Abstract
Hantavirus cardiopulmonary syndrome is a zoonotic illness associated with a systemic inflammatory immune response, capillary leak, noncardiogenic pulmonary edema, and shock in humans. Cytokines, including TNF, IFN-γ, and lymphotoxin, are thought to contribute to its pathogenesis. In contrast, infected rodent reservoirs of hantaviruses experience few or no pathologic changes and the host rodent can remain persistently infected for life. Generally, it is unknown why such dichotomous immune responses occur between humans and reservoir hosts. Thus, we examined CD4+ T cell responses from one such reservoir, the deer mouse (Peromyscus maniculatus), infected with Sin Nombre virus. Proliferation responses to viral nucleocapsid antigen were relatively weak in T cells isolated from deer mice, regardless of acute or persistent infection. The T cells from acutely infected deer mice synthesized a broad spectrum of cytokines, including IFN-γ, IL-4, IL-5, and TGF-β1, but not TNF, lymphotoxin, or IL-17. However, in T cells from persistently infected deer mice, only TGF-β1 was expressed by all lines, whereas some expressed reduced levels of IFN-γ or IL-5. The Forkhead box P3 transcription factor, a marker of some regulatory T cells, was expressed by most of these cells. Collectively, these data suggest that TGF-β1-expressing regulatory T cells may play an important role in limiting immunopathology in the natural reservoir host, but this response may interfere with viral clearance. Such a response may have arisen as a mutually beneficial coadaptive evolutionary event between hantaviruses and their rodent reservoirs, so as to limit disease while also allowing the virus to persist.
Keywords: hantavirus, TGF, zoonosis, immunopathology, Peromyscus
Hantaviruses are zoonotic rodent-borne, negative-strand RNA viruses with tripartite genomes that can cause two diseases in humans: hemorrhagic fever with renal syndrome in Eurasia and hantavirus cardiopulmonary syndrome (HCPS) in the Americas (1). These diseases have variable case–fatality ratios, with that of HCPS ≈35%. The principal target of hantaviruses is the capillary endothelium, which results in a capillary leak syndrome and edema but without conspicuous cytopathic effect (2). In HCPS, the escape of plasma from the blood can result in cardiogenic shock from severe hypotension. In fatal cases, the intense antiviral immune response is suspected of contributing to pathogenesis in HCPS. Mononuclear cell infiltrates are found in autopsy lung specimens from HCPS patients, and many of these secrete proinflammatory cytokines, including TNF, lymphotoxin (LT), IL-2, IL-4, and IFN-γ (3). CD4+ and CD8+ cytotoxic T lymphocytes (CTLs) have been isolated from Sin Nombre virus (SNV) patients, and a prominent role for CD8+ CTLs has been suggested for the severity of HCPS (4, 5).
In contrast, rodent reservoirs infected with hantaviruses exhibit little or no pathology and can remain persistently infected for life (6, 7). Each hantavirus species is associated with a single principal rodent host species (8), suggesting that hantaviruses and rodents share a lengthy coevolutionary history. Deer mice (Peromyscus maniculatus) are the principal reservoir hosts for SNV, which causes the great majority of HCPS cases in North America (9–11). Morphologic examination of the cells that express viral antigen in the tissues of infected deer mice reveals that they, as with infected human cells, are of endothelial origin (12). However, in the deer mouse model, capillary leakage has not been observed (6, 7). Deer mice that are persistently infected with SNV fall into two discrete categories: those with a widely disseminated pattern of infection that is associated with RNA viremia and those with a more limited form of infection (“restricted” pattern), the latter of which retain viral RNA in at least some tissues but from which recovery of infectious SNV is more difficult (7). However, for both patterns of infection, rodents remain healthy and viral RNA and antigen can be detected in multiple tissues, including the lungs, heart, spleen, kidneys, and brown fat. Deer mice produce antibodies that neutralize SNV; however, those antibodies are insufficient to clear the virus. The consistent presence of antiviral IgG also indicates a role for helper T cells in the containment of infection, because its production requires T cell-mediated class switching. Moreover, high-titered neutralizing IgG indicates affinity maturation occurs during infection, also implying a role for helper T cells during deer mouse immune responses to SNV. Despite such T cell mobilization to SNV infection, the virus eludes a sterilizing immune response and establishes persistence.
Several contributory mechanisms that should favor the persistence of viruses in host organisms have been identified, including interference with peptide processing, inhibition of MHC biosynthesis, sequestration in immunologically privileged sites, interference with cytokine responses, interference with bone marrow antigen-presenting cell (APC) development, and inhibition of cytolytic responses (13–23). However, most of the viruses that use those strategies have large genomes, unlike hantaviruses, that encode polypeptides that target these immunological pathways. Of the four polypeptides encoded by hantaviruses, none has been reported to possess such immunomodulating activities in their reservoirs, although some can affect type I IFN responses in human cells (24). In addition, hantaviruses that cause HCPS possess an immunoreceptor tyrosine-based activation motif in the GN (or G1) glycoprotein that may influence immune cells or infected endothelial cells (25). However, it is unknown whether this occurs in infected deer mice.
Some pathogenic human virus infections have been associated with subsets of immunosuppressive CD4+ T cells, collectively referred to as regulatory T cells, that differentially express TGF-β1, IL-10, and Forkhead box (Fox)P3 transcription factor, and suppress inflammatory responses in a contact- and cytokine-dependent manner (26–28). Included among these subsets are inducible regulatory T cells that express FoxP3 and TGF-β1, and T helper (Th)3 cells that express TGF-β1 but not FoxP3 (29). Such suppression can lead to chronic infection with several viruses, including HIV, herpes simplex virus, hepatitis C virus, and EBV (30).
As a first step, we sought to identify possible genes that correlated with persistence without immunopathology associated with infection by SNV in deer mice. We generated CD4+ T cell lines specific for recombinant SNV nucleocapsid antigen (N-Ag), which has been shown previously to be a helper T cell antigen in deer mice (31), from both acutely infected and persistently infected deer mice. Whereas T cells from acutely infected deer mice expressed a broad array of cytokines, T cells from persistently infected deer mice resembled regulatory T cells. These results suggest that a regulatory T cell-mediated antiinflammatory immune response has evolved in hantavirus–rodent interactions that limits immunopathology but may promote persistent infection. Ostensibly, this contributes to the natural transmission and maintenance of a lethal zoonotic agent.
Results
Deer Mouse T Cell Cultures Do Not Support the Propagation of SNV.
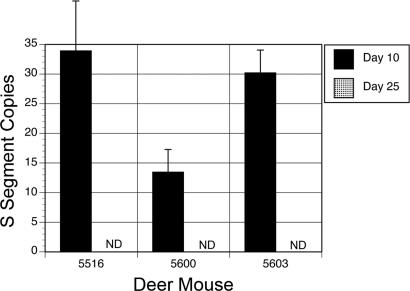
We infected deer mice with 20 animal ID50 of SNV and harvested T cells from acutely infected (days 9–10 postinfection) or persistently infected (days 43–45 postinfection) animals (6, 7). Viral S segment was detected by RT-PCR in the lungs of all infected deer mice but not in uninfected controls (data not shown) (32). T cell lines established from three persistently infected deer mice were sampled at days 10 and 25 of culture for the abundance of viral S segment genomic RNA by using a TaqMan real-time PCR assay. Fewer than 50 copies of viral RNA (sensitivity of 5 copies) were detected at day 10 of culture, and no copies were detected by day 25 (Fig. 1). Sampling of all other T cell culture supernatants and T cell RNA also failed to detect viral RNA, indicating that the T cell lines were not susceptible to SNV.
Fig. 1.
T cells from SNV-infected deer mice are not persistently infected. T cell lines were established from three deer mice (DM5516, DM5600, and DM5603) and sampled at days 10 and 25 of culture. TaqMan real-time PCR was used to quantify copies of viral S segment. By day 25, viral RNA could not be detected (ND).
Molecular Phenotype of T Cell Lines Isolated from SNV-Infected Deer Mice.
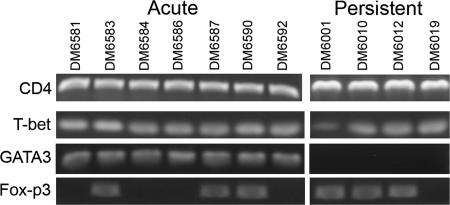
T cell lines were profiled 7 days after in vitro antigenic stimulation for the expression of CD4, CD8, T-bet, GATA3, and FoxP3 by RT-PCR (Fig. 2). All lines from acutely and persistently infected deer mice were CD4+/CD8− and T-bet+, whereas only lines from acutely infected deer mice expressed GATA3. FoxP3 expression was differential: three of the seven lines from acutely infected and three of the four lines from persistently infected deer mice. We were unable to determine whether the cells expressed other markers useful for identifying regulatory T cell subsets (33), including CD25, CTL-associated antigen-4, or glucocorticoid-induced TNF receptor family-related gene (28), because sequences for these genes are not available for the deer mouse.
Fig. 2.
Molecular phenotypes of cultured T cells from infected deer mice. RNA from cultured T cells was reverse transcribed for PCR to detect the expression of CD4, T-bet, GATA3, and FoxP3. All cells were CD4+ and T-bet+, whereas only T cells from acutely infected deer mice expressed GATA3. FoxP3 expression was detected in some of the acutely and persistently infected deer mice.
Proliferation Responses of T Cells from Acutely and Persistently Infected Deer Mice.
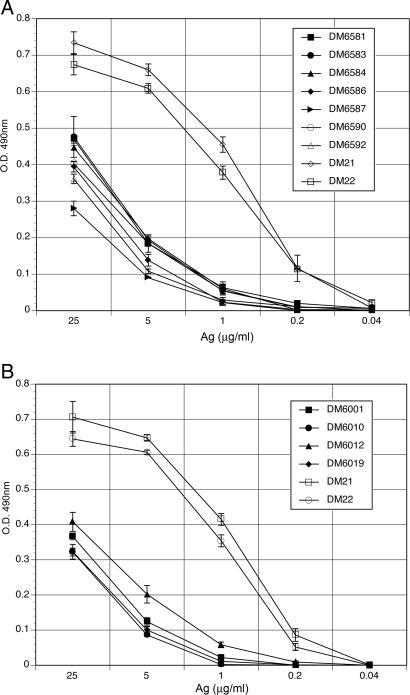
T cell lines from both acutely and persistently infected deer mice exhibited weak proliferation responses to recombinant N-Ag (Fig. 3). By comparison, T cells from control deer mice immunized with an irrelevant antigen (keyhole limpet hemocyanin [KLH]) proliferated vigorously, even at low concentrations. Maximal proliferation of T cells from infected deer mice challenged with N-Ag were approximately half that of the T cells from the uninfected control deer mice immunized and challenged with KLH. In addition, dilution of the N-Ag resulted in a rapid diminution of proliferative responses, such that at a dosage of 1 μg/ml, proliferation was no longer detected. In contrast, proliferation of the control T cell lines from KLH-immunized deer mice were approximately half maximal at 1 μg/ml of KLH, similar to other KLH-specific deer mouse T cell lines produced by us (34, 35).
Fig. 3.
T cells from SNV-infected deer mice exhibit weak recall proliferation responses to N-Ag. T cell lines established from acutely infected deer mice (A) and persistently infected deer mice (B) were cultured in duplicate with autologous bone-marrow-derived APCs at various concentrations of antigen for 3 days; after which, proliferation was assessed by MTS assay. The means and SDs were plotted and compared with T cells from control deer mice immunized with KLH (DM21 and DM22).
Cytokine Profiles of T Cells from Acutely and Persistently Infected Deer Mice.
Because proinflammatory cytokines are thought to be important in the pathogenesis of HCPS, we examined the profiles of cytokine genes expressed by T cells from infected deer mice. We previously developed an assay for the detection of changes in cytokine gene expression between antigen-stimulated and unstimulated T cells from a given animal by using real-time PCR (35). This allows the calculation of the relative template abundance of each cytokine transcript in response to in vitro antigenic stimulation.
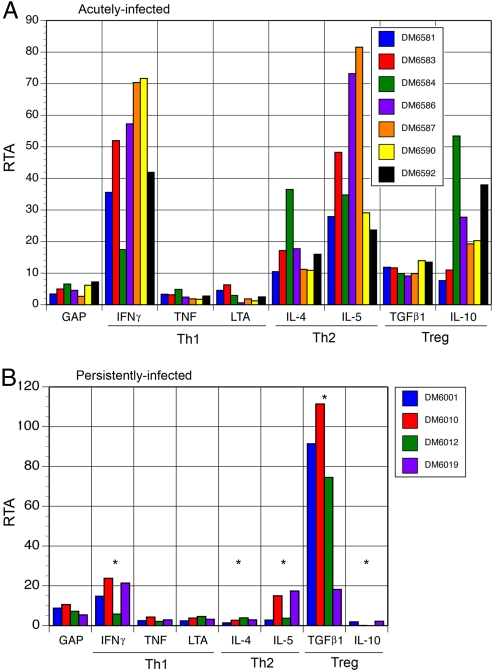
Polyclonal T cells isolated from acutely infected deer mice expressed several type 1 (IFN-γ), type 2 (IL-4 and IL-5), and regulatory T cell (TGF-β1 and IL-10) cytokines (Fig. 4A). None of the lines expressed IL-17, a marker of inflammatory Th17 cells (36), by conventional reverse-transcription PCR (data not shown). Most of the lines had more than 20-fold increased expression of IFN-γ, IL-5, and IL-10, whereas IL-4 and TGF-β1 expression was increased more than 10-fold in most animals. The change in expression levels of the type 1 cytokines TNF and LT did not exceed that of the control gene (GAPDH) in any of the T cell lines.
Fig. 4.
Cytokine responses of T cell lines from acutely and persistently infected deer mice. (A) T cells from acutely infected deer mice expressed IFN-γ, IL-4, IL-5, TGF-β1, and IL-10 but not TNF or LT. (B) Lines from persistently infected deer mice expressed TGF-β1 but not TNF, LT, IL-4, or IL-10. DM6001 also expressed IFN-γ and deer mice DM6010 and DM6019 also expressed IFN-γ and IL-5. Expression levels denoted by * are significantly different (P < 0.05) from the same cytokine expressed by T cells from acutely infected deer mice. RTA, relative template abundance.
The T cell lines from persistently infected deer mice exhibited diminished cytokine responses upon challenge with antigen (Fig. 4B). Three of the lines exhibited 15- to 24-fold increases in IFN-γ expression, and two lines had ≈15-fold increases in IL-5 expression. All four lines had at least 18-fold increases in TGF-β1 expression, with three of the lines increasing expression by 74-, 91-, and 111-fold. Antigen-induced changes of TNF, LT, IL-4, and IL-10 were near background (GAPDH). None of these T cells expressed IL-17 either.
Significant decreases (two-tailed t test) occurred in most cytokine expression levels in T cells from persistently infected deer mice compared with T cells from acutely infected deer mice, including IFN-γ (P = 0.011), IL-10 (P = 0.016), IL-4 (P = 0.012), and IL-5 (P = 0.017). However, TGF-β1 expression was significantly increased in T cell lines from persistently infected deer mice (P = 0.002). No significant changes occurred with TNF (P = 0.919) or LT (P = 0.191).
Discussion
Viruses that establish persistent infections must evade sterilizing host immune responses. Many such mechanisms have evolved and often use virally encoded proteins that modulate innate and adaptive immune responses (13–15, 19, 22, 23, 37–39). However, hantaviruses are among the simplest of viruses, with only three genes encoding four polypeptides. Although HCPS-causing hantavirus GN polypeptides have some immunoreceptor tyrosine-based activation motif activities (25) and some hantaviruses can impair type I IFN responses in human cells (24) in vitro, no such activities have been demonstrated in vivo in reservoir hosts.
Studying hantavirus infections of natural reservoirs has been challenging because of the near absence of immunologic and genetic reagents for use in the unusual rodent host species. Most monoclonal antibodies to house mouse (Mus musculus) cytokines, CD, and other cell surface antigens do not cross-react with deer mouse orthologs (ref. 40; unpublished observations). Consequently, we developed molecular assays to overcome these limitations (35, 41, 42).
Our first hypothesis was that SNV may infect and impair T cells because activated T cells can express CD61 (β3 integrin) (43), an entry determinant for hantaviruses (44). This might result in the disabling of virus-specific T cells during the acute phase of the adaptive immune response. Theoretically, such an effect could result in a hole in the T cell repertoire (45); SNV-specific T cells could be depleted. In addition, it may provide a means for functionally disabling T cells or modulating their activities; the immunoreceptor tyrosine-based activation motif of GN has been shown to bind to zeta-associated protein 70 (25), a T cell tyrosine kinase necessary for CTL and Th cell functions. However, we were able to establish CD4+ T cell lines from infected deer mice and demonstrate that they are not susceptible to SNV, suggesting that disabling or modulating events are unlikely for CD4+ T cells. This does not discount the possibility that the CTLs could be infected, such that GN could impair or influence their activities through binding to zeta-associated protein 70. Other studies will be required to examine this possibility.
We did not detect virus in bone marrow sections by immunohistochemistry or real-time PCR of bone marrow cultures (data not shown), indicating that it is unlikely that SNV impairs APC development in the bone marrow. In addition, T cell infiltrates are not present in the lungs of infected deer mice (6); thus we used spleen cells as a source of T cells.
We established polyclonal T cell lines from all seven acutely infected deer mice but from only four of the nine persistently infected deer mice, despite the presence of viral RNA in the lungs of all deer mice (data not shown). Although it is possible that technical reasons could account for the fewer number of lines established from persistently infected deer mice, we believe that this is unlikely because of the success rate in the acutely infected deer mice and because we have established T cell lines from 16 of 16 deer mice immunized with KLH (34, 35; unpublished data). We favor the hypothesis that after more than a month postinfection, the T cell responses have waned, despite viral persistence, making T cell recovery more difficult; or that persistence is mediated by nonculturable T cells in some infected deer mice. It is also possible that SNV affects T cells in some as yet uncharacterized manner during persistence and that this has led indirectly to these observations as well.
All of the T cell lines expressed CD4 and the Th1 transcription factor T-bet, a transcription factor normally associated with the synthesis of inflammatory cytokines, such as IFN-γ. IFN-γ was expressed by all seven of the lines from the acutely infected deer mice but at low levels in the four lines from persistently infected deer mice. The influence of T-bet on the expression of IFN-γ may be altered during persistence and warrants further investigation. The Th2 transcription factor GATA3 was expressed by only acutely infected deer mouse T cells (46). Concordant with this was a lack of IL-4 expression, which is dictated by GATA3 expression (46), from T cells of persistently infected deer mice. Despite the absence of GATA3 expression in all of the lines from persistently infected deer mice, two expressed IL-5, suggesting that the expression of GATA3 is not required for IL-5 expression in deer mouse T cells. The transcription factor FoxP3, which is associated with some but not all regulatory T cell subsets (27, 33), was differentially expressed by T cells from acutely and persistently infected deer mice, including all three of the high-expressing TGF-β1 lines from persistently infected deer mice. These three T cell lines resemble inducible regulatory T cells (TGF-β1+/FoxP3+), whereas the other resembles Th3 (TGF-β1+/FoxP3−) regulatory T cell subsets (29) and support a potential role for regulatory T cells in the in vivo response of deer mice to SNV.
The proliferative responses of deer mouse T cells to viral N-Ag were weak to moderate compared with T cell responses in deer mice immunized with the irrelevant antigen KLH presented here and elsewhere (34, 35). The SNV N-Ag is capable of inducing vigorous T cell proliferation in immunized BALB/c mice (47), as does N-Ag from Puumala virus (48), and in deer mice immunized with a DNA vaccine encoding the N-Ag (31). It is possible that during the coadaptation of hantaviruses to their rodent reservoirs, natural selection favored rodents able to produce viral polypeptides that limited T cell signaling through the T cell receptor. In other T cell peptide studies using inbred laboratory house mice, poor peptide binding to MHC molecules has been shown to reduce the proliferative magnitude and cytokine profiles of T cell responses (49–52). Whereas high-density peptides induced strong proliferation and Th1 cytokine responses, low-density peptides induced weak proliferation and Th2 cytokines. In this manner, it is evident that antigenic peptides can dictate the quality of an immune response by virtue of their affinity for MHC molecules (52). Supporting this contention, de Carvalho Nicacio et al. found MHC-specific differences in proliferation magnitude to recombinant Puumala virus N-Ag in inbred strains of laboratory house mice (48). In addition, some viral peptides are antagonistic to T cell responses, such that they can impact effector T cell maturation (53–55). Such antagonism can lead to regulatory T cell activation and bystander suppression of an inflammatory immune response (56). It is also conceivable that selective pressures have influenced the evolution of T cell receptor variable region gene segments for those that interact weakly with SNV peptides, which would result in T cells with less proliferative capacities to viral antigens. Whether these events occur in rodent reservoirs infected with hantaviruses is unknown and will require further investigation.
No mAbs to deer mouse cytokines are available and because deer mice are ≈25 million years divergent from laboratory house mice and laboratory rats (57), mAbs for detecting cytokines in these species do not cross-react with deer mouse cytokines (unpublished results). Thus, we used real-time PCR to assess changes in cytokine gene expression levels induced by antigenic stimulation of the T cell lines. Although these data may not precisely reflect translation and secretion of cytokines, they provide insight into future directions to further explore the T cell responses to antigenic stimuli.
The polyclonal T cell lines from acutely infected deer mice produced both type 1 and type 2 cytokines, including IFN-γ, IL-4, and IL-5, likely reflecting a polyclonal response and paralleling that of studies of house mice immunized with recombinant Puumala virus N-Ag (48). Absent in the response were the type 1 cytokines TNF and LT, suggesting that these cytokines have no or limited roles during deer mouse CD4+ T cell responses to SNV. Both of these cytokines are produced in fatal cases of HCPS (3) and can be expressed by deer mouse T cells (35). TNF is suspected of causing the capillary leak syndrome in humans, and its absence in the deer mouse lines implies that virus-specific CD4+ T cells are not a source of TNF in deer mice. However, it is possible that other cells in infected deer mice produce TNF, although this seems unlikely because no pulmonary inflammation is observed in deer mice infected with SNV (6, 7).
Both TGF-β1 and IL-10 were expressed by the T cells from acutely infected deer mice. These cytokines are differentially expressed by regulatory T cell subsets and, in conjunction with cognate signals, are instrumental in suppressing inflammation (27, 29, 33). Their presence early in infection suggests that they may limit inflammatory responses during acute infection. Collectively, these data indicate that a polarizing immune response does not occur during the early adaptive phase of the immune response to SNV.
TGF-β1 was expressed by all four T cell lines from persistently infected deer mice, whereas IFN-γ and IL-5 were differentially expressed by the lines. Expression of both IL-4 and IL-10 was absent in the lines, suggesting a down-regulation of these genes during persistence. Of note, three of the lines (DM6001, DM6010, and DM6012) expressed high levels of TGF-β1 (Fig. 4A) and also expressed FoxP3 (Fig. 2), suggestive of the inducible regulatory T cell phenotype. The other line (DM6019) expressed modest levels of TGF-β1, yet higher than T cells from acutely infected deer mice, but no FoxP3, and resembles the Th3 phenotype. Low to moderate levels of IFN-γ and IL-5 were also expressed by some of the T cells from persistently infected deer mice. IFN-γ has a critical role in inflammation by its induction of TNF (58), which appears to be minimized in helper T cells from deer mice infected with SNV. IL-5 is an important B cell-stimulating factor that induces the secretion of IgG and IgA from antigen-activated B cell subsets (59) and likely contributes to IgG persistence in infected deer mice.
The findings presented here suggest a prominent role for an antiinflammatory adaptive response that limits immunopathology, but that also may allow persistent infection to develop. Although we demonstrated predominant TGF-β1 mRNA expression in T cells from persistently infected deer mice, we were unable to assess cytokine protein levels because commercially available antibodies for the laboratory mouse do not cross-react. Nonetheless, our cytokine gene expression findings are identical to those of Easterbrook et al. (60) in which they report elevated transcription (and translation) of TGF-β, but decreased transcription of IL-10 and TNF in rats (Rattus norvegicus) persistently infected with Seoul hantavirus. All of their T cells expressed FoxP3, as did all but one of our T cell lines from persistently infected deer mice. Together, these results suggest that activation of regulatory T cell subsets may be a common mechanism mediating persistence of hantaviruses in their rodent reservoirs; although further examination of cytokine protein responses in deer mice and responses in additional reservoirs and their hantaviruses is needed.
The generation of regulatory T cells is initiated by the innate immune response and, for some regulatory T cell subsets, is dictated by TGF-β1-producing cells (28, 33). It is likely that early events during the innate response drive the development of the antiinflammatory adaptive response, thus a closer examination of the acute phase of infection will be required to fully understand hantavirus-reservoir relationships. In particular, the role of chemokines should be examined because no pulmonary leukocyte infiltration occurs in deer mice, which contrasts with that observed in human infections.
These results suggest an immunological détente may have co-evolved in the rodent-hantavirus relationship that is mediated by regulatory T cell responses, and lends itself to the hypothesis that this response limits inflammation and immunopathology. Importantly, the nature of this response likely contributes to zoonotic persistence and ecology of hantaviruses in rodent reservoirs. The dichotomy of immune responses between humans and rodent reservoirs also suggests that TGF-β1 may be a potential therapeutic agent for treating immunopathologies caused by hantaviruses, which could abrogate the fatal inflammation associated with HCPS. Hamster models of HCPS are available that have similar pathogenesis to human HCPS (61–63) and could be used to test this hypothesis. The use of corticosteroid antiinflammatory approaches to hantavirus disease has had recent success (64); however, further work will be required to determine whether antiinflammatory cytokine therapies can be used safely and effectively without causing persistent infection.
Materials and Methods
Deer Mouse Infections.
All methods were approved by the University of Northern Colorado and University of New Mexico Institutional Animal Care and Use Committees and were conducted in accordance with the Animal Welfare Act according to a set of strict, written standard operating procedures (65, 66). Deer mice, of both sexes and of 6 to 10 weeks of age, were inoculated intramuscularly with 20 animal ID50 of SNV77734 and housed in sheltered outdoor nest boxes at the Sevilleta National Wildlife Refuge as previously described (6). The deer mice were killed by cervical dislocation at the refuge site on days 9 or 10 (acute infection) or days 43–45 (persistent infection) and packed on ice for transport to the University of New Mexico Department of Pathology for tissue processing in biosafety cabinets. Spleens and bone marrow cells were recovered from infected deer mice and made into single-cell suspensions, and aliquots were frozen in 5% FBS–RPMI medium 1640 containing 5% DMSO at −70°C for subsequent cell culture. RNA was extracted from lungs by using an RNeasy Mini kit (Qiagen, Valencia, CA) and a BeadBeater (BioSpec Products, Inc., Bartlesville, OK), and the presence of viral S segment was verified by RT-PCR; the detection of viral S segment has been shown previously to reflect similarly virus isolation methods in deer mice (32).
Quantification of Viral RNA from Cultures.
Culture supernatants were collected, and RNA was extracted by using the QIAamp Viral RNA mini kit (Qiagen) for supernatants and VersaGene Cellular RNA kit (Gentra Systems, Minneapolis, MN) for T cells. The TaqMan assay (Applied Biosystems, Foster City, CA) has been previously described (6). Briefly, cDNA was made from the S segment, followed by real-time PCR amplification of samples and plasmid standards of 106, 104, and 102 copies per reaction. Viral RNA copy number was determined by regression analysis of the standard. For routine screening of T cell lines, conventional RT-PCR was used.
Generation of Bone-Marrow-Derived APCs.
Bone marrow cells from each deer mouse were cultured in 35-mm bacterial Petri dishes in 3 ml of APC medium (10% FBS–RPMI medium 1640 supplemented with 10 ng/ml recombinant mouse granulocyte–macrophage colony-stimulating factor; R&D Systems, Minneapolis, MN) (34). At 2- or 3-day intervals, half of the medium was removed and replaced with fresh APC medium for 10–14 days, which produced large numbers of competent APCs that were used to establish T cell lines and in T cell stimulation experiments (detailed below).
Establishment of Antigen-Specific T Cell Lines.
T cells were produced and maintained as previously described (34). Bone-marrow-derived APCs treated with mitomycin C were used to stimulate autologous splenic T cells with 5 μg/ml full-length recombinant N-Ag (67) with a C-terminal His tag and produced in Escherichia coli. After 4 days, the T cells were restimulated with fresh N-Ag and APC. The cultures were fed at 2-day intervals by replacement with 5% FBS–RPMI medium 1640 containing 10 ng/ml recombinant human IL-2 (R&D Systems). Cells were split 1:2 as wells approached confluence. After 2 weeks of culture, T cells were collected, washed, and restimulated for continued in vitro adaptation and expansion. The cultures expressed CD4 but not CD8 mRNA by RT-PCR by using primers listed in supporting information (SI) Table 1. Additionally, KLH-specific T cells were generated with single-dose immunizations (10 μg in CFA) in other deer mice to assess proliferation and cytokine responses in uninfected animals (34). These lines were derived in the same manner as those from infected deer mice.
T Cell Proliferation Assay.
KLH or N-Ag at various concentrations, 2 × 105 T cells, and 104 autologous bone-marrow-derived APCs were cultured in 5% FBS–RPMI medium 1640 in 96-well plates for 72 h under 7% CO2; then proliferation was assessed by MTS assay (CellTiter-96 Aq, Promega, Madison, WI). Means and SDs were calculated from duplicate samples with the −K (no antigen) subtracted from each mean.
Real-Time PCR Detection of Cytokine Gene Expression.
The real-time PCR assay for detecting cytokine gene expression has been described previously (35). For these experiments, 106 T cells and 105 bone-marrow-derived APCs, with or without 5 μg/ml N-Ag, were cultured in 48-well plates for 42 h; then total RNA was extracted (VersaGene RNA Cell kit). Messenger RNA was reverse transcribed (iScript; Bio-Rad, Hercules, CA) and used as template cDNA. Real-time PCR with primers listed in SI Table 1 was performed in triplicate in 25-μl volumes by using the iQ SYBR Green kit (Bio-Rad) for 50 cycles with a MyiQ real-time thermal cycler (Bio-Rad). The relative template abundance was determined for each T cell line by comparing gene expression in antigen-stimulated and unstimulated cell cultures. Cycle threshold means were subtracted from 50 to produce adjusted cycle threshold means and SDs from triplicate samples. The adjusted mean of each −K sample was subtracted from the adjusted mean of the antigen-stimulated sample from the same deer mouse T cell line to provide the cycle difference. Finally, the relative template abundance was determined by calculating 2CD, where CD indicates cycle difference. Differences between acute and persistent gene expression levels in the groups of deer mice were evaluated for statistical significance by using Student's two-tailed t test.
Supplementary Material
Acknowledgments
We thank Barry J. Beaty, Charles H. Calisher, and Sabra Klein for helpful discussions and support of this work; Joseph S. Murray for critical review of the manuscript; Richard Jurin for assistance with statistical analysis; Mariana Acuna for assistance in processing bone marrow from deer mice; and Surendra P. Singh for guidance (T.S.). Funding was provided by National Institutes of Health (NIH) Contract AI25489 and NIH Grant AI054461 (to T.S.) and NIH Grants 2 U19 AI45452, U01 AI 56618, 1U01 AI054779, and R56 AI034448 (to B.H.); the βββ Biological Honor Society (L.O.); and the University of Northern Colorado (T.S.).
Abbreviations
- APC
antigen-presenting cell
- CTL
cytotoxic T lymphocyte
- Fox
Forkhead box
- HCPS
hantavirus cardiopulmonary syndrome
- KLH
keyhole limpet hemocyanin
- LT
lymphotoxin
- N-Ag
recombinant Sin Nombre virus nucleocapsid antigen
- SNV
Sin Nombre virus
- Th
T helper.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707454104/DC1.
References
- 1.Schmaljohn C, Hjelle B. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, et al. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]
- 3.Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, Waite DC, Koster FT, Ennis FA. J Infect Dis. 1999;179:295–302. doi: 10.1086/314597. [DOI] [PubMed] [Google Scholar]
- 4.Ennis FA, Cruz J, Spiropoulou CF, Waite D, Peters CJ, Nichol ST, Kariwa H, Koster FT. Virology. 1997;238:380–390. doi: 10.1006/viro.1997.8827. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA. J Immunol. 2004;172:3297–3304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- 6.Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Proc Natl Acad Sci USA. 2000;97:10578–10583. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botten J, Mirowsky K, Kusewitt D, Ye C, Gottlieb K, Prescott J, Hjelle B. J Virol. 2003;77:1540–1550. doi: 10.1128/JVI.77.2.1540-1550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calisher CH, Mills JN, Root JJ, Beaty BJ. J Am Vet Med Assoc. 2003;222:163–166. doi: 10.2460/javma.2003.222.163. [DOI] [PubMed] [Google Scholar]
- 9.Childs JE, Ksiazek TG, Spiropoulou CF, Krebs JW, Morzunov S, Maupin GO, Gage KL, Rollin PE, Sarisky J, Enscore RE, et al. J Infect Dis. 1994;169:1271–1280. doi: 10.1093/infdis/169.6.1271. [DOI] [PubMed] [Google Scholar]
- 10.Hjelle B, Jenison S, Torrez-Martinez N, Yamada T, Nolte K, Zumwalt R, MacInnes K, Myers G. J Virol. 1994;68:592–596. doi: 10.1128/jvi.68.2.592-596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 12.Green W, Feddersen R, Yousef O, Behr M, Smith K, Nestler J, Jenison S, Yamada T, Hjelle B. J Infect Dis. 1998;177:1696–1700. doi: 10.1086/515325. [DOI] [PubMed] [Google Scholar]
- 13.Alcami A. Nat Rev Immunol. 2003;3:36–50. doi: 10.1038/nri980. [DOI] [PubMed] [Google Scholar]
- 14.Alcami A, Koszinowski UH. Mol Med Today. 2000;6:365–372. doi: 10.1016/S1357-4310(00)01775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basta S, Bennink JR. Viral Immunol. 2003;16:231–242. doi: 10.1089/088282403322396064. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky FM, Lem L, Solache A, Bennett EM. Immunol Rev. 1999;168:199–215. doi: 10.1111/j.1600-065x.1999.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 17.Diamond MS. Immunol Cell Biol. 2003;81:196–206. doi: 10.1046/j.1440-1711.2003.01157.x. [DOI] [PubMed] [Google Scholar]
- 18.Dunn C, Chalupny NJ, Sutherland CL, Dosch S, Sivakumar PV, Johnson DC, Cosman D. J Exp Med. 2003;197:1427–1439. doi: 10.1084/jem.20022059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandvaux N, tenOever BR, Servant MJ, Hiscott J. Curr Opin Infect Dis. 2002;15:259–267. doi: 10.1097/00001432-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hegde NR, Tomazin RA, Wisner TW, Dunn C, Boname JM, Lewinsohn DM, Johnson DC. J Virol. 2002;76:10929–10941. doi: 10.1128/JVI.76.21.10929-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hewitt EW. Immunology. 2003;110:163–169. doi: 10.1046/j.1365-2567.2003.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 23.Vossen MT, Westerhout EM, Soderberg-Naucler C, Wiertz EJ. Immunogenetics. 2002;54:527–542. doi: 10.1007/s00251-002-0493-1. [DOI] [PubMed] [Google Scholar]
- 24.Alff PJ, Gavrilovskaya IN, Gorbunova E, Endriss K, Chong Y, Geimonen E, Sen N, Reich NC, Mackow ER. J Virol. 2006;80:9676–9686. doi: 10.1128/JVI.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geimonen E, LaMonica R, Springer K, Farooqui Y, Gavrilovskaya IN, Mackow ER. J Virol. 2003;77:1638–1643. doi: 10.1128/JVI.77.2.1638-1643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson SJ, Hasenkrug KJ. Springer Semin Immunopathol. 2006;28:51–62. doi: 10.1007/s00281-006-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouse BT, Suvas S. J Immunol. 2004;173:2211–2215. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 28.Wahl SM. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Bettelli E, Oukka M, Kuchroo VK. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 30.Rouse BT, Sarangi PP, Suvas S. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 31.Bharadwaj M, Mirowsky K, Ye C, Botten J, Masten B, Yee J, Lyons CR, Hjelle B. J Gen Virol. 2002;83:1745–1751. doi: 10.1099/0022-1317-83-7-1745. [DOI] [PubMed] [Google Scholar]
- 32.Prescott J, Ye C, Sen G, Hjelle B. J Virol. 2005;79:15007–15015. doi: 10.1128/JVI.79.24.15007-15015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izcue A, Coombes JL, Powrie F. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 34.Davenport BJ, Willis DG, Prescott J, Farrell RM, Coons TA, Schountz T. BMC Immunol. 2004;5:23. doi: 10.1186/1471-2172-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oko L, Aduddell-Swope B, Willis D, Hamor R, Coons TA, Hjelle B, Schountz T. BMC Immunol. 2006;7:18. doi: 10.1186/1471-2172-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Bernet J, Mullick J, Singh AK, Sahu A. J Biosci. 2003;28:249–264. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn YS. Curr Opin Immunol. 2003;15:443–449. doi: 10.1016/s0952-7915(03)00076-1. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzo ME, Ploegh HL, Tirabassi RS. Semin Immunol. 2001;13:1–9. doi: 10.1006/smim.2000.0290. [DOI] [PubMed] [Google Scholar]
- 40.Vaughn J, Schountz T. BIOS. 2003;74:79–86. [Google Scholar]
- 41.Herbst MM, Prescott J, Palmer AD, Schountz T. Cytokine. 2002;17:203–213. doi: 10.1006/cyto.2001.0998. [DOI] [PubMed] [Google Scholar]
- 42.Schountz T, Green R, Davenport B, Buniger A, Richens T, Root JJ, Davidson F, Calisher CH, Beaty BJ. BMC Immunol. 2004;5:1. doi: 10.1186/1471-2172-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerber DJ, Pereira P, Huang SY, Pelletier C, Tonegawa S. Proc Natl Acad Sci USA. 1996;93:14698–14703. doi: 10.1073/pnas.93.25.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. Proc Natl Acad Sci USA. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buus S, Sette A, Colon SM, Miles C, Grey HM. Science. 1987;235:1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- 46.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 47.Bharadwaj M, Lyons CR, Wortman IA, Hjelle B. Vaccine. 1999;17:2836–2843. doi: 10.1016/s0264-410x(99)00096-1. [DOI] [PubMed] [Google Scholar]
- 48.de Carvalho Nicacio C, Sallberg M, Hultgren C, Lundkvist A. J Gen Virol. 2001;82:129–138. doi: 10.1099/0022-1317-82-1-129. [DOI] [PubMed] [Google Scholar]
- 49.Schountz T, Kasselman JP, Martinson FA, Brown L, Murray JS. J Immunol. 1996;157:3893–3901. [PubMed] [Google Scholar]
- 50.Janeway CA, Jr, Bottomly K. Semin Immunol. 1996;8:108–115. doi: 10.1006/smim.1996.0013. [DOI] [PubMed] [Google Scholar]
- 51.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. J Exp Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray JS. Immunol Today. 1998;19:157–163. doi: 10.1016/s0167-5699(97)01237-1. [DOI] [PubMed] [Google Scholar]
- 53.Schountz T, Kasselman JP, Ford SR, Murray JS. Cell Immunol. 1996;168:193–200. doi: 10.1006/cimm.1996.0066. [DOI] [PubMed] [Google Scholar]
- 54.Sette A, Alexander J, Ruppert J, Snoke K, Franco A, Ishioka G, Grey HM. Annu Rev Immunol. 1994;12:413–431. doi: 10.1146/annurev.iy.12.040194.002213. [DOI] [PubMed] [Google Scholar]
- 55.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larkin J, III, Picca CC, Caton AJ. Eur J Immunol. 2007;37:139–146. doi: 10.1002/eji.200636577. [DOI] [PubMed] [Google Scholar]
- 57.Crew MD, Bates LM, Douglass CA, York JL. Immunogenetics. 1996;44:177–185. [PubMed] [Google Scholar]
- 58.Nedwin GE, Svedersky LP, Bringman TS, Palladino MA, Jr, Goeddel DV. J Immunol. 1985;135:2492–2497. [PubMed] [Google Scholar]
- 59.Takatsu K. Nippon Rinsho. 2004;62:1941–1951. [PubMed] [Google Scholar]
- 60.Easterbrook JD, Zink MC, Klein SL. Proc Natl Acad Sci USA. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooper JW, Larsen T, Custer DM, Schmaljohn CS. Virology. 2001;289:6–14. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- 62.Wahl-Jensen V, Chapman J, Asher L, Fisher R, Zimmerman M, Larsen T, Hooper JW. J Virol. 2007;81:7449–7462. doi: 10.1128/JVI.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milazzo ML, Eyzaguirre EJ, Molina CP, Fulhorst CF. J Infect Dis. 2002;186:1390–1395. doi: 10.1086/344735. [DOI] [PubMed] [Google Scholar]
- 64.Seitsonen E, Hynninen M, Kolho E, Kallio-Kokko H, Pettila V. Eur J Clin Microbiol Infect Dis. 2006;25:261–266. doi: 10.1007/s10096-006-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Botten J, Nofchissey R, Kirkendoll-Ahern H, Rodriguez-Moran P, Wortman IA, Goade D, Yates T, Hjelle B. J Mammal. 2000;81:250–259. [Google Scholar]
- 66.Mills J, Yates T, Childs J, Parmenter RR, Ksiazek T, Rollin P, Peters CJ. J Mamm. 1995;76:716–722. [Google Scholar]
- 67.Hjelle B, Jenison S, Torrez-Martinez N, Herring B, Quan S, Polito A, Pichuantes S, Yamada T, Morris C, Elgh F, Lee HW, Artsob H, Dinello R. J Clin Microbiol. 1997;35:600–608. doi: 10.1128/jcm.35.3.600-608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.