reduced expression of TNF during persistent Seoul virus infection. Consistent with the low expression of TNF mRNA, synthesis of TNF protein was at or below the limit of detection during Seoul virus infection (data not shown).
Fig. 4.
Regulatory T cells contribute to reduced expression of TNF in the lungs during persistent Seoul virus infection. At days 0, 7, and 30 after inoculation with Seoul virus, expression of TNF (a) and IL-6 (b) was measured in the lungs by real-time RT-PCR in vehicle-treated and anti-CD25 mAb-treated male rats. The expression of each cytokine was normalized to the expression of GAPDH. Lung samples were collected from uninfected rats at the same time points as infected rats and are labeled as day 0 p.i. The vertical bars indicate means ± SEM, and statistically significant differences between infected and uninfected rats within each treatment group are indicated (*, P < 0.05).
In the spleen, there was no effect of infection on the expression of TNF, IL-6, or IL-1β in vehicle-treated rats; functional inactivation of regulatory T cells, however, caused an elevation of TNF, IL-6, and IL-1β expression during persistent infection (i.e., day 30 p.i.) (SI Fig. 7 c and d and data not shown; TNF, P = 0.004; IL-6, P = 0.005; IL-1β, P = 0.017). Taken together, these data suggest that functional regulatory T cells suppress the expression of proinflammatory cytokines.
Functional Inactivation of Regulatory T Cells Reduces Seoul Virus Persistence and Delays Shedding of Viral RNA.
To determine whether regulatory T cells contribute to Seoul virus persistence and shedding, viral RNA copies in the lungs and saliva were measured during acute (day 7 p.i.) and persistent (day 30 p.i.) infection. Inactivation of regulatory T cells significantly reduced the amount of Seoul virus RNA in the lungs, with this effect being most pronounced during the persistent phase of infection (Fig. 5; P = 0.015). Seoul virus RNA is shed in the saliva of infected rats and previous studies illustrate that hantavirus shedding is prominent during the acute phase of infection (i.e., within the first 2 weeks after inoculation) (14). In the present study, ≈50% of the vehicle-treated rats shed Seoul virus 7 (6/10) and 30 (5/10) days p.i. Functional inactivation of regulatory T cells reduced the proportion of male rats shedding viral RNA in saliva 7 (1/10) but not 30 (4/10) days p.i. (P = 0.057). Among the rats that were shedding, there were no differences in the amount of viral RNA shed between vehicle- and anti-CD25 mAb-treated rats at day 7 p.i. (vehicle, 716 ± 187 copies per microliter; anti-CD25 mAb, 109 copies per microliter) or day 30 p.i. (vehicle, 554 ± 176 copies per microliter; anti-CD25 mAb, 1,705 ± 787 copies per microliter). Regulatory T cell inactivation delayed shedding of genomic RNA and may reduce the overall period of infectiousness.
Fig. 5.
Presence of Seoul virus RNA is reduced after inactivation of regulatory T cells. Male rats were administered anti-CD25 mAb or vehicle alone and were inoculated with Seoul virus. At days 7 and 30 p.i., Seoul virus RNA was measured in the lungs by real-time RT-PCR. Because the amount of viral RNA in the lungs was not normally distributed, the median values are graphed, and scale bars indicate the 25th and 75th quartiles; statistically significant differences between vehicle-treated and anti-CD25-treated rats are indicated (*, P < 0.05).
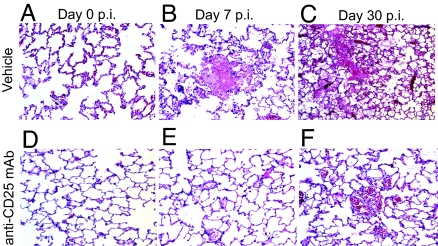
Inactivation of Regulatory T Cells Reduces Subclinical Lung Pathology Observed During Seoul Virus Infection.
It is generally well established that hantaviruses do not cause observable disease in their reservoir hosts, but whether subclinical pathologic changes occur has not been well defined (8, 18). In other infectious disease systems, the manipulation of regulatory T cells affects the development of proinflammatory mediated pathologic changes (12, 13); thus, we examined the effects of regulatory T cell inactivation on gross markers of subclinical pathology, such as weight gain and body temperature, during Seoul virus infection. Over the course of Seoul virus infection, inactivation of regulatory T cells resulted in a faster rate of weight gain and reduced rectal body temperatures as compared with vehicle-treated rats (SI Fig. 8 a and b; body mass, P < 0.001; body temperature, P = 0.01). To assess whether removal of regulatory T cells would result in host-mediated proinflammatory lesions in response to infection, lung tissue was examined for evidence of pathologic alterations. Neither proinflammatory infiltration nor lesions were observed in either vehicle-treated or anti-CD25-treated rats. During both acute and persistent Seoul virus infection, subclinical multifocal random areas of acute hemorrhage and edema were observed throughout the lung parenchyma, accompanied by necrosis of alveolar cell walls and dilation of perivascular lymphatics (Fig. 6 B and C). No pathology was observed in either vehicle-treated or anti-CD25-treated uninfected rat lungs (Fig. 6 A and D). The average percentage of lung tissue displaying hemorrhage and edema increased over the course of Seoul virus infection (day 7 p.i., 7.2%; day 30 p.i., 22.3%) in vehicle-treated rats (P < 0.001). Functional inactivation of regulatory T cells reduced the average amount of hemorrhage and edema observed in the lungs at days 7 p.i. (0.4%) and 30 p.i. (8.9%) (Fig. 6 E and F; P < 0.001). The proportion of pathologic areas in the lungs did not correlate with the amount of viral RNA present in the lungs or the expression of proinflammatory (i.e., TNF, IL-6, and IL-1β) or regulatory (i.e., TGF-β and IL-10) cytokines in the lungs. Pathological lesions were not observed in the spleen.
Fig. 6.
Rats with inactivated regulatory T cells display less subclinical pathology in the lungs as compared with vehicle-treated rats during Seoul virus infection. On days 0, 7, and 30 p.i., lungs were inflated with 10% formaldehyde and, after 24 h, were prepared for H&E staining for pathologic examination. Lungs were collected from uninfected rats at the same time as infected rats, designated as day 0 p.i. (A–C) Representative H&E-stained lung sections for vehicle-treated rats at days 0, 7, and 30 p.i., respectively. (D–F) Lung sections from rats treated with anti-CD25 mAb at the same time points. In B, C, and F, hemorrhage and edema in alveolar spaces are shown.
Discussion
Regulatory T cells contribute to Seoul virus persistence in Norway rats. In the present study, numbers of CD4+CD25+FoxP3+ regulatory T cells and the transcription of FoxP3 and TGF-β were elevated, and the expression of TNF and IL-10 was reduced in the lungs of male rats during the persistent phase of Seoul virus infection. Functional inactivation of regulatory T cells reduced numbers of Seoul virus RNA copies in the lungs and the proportion of rats shedding viral RNA in saliva. Expression of TNF, IL-10, and TGF-β, as well as synthesis of TGF-β, in the lungs was quenched by inactivation of regulatory T cells during persistent infection. Subclinical pathologic symptoms, as measured by body temperature, weight gain, and hemorrhage and edema in the lungs, also were reduced by inactivation of regulatory T cells during Seoul virus infection.
Hantaviruses persistently infect their rodent hosts (10). The mechanisms mediating hantavirus persistence remain unknown, but several hypotheses have been postulated, including immune evasion, direct suppression or alteration of host immune responses, and induction of host regulatory/antiinflammatory responses (10). T cell responses are necessary for control of Seoul virus replication because nude rats that are infected with Seoul virus have higher viral titers than their T cell competent counterparts and eventually succumb to infection (19). Several RNA viruses suppress host cellular immune responses to cause persistent infection. Lymphocytic choriomeningitis virus is a noncytopathic RNA virus that persistently infects its rodent reservoir (i.e., Mus spp.) by “exhausting” and down-regulating the CD8+ T cell population by continuous exposure to a high amounts of viral antigen (20). Haantan virus persistence in newborn BALB/c mice is correlated with a reduction of antigen-specific IFN-γ-producing CD8+ T cells (21). Mus species are not natural hosts for hantaviruses; therefore, whether CD8+ T cell down-regulation is involved in persistent hantavirus infection of rodent reservoir hosts requires further investigation. Hepatitis C virus (HCV) and Friend leukemia virus persistence reflects regulatory T cell suppression of virus-specific CD8+ T cell activity (i.e., production of IFN-γ and TNF) (22, 23). Similar to Friend leukemia virus and HCV, expression of TNF was reduced during the persistent phase of Seoul virus infection, and this depended on functional regulatory T cells. Whether the production of TNF by hantavirus-specific CD8+ T cells is suppressed by regulatory T cells during persistent infection requires additional investigation.
Regulatory T cells are exploited by a broad range of pathogens, including several viruses, bacteria, and parasites, to establish and maintain persistent infection (11, 12). Regulatory T cells suppress proinflammatory responses that are necessary to eliminate the pathogen but that also can cause potentially fatal proinflammatory-mediated pathogenesis (11, 12). For example, during Leishmaina major persistence in mice, CD4+CD25+ regulatory T cells, found at the site of infection (i.e., the dermis), suppress the ability of CD4+CD25− effector T cells to eliminate the parasite (13). Proinflammatory dermal lesions, however, are reduced, and protection against reinfection is maintained by regulatory T cell responses (13, 24). The relationship between host regulatory T cells and the pathogen appears to represent a coevolved balance that maintains homeostasis in the host. The present study illustrates that, like L. major, HCV, and Friend leukemia virus, regulatory T cells contribute to the persistence of Seoul virus in its natural reservoir host, Norway rats. Functional inactivation of regulatory T cells reduced the amount of virus in the lungs during the acute phase and even more so during the persistent phase of Seoul virus infection in male rats. These data present a mechanism of hantavirus persistence in a natural reservoir host species. Because regulatory T cells suppress the function and chemotaxis of several immune cell populations, including CD8+ T cells and macrophages (25, 26), this represents a likely effector mechanism in our hantavirus model system.
Regulatory T cell activity often is mediated by secreted or membrane-bound TGF-β and IL-10 (13, 22, 26). TGF-β maintains regulatory T cell populations and contributes to their activity during chronic HCV infection in humans (22, 27). Similar to HCV, expression and synthesis of TGF-β was elevated in the lungs during the persistent phase of Seoul virus infection and reflected a concomitant increase in regulatory T cell numbers and FoxP3 expression and suppression of TNF mRNA. Furthermore, the increased amounts of TGF-β mRNA and protein in the lungs during Seoul virus infection were dependent on functional regulatory T cells. Our data support and expand observations from deer mice infected with Sin Nombre virus, in which FoxP3 and TGF-β expression is elevated after antigen-specific stimulation of T cells isolated during persistent infection (28). Because many cells can produce TGF-β (29), whether regulatory T cells are producing TGF-β in the lungs and whether TGF-β mediates the effector functions of regulatory T cells during infection remain to be investigated.
Regulatory T cell-mediated pathogen persistence that depends on IL-10 has been reported for several pathogens, including persistent L. major infection in mice (17). In contrast to L. major, expression of IL-10 was reduced in the lungs during persistent Seoul virus infection in rats; reduced expression during infection depended on functional regulatory T cells. Expression of IL-10 was not correlated with the amount of Seoul virus RNA in the lungs. Although seemingly contradictory, one possible explanation is that regulatory T cells may suppress T helper 1 cells (i.e., CD4+T-bet+) or other cells (i.e., macrophages) that also produce IL-10 during infection (30).
Regulatory T cells contribute to detrimental pathogen persistence, but they also protect the host by suppressing proinflammatory responses elicited in response to infection (11, 12). In the absence of functional regulatory T cells, expression of TNF in the lungs was no longer reduced and expression of IL-6 and IL-1β in the spleen was elevated during persistent infection as compared with uninfected rats. Excessively high proinflammatory responses contribute to the development of hantavirus cardiopulmonary syndrome and hemorrhagic fever with renal syndrome in humans (9). Rodent hosts do not exhibit overt signs of disease during hantavirus infection, but whether subclinical pathological changes occur has not been well defined (8, 9, 18). During the acute and persistent phases of Seoul virus infection, subclinical, focal, and random hemorrhage and edema were observed in the lungs of infected rats. Surprisingly, the extent of subclinical pathologic foci was reduced after regulatory T cell inactivation. In the present study, functional inactivation of regulatory T cells resulted in greater weight gain and improved thermoregulation throughout Seoul virus infection. These data suggest that regulatory T cells contribute to subclinical pathologic observations in rats during Seoul virus infection. Neither proinflammatory lesions nor infiltration was observed in the lungs of either vehicle-treated or regulatory T cell-inactivated rats during infection. Expression of genes that encode proinflammatory cytokines also was not correlated with development of subclinical pathologic outcomes. Because hantaviruses are noncytopathic, the virus itself likely does not contribute to pathologic changes (31). The etiology of hemorrhage and edema in the lungs of rats remains unknown. Whether direct infection of pulmonary endothelial cells alters permeability to cause leakage of blood and fluid into alveolar spaces requires investigation. Currently, the only animal models of hantavirus disease in humans are Andes virus and Maporal virus infection of Syrian hamsters (32). Whether Seoul virus infection of Norway rats models subclinical or less severe hantavirus disease in humans should be considered.
Functional inactivation or depletion of CD25 is the most commonly used method to evaluate the role of regulatory T cells in various model systems (13). Activated T cells, however, also express CD25, as well as other regulatory T cell markers (i.e., glucocorticoid-induced TNF receptor family-related gene and cytotoxic T lymphocyte-associated antigen-4) (33). Administration of anti-CD25 mAb renders regulatory T cells nonfunctional, but the effect on CD25+ activated T cells has not been reported (17). Analysis of the CD4+CD25+ population during acute and persistent Seoul virus infection indicated that >90% of these cells were also FoxP3+ and, thus, regulatory T cells. Additionally, there was no effect of Seoul virus infection on activated CD4+CD25+FoxP3− T cells. This analysis illustrates that CD25+FoxP3−-activated T cells do not contribute substantially to the proportion of CD4+CD25+ T cells during Seoul virus infection.
Transmission of zoonotic pathogens, including hantaviruses, to humans requires contact with infected reservoir hosts. Consequently, the ability of a virus to persist in its animal host increases the likelihood of transmission. Hantaviruses are shed in saliva and excrement and transmission occurs by aerosolization of virus in urine and feces or introduction of virus in saliva through a bite wound (7). Regulatory T cells not only contribute to the persistence of Seoul virus in the lungs of rats, but also to the duration of shedding in saliva. Taken together, our data illustrate that by contributing to the persistence of Seoul virus in their reservoir rodent hosts, regulatory T cells may increase the potential for zoonotic transmission of hantaviruses to humans.
Methods
Animals.
Adult male (60–70 days of age) Long Evans rats (Rattus norvegicus) were purchased from Charles River Laboratories (Raleigh, NC) and housed individually in a pathogen-free biosafety level 3 animal facility. Because Seoul virus persists to a greater extent in males than females (S.L.K. and M. Hannah, unpublished data), only male rats were used in the present study to evaluate the role of regulatory T cells in virus persistence. The Johns Hopkins Animal Care and Use Committee (RA04H338) and Office of Health, Safety, and the Environment (P9902030108) approved all procedures described in this report.
Virus and Infection Protocols.
Animals were inoculated i.p. with 104 pfu of purified Seoul virus (strain SR-11) suspended in Eagle's minimal essential medium (n = 72) or were inoculated i.p. with Eagle's minimal essential medium alone (n = 20). Seoul virus was obtained from the U.S. Army Medical Research Institute of Infectious Diseases (Fort Detrick, MD), where the virus was isolated from neonatal rat brains and passaged four times in VeroE6 cells. Rectal temperature and body mass were measured every 3 days during the course of infection for the indicated experiment. At several time points, including days 3, 7, 15, 30, and 40 p.i. (n = 8–10 per time point per treatment), animals were anesthetized and saliva was collected after an i.p. injection of 2.5 mg/kg pilocarpine HCl (Sigma, St. Louis, MO). Rats were killed, and lung and spleen tissues were collected. Samples also were collected from uninfected male rats (designated as day 0 p.i.) and processed at the same time points as infected rats.
Anti-Rat CD25 Treatment.
A hybridoma cell line that produces anti-rat CD25 IgG mAb (NDS-63) was provided by Margaret Dallman and Kathryn Wood (University of Oxford, Oxford, U.K.). Rats were injected i.p. with anti-CD25 mAb in 0.9% sterile saline (2 mg per rat) or vehicle alone 1 day before and 1 day after inoculation with Seoul virus for the initial inactivation of CD25+ regulatory T cells and subsequently at 10 and 20 days after Seoul virus inoculation to maintain functional inactivation (Y. Belkaid, personal communication).
Real-Time RT-PCR for Seoul Virus Detection and Cytokine Expression.
RNA was isolated from lungs, spleen, and saliva as described previously (14). First-strand Seoul virus cDNA was synthesized by using a gene-specific primer (SuperScript III; Invitrogen, Carlsbad, CA). An 81-bp nucleotide sequence of the negative strand of the S segment of the Seoul virus was amplified by using the Platinum Quantitative PCR Supermix-UDG with ROX (Invitrogen). A standard curve ranging from 106 to 10 copies of SR-11 S segment in pWRG7077 was run on each plate. For cytokine expression, custom primer and probe sets were generated for GAPDH, IL-1β, IL-6, TNF, IL-10, TGF-β1, and FoxP3 by using Primer Express 2.0 software and the protocol for the TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA). A standard curve, ranging from 2.5 ng/μl to 25 pg/μl cDNA, was run on each plate.
Cytokine ELISAs.
Lung and spleen tissues were homogenized, and supernatants were collected. TNF and active TGF-β1 protein concentrations were measured according to the protocol of the manufacturer (R&D Systems, Minneapolis, MN).
FACS Analyses.
Lung tissue was digested in collagenase (1 mg/ml; Invitrogen) and DNase (3 μg/μl; Roche, Indianapolis, IN) to produce a single-cell suspension. After red blood cell lysis, lung and spleen cells were stained for viability by using ethidium monoazide (Invitrogen), FITC–anti-rat CD4 mAb (clone OX-35), and phycoerythrin–anti-rat CD25 mAb (clone OX-39; BD Biosciences, San Diego, CA). After fixation and permeabilization, cells were labeled with allophycocyanin–anti-rat FoxP3 (eBiosceinces, San Diego, CA). Cells were identified by using CellQuest Pro (BD Biosciences), and the data were analyzed by using FlowJo software (Treestar, Ashland, OR).
Lung Pathologic Analyses.
After 24 h of fixation in 10% formaldehyde, inflated lung and spleen samples were embedded in paraffin, cut into 5-μm sections, and mounted on glass slides. The slides were H&E stained to evaluate pathologic observations by using light microscopy.
Statistical Analyses.
Cytokine gene expression and synthesis, percentages of regulatory T cells, and copies of Seoul virus RNA were assessed by using one-way ANOVA with one between-group variable (day p.i. or treatment). Differences in body weight and temperature across time and by treatment were determined by multivariate ANOVA. Differences in the proportion of rats shedding Seoul virus RNA in saliva were determined by χ2 analysis. In cases in which the data violated the assumptions of normality, nonparametric statistics were used. Significant interactions were further analyzed by using the Tukey or Dunn method for pairwise multiple comparisons. Mean differences were considered statistically significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank Connie Schmaljohn, Cindy Rossi, and Kristen Spik (U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD) for providing hantavirus reagents. We also thank Michele Hannah and Jenifer Kaplan for technical assistance and Greg Glass for discussions about these data. We acknowledge the Becton Dickinson Immune Function Laboratory (The Johns Hopkins Bloomberg School of Public Health) for use of the FACS equipment and analysis of FACS data. Financial support was provided by National Institutes of Health Grant R01 AI 054995 (to S.L.K.).
Abbreviations
- Fox
Forkhead box
- HCV
hepatitis C virus
- p.i.
postinoculation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707453104/DC1.
References
- 1.King LJ. Committee on Assessing the Nation's Framework for Addressing Animal Diseases. Washington, DC: Natl Acad Press; 2005. pp. 1–4. [Google Scholar]
- 2.Morens DM, Folkers GK, Fauci AS. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khaiboullina SF, St Jeor SC. Viral Immunol. 2002;15:609–625. doi: 10.1089/088282402320914548. [DOI] [PubMed] [Google Scholar]
- 4.Plyusnin A, Morzunov SP. Hantaviruses. 2001;256:47–75. doi: 10.1007/978-3-642-56753-7_4. [DOI] [PubMed] [Google Scholar]
- 5.Korch GW, Childs JE, Glass GE, Rossi CA, Leduc JW. Am J Trop Med Hyg. 1989;41:230–240. doi: 10.4269/ajtmh.1989.41.230. [DOI] [PubMed] [Google Scholar]
- 6.Kariwa H, Fujiki M, Yoshimatsu K, Arikawa J, Takashima I, Hashimoto N. Arch Virol. 1998;143:365–374. doi: 10.1007/s007050050292. [DOI] [PubMed] [Google Scholar]
- 7.Lee HW, Lee PW, Baek LJ, Song CK, Seong IW. Am J Trop Med Hyg. 1981;30:1106–1112. doi: 10.4269/ajtmh.1981.30.1106. [DOI] [PubMed] [Google Scholar]
- 8.Lee PW, Yanagihara R, Gibbs CJ, Gajdusek DC. Arch Virol. 1986;88:57–66. doi: 10.1007/BF01310890. [DOI] [PubMed] [Google Scholar]
- 9.Childs JE, Glass GE, Korch GW, Leduc JW. J Wildlife Dis. 1989;25:469–476. doi: 10.7589/0090-3558-25.4.469. [DOI] [PubMed] [Google Scholar]
- 10.Meyer BJ, Schmaljohn CS. Trends Microbiol. 2000;8:61–67. doi: 10.1016/s0966-842x(99)01658-3. [DOI] [PubMed] [Google Scholar]
- 11.Mills KHG. Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 12.Belkaid Y, Rouse BT. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 14.Klein SL, Bird BH, Glass GE. Am J Trop Med Hyg. 2001;65:57–63. doi: 10.4269/ajtmh.2001.65.57. [DOI] [PubMed] [Google Scholar]
- 15.Tellides G, Dallman MJ, Morris PJ. Transplant Proceed. 1989;21:997–998. [PubMed] [Google Scholar]
- 16.Wood MJA, Sloan DJ, Dallman MJ, Charlton HM. J Exp Med. 1993;177:597–603. doi: 10.1084/jem.177.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz, Ziegler SF, Miller SD. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 18.Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Proc Natl Acad Sci USA. 2000;97:10578–10583. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohmae K, Okabe M, Nishimune Y. J Infect Dis. 1994;170:1589–1592. doi: 10.1093/infdis/170.6.1589. [DOI] [PubMed] [Google Scholar]
- 20.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 21.Araki K, Yoshimatsu K, Lee BH, Kariwa H, Takashima I, Arikawa J. J Virol. 2003;77:8408–8417. doi: 10.1128/JVI.77.15.8408-8417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera R, Tu ZK, Xu YL, Firpi RJ, Rosen HR, Liu C, Nelson DR. Hepatology. 2004;40:1062–1071. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 23.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich ARM, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, et al. Immunity. 2004;20:293–303. doi: 10.1016/s1074-7613(04)00054-8. [DOI] [PubMed] [Google Scholar]
- 24.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 25.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan D, Wang Y, Qin X, Wang Y, Zheng G, Wang YM, Alexander SI, Harris DC. J Am Soc Nephrol. 2006;17:2731–2741. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- 27.Marie JC, Letterio JJ, Gavin M, Rudensky AY. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schountz T, Prescott J, Cogswell AC, Oko L, Mirowsky K-M, Fuenzalida AG, Hjelle B. Proc Natl Acad Sci USA. 2007;104:15496–15501. doi: 10.1073/pnas.0707454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massague J. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 30.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardestam J, Klingstrom J, Mattsson K, Lundkvist A. J Med Virol. 2005;76:234–240. doi: 10.1002/jmv.20347. [DOI] [PubMed] [Google Scholar]
- 32.Campen MJ, Milazzo ML, Fulhorst CF, Akata CJO, Koster F. Virology. 2006;356:45–49. doi: 10.1016/j.virol.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 33.Stephens LA, Barclay AN, Mason D. Int Immunol. 2004;16:365–375. doi: 10.1093/intimm/dxh033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.