Abstract
Human mitochondrial tRNA (hmt-tRNA) mutations are associated with a variety of diseases including mitochondrial myopathies, diabetes, encephalopathies, and deafness. Because the current understanding of the precise molecular mechanisms of these mutations is limited, there is no efficient method to treat their associated mitochondrial diseases. Here, we use a variety of known mutations in hmt-tRNAPhe to investigate the mechanisms that lead to malfunctions. We tested the impact of hmt-tRNAPhe mutations on aminoacylation, structure, and translation elongation-factor binding. The majority of the mutants were pleiotropic, exhibiting defects in aminoacylation, global structure, and elongation-factor binding. One notable exception was the G34A anticodon mutation of hmt-tRNAPhe (mitochondrial DNA mutation G611A), which is associated with MERRF (myoclonic epilepsy with ragged red fibers). In vitro, the G34A mutation decreases aminoacylation activity by 100-fold, but does not affect global folding or recognition by elongation factor. Furthermore, G34A hmt-tRNAPhe does not undergo adenosine-to-inosine (A-to-I) editing, ruling out miscoding as a possible mechanism for mitochondrial malfunction. To improve the aminoacylation state of the mutant tRNA, we modified the tRNA binding domain of the nucleus-encoded human mitochondrial phenylalanyl-tRNA synthetase, which aminoacylates hmt-tRNAPhe with cognate phenylalanine. This variant enzyme displayed significantly improved aminoacylation efficiency for the G34A mutant, suggesting a general strategy to treat certain classes of mitochondrial diseases by modification of the corresponding nuclear gene.
Keywords: aminoacyl–tRNA synthetase, translation, mitochondria
Human mitochondrial (hmt)-tRNAs are essential for protein synthesis in mitochondria. Since the completion of the hmt genome sequence (1), a variety of diseases have been directly linked to hmt DNA point mutations, with over half being coupled to changes in hmt-tRNA genes (MITOMAP: A Human Mitochondrial Genome Database; http://www.mitomap.org). Diseases associated with hmt-tRNA point mutations include MERRF (myoclonic epilepsy with ragged red fibers), MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes), and deafness (2–4). Pathogenic mutations of hmt-tRNAs have been shown to affect global tRNA structure (5–8), tRNA processing (9–13), modification (14–20), aminoacylation (7, 21–26), and translation efficiency (27–29). However, little is known about the precise pathogenic mechanisms of the vast majority of hmt-tRNA mutations, hindering the development of appropriate treatments.
Currently, treatments for mitochondrial diseases are largely inadequate (30). In contrast to traditional treatments, gene therapy methods provide promising tools to cure mitochondrial disorders, although several hurdles remain to be resolved (reviewed in refs. 30 and 31). One major hindrance is the inability to deliver exogenous DNA to mammalian mitochondria in a heritable manner (30). A characteristic of mitochondrial diseases is heteroplasmy; in these patients, mitochondria are normally found to contain both WT and mutant copies of the genome. Symptoms will not appear until mutant mitochondrial DNA reaches a sufficiently high level, a phenomenon called the threshold effect (30). Several gene therapy methods have thus aimed at reducing the ratio of mutant to WT mitochondrial genomes (32, 33). Nevertheless, these approaches are limited by the delivery and toxicity of selected drugs. A more direct strategy to treat diseases caused by hmt-tRNA mutations is to import functional tRNAs into mitochondria. Pioneering studies by Tarassov and colleagues (34, 35) demonstrated that an altered yeast cytoplasmic tRNALys can be imported into human mitochondria in vitro and in vivo. The imported tRNA participates in mitochondrial protein synthesis and partially rescues a pathogenic mutation in hmt-tRNALys. One limitation is that only a few tRNA acceptor species can be imported via this pathway. Another study recently showed that the Leishmania mitochondrial RNA import complex, when delivered into human cells, is able to import certain cytoplasmic tRNAs (36). However, the RNA import complex is a large (≈600 kDa) multisubunit complex with many unknown components, and its delivery into target cells presents a considerable challenge. In contrast to tRNAs, the majority of mitochondrial proteins are nucleus-encoded, synthesized in the cytoplasm, and then imported into mitochondria (37). It is thus plausible to import allotopically expressed proteins to rescue the function of mutant tRNAs.
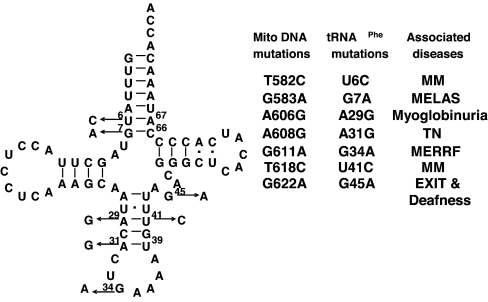
A number of mitochondrial diseases have recently been ascribed to point mutations in hmt-tRNAPhe (Fig. 1) (2–4, 38–42), including a G34A anticodon variant associated with MERRF syndrome. Anticodon pathogenic mutations are rare in hmt-tRNAs, the only other known example being the G36A mutation of hmt-tRNAPro (G15990A) for which the pathogenic mechanism remains obscure (43). In this study, we investigated the impact of hmt-tRNAPhe pathogenic mutations on both global tRNA structure and different steps in translation. All pathogenic mutants displayed pleiotropic phenotypes, with the exception of the G34A anticodon mutation, which solely affected aminoacylation. In an attempt to rescue the aminoacylation defect of the G34A hmt-tRNAPhe, we modified the nucleus-encoded human mitochondrial phenylalanyl-tRNA synthetase (hmt-PheRS), which catalyzes aminoacylation of hmt-tRNAPhe. The resulting enzyme variants showed significantly improved aminoacylation efficiency for the G34A mutant, suggesting a potential route to treat this presentation of myoclonic epilepsy with ragged red fibers (MERFF) syndrome.
Fig. 1.
Pathogenic mutations of human mitochondrial tRNAPhe. Pathogenic mutations are indicated by arrows on the left and are listed on the right. MM, mitochondrial myopathy; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes; TN, tubulointerstitial nephritis; MERRF, myoclonic epilepsy with ragged red fibers; EXIT, exercise intolerance.
Results
Hmt-tRNAPhe Pathogenic Mutations Cause Aminoacylation Defects.
Several mitochondrial diseases have been associated with point mutations in hmt-tRNAPhe (Fig. 1) (2–4, 38–42). To investigate the pathogenic mechanisms of these mutations, we used the corresponding WT and mutant forms of in vitro-transcribed hmt-tRNAPhe in aminoacylation experiments. All seven pathogenic mutations decreased the aminoacylation efficiency by hmt-PheRS, although to different extents (Table 1). The transcript of the A29G (A606G) mutant was not chargeable; thus, its kinetic parameters were not determined. Mutations G7A (G583A), G34A (G611A), and U41C (T618C) resulted in significant losses in aminoacylation efficiency, whereas U6C (T582C), A31G (A608G), and G45A (G622A) only decreased activity by 2- to 4-fold. To probe whether these nucleotides are identity elements for the cognate hmt-PheRS, we introduced compensatory mutations in the stem regions to restore Watson–Crick base paring. U6C/A67G, A29G/U41C, and A31G/U39C double mutants exhibited fully restored aminoacylation activity compared with the WT hmt-tRNAPhe, suggesting that the original pathogenic mutations decrease the aminoacylation activity by affecting the global or local tRNA structure rather than by changing PheRS identity elements. G7A/C66U hmt-tRNAPhe displayed partially rescued aminoacylation activity compared with the G7A mutant, perhaps because of the lower thermodynamic stability of the A:U pair compared with the G:C pair. However, we cannot exclude the possibility that G7 or C66 of hmt-tRNAPhe is directly recognized by hmt-PheRS. G45 is required for tertiary structure formation in bovine mt-tRNAPhe (44); the mild aminoacylation deficiency of G45A hmt-tRNA is likely due to a tertiary structure alteration. The anticodon nucleotide G34 is a well characterized identity element for both Escherichia coli and yeast PheRSs, but is not expected to be a significant structural determinant (45, 46). The severe loss observed in aminoacylation efficiency indicates that G34 is also an identity element for hmt-PheRS.
Table 1.
Steady-state kinetics of phenylalanylation of hmt-tRNAPhe variants by WT hmt-PheRS
| tRNAPhe | kcat, min−1 | Km, μM | kcat/Km, μM·min−1 | Relative, kcat/Km | Loss, -fold | Charging level, % |
|---|---|---|---|---|---|---|
| WT | 2.3 ± 0.2 | 0.6 ± 0.1 | 4.1 ± 1.0 | 100 | 1.0 | 20 |
| U6C | 1.5 ± 0.2 | 1.6 ± 0.3 | 1.0 ± 0.3 | 24 | 4.1 | 15 |
| G7A | ND | ND | 0.052 ± 0.001* | 1.3 | 79 | 10 |
| A29G | ND | ND | ND | ND | ND | <1 |
| A31G | 2.3 ± 0.3 | 1.3 ± 0.5 | 1.9 ± 0.7 | 46 | 2.2 | 10 |
| G34A | ND | ND | 0.046 ± 0.005* | 1.1 | 89 | 15 |
| U41C | ND | ND | 0.24 ± 0.002* | 5.9 | 17 | 10 |
| G45A | 2.8 ± 0.3 | 2.1 ± 0.3 | 1.3 ± 0.1 | 32 | 3.2 | 15 |
| U6C/A67G | 1.9 ± 0.1 | 0.4 ± 0.1 | 4.9 ± 1.0 | 120 | 0.8 | 20 |
| G7A/C66U | ND | ND | 0.69 ± 0.08* | 17 | 5.9 | 10 |
| A29G/U41C | 2.8 ± 0.6 | 0.7 ± 0.1 | 3.9 ± 0.5 | 95 | 1.1 | 15 |
| A31G/U39C | 2.7 ± 0.4 | 0.7 ± 0.1 | 3.9 ± 0.9 | 95 | 1.1 | 15 |
The kcat and Km values are for hmt-tRNAPhe. For charging levels, all tRNA concentrations used represent active fractions as determined from plateau levels. The kcat/Km is estimated by using subsaturating tRNA concentrations. ND, not determined.
*The Km values are too high to be accurately determined.
Effect of Pathogenic Mutations on tRNAPhe Structure.
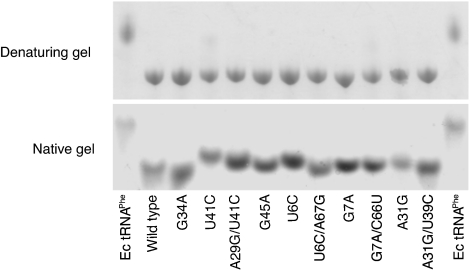
To provide some preliminary insights into whether the pathogenic mutations affect tRNAPhe structure, we tested the migration patterns of the WT and mutant tRNAsPhe by native gel electrophoresis. Mutations that perturb global structure are expected to slow tRNA migration on partially denaturing gels (47, 48). Pathogenic mutations in the stem regions, as well as G45A, resulted in migration retardation on a native gel, whereas on a denaturing gel all tRNA mutants migrated at the same rate as the WT (Fig. 2). This is consistent with predictions from our aminoacylation data, and from previous studies (44), that these mutations primarily affect the secondary or tertiary structure of hmt-tRNAPhe. As expected, the G34A mutant displayed the same migration pattern as the WT, consistent with the role of G34 as a PheRS identity element not involved in global structure.
Fig. 2.
Effects of pathogenic mutations on electrophoretic mobility. Fifty picomoles of each tRNA variant was loaded on the gel, respectively. Migration is directed from top to bottom. Except for the G34A mutant, all other hmt-tRNAPhe pathogenic mutants migrate slower than WT on a native gel, indicating alterations in the global structure.
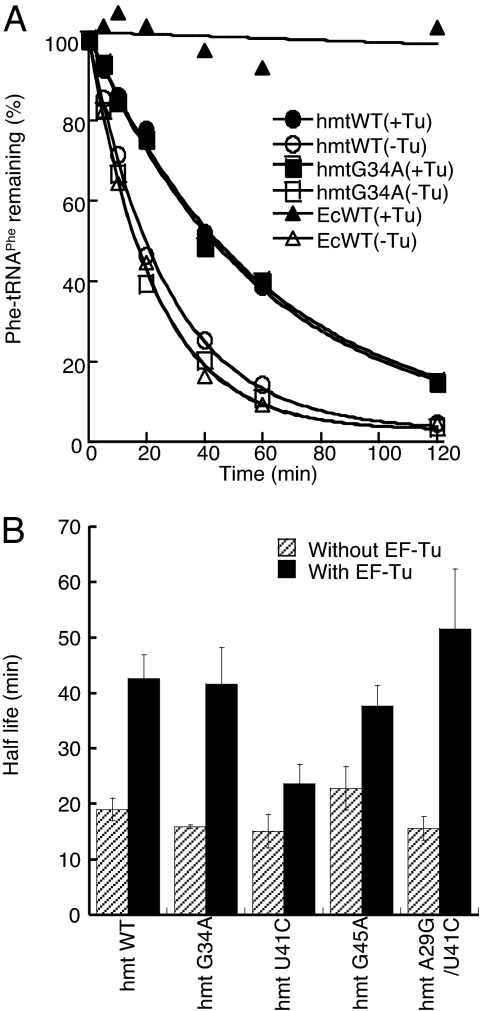
The G34A Mutation Does Not Influence EF-Tu Binding.
Pathogenic mutations in the anticodons of hmt-tRNAs are rare, leading us to systematically investigate the impact of mutation G34A on various aspects of tRNA function beyond aminoacylation. Once the amino acid is attached to the tRNA by its cognate synthetase, EF-Tu delivers the aminoacyl-tRNA to the ribosome for peptide elongation. To analyze the binding of Phe-tRNAPhe to EF-Tu, we monitored the hydrolysis of Phe-tRNAPhe variants in the presence and absence of bovine mitochondrial EF-Tu, which shares 95% sequence identity with its human counterpart (49) [Fig. 3 and supporting information (SI) Table 4]. No significant difference was observed compared with WT, indicating that the G34A mutation does not affect EF-Tu recognition. In contrast, mutation U41C significantly reduced the half-life of Phe-tRNAPhe in the presence of EF-Tu. The protection defect was rescued by a compensatory change in the stem region that restored base pairing, indicating that the reduced EF-Tu binding efficiency resulted from altered global or local tRNA structure. Overall, EF-Tu protects mitochondrial Phe-tRNAPhe less efficiently than it does E. coli Phe-tRNAPhe, presumably because the hmt-tRNAPhe transcripts are thermodynamically less stable than their bacterial counterparts (reviewed in ref. 6).
Fig. 3.
Mutation G34A does not affect EF-Tu recognition. (A) Phe-tRNAPhe hydrolysis in the presence and absence of bovine mitochondrial EF-Tu. Data points are the average of three independent experiments. (B) Quantification of the half-lives of Phe-tRNAPhe.
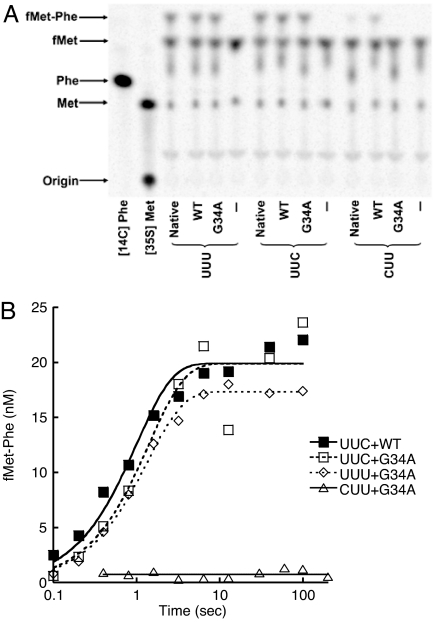
The G34A Mutation Does Not Affect Decoding of Phe Codons in Vitro.
Because the mutation G34A occurs at the anticodon, we next questioned whether this mutation affects decoding of Phe codons. According to the wobble hypothesis, the AAA anticodon of the G34A tRNAPhe mutant may not be able to read the UUC Phe codon efficiently. To analyze the effect of G34A on the decoding step of protein synthesis, we measured the rate of dipeptide formation in single-turnover reactions as described in ref. 50. Because no in vitro mitochondrial decoding system has been established, we used the well characterized E. coli system instead. 70S initiation complexes containing formyl-[35S]Met-tRNAfMet in the P site and codon UUU, UUC, or CUU in the A site were prepared. Separately, ternary complexes (EF-Tu·GTP·Phe-tRNAPhe) without or with mutation G34A were prepared. Initiation complexes were rapidly mixed with ternary complex by using a quench-flow apparatus, and the amount of fMet-Phe formed was determined at various time points. In vitro-transcribed E. coli tRNAPhe (WT) showed only a ≈2-fold decrease in the apparent rate of dipeptide formation compared with native tRNA for both UUU and UUC codons (Fig. 4 and Table 2), suggesting that modifications of tRNAPhe only modestly affect the rate of decoding. Contrary to our expectations, G34A Phe-tRNAPhe decoded the UUC codon as well as WT Phe-tRNAPhe. To investigate the possibility that decoding fidelity was compromised under our experimental conditions, we tested dipeptide formation in response to a CUU codon (encoding Leu). The decoding rates of the CUU codon by both WT and G34A tRNAPhe were dramatically reduced (Fig. 4 and Table 2). Thus, whereas the C×A mismatch at the third position of the codon-anticodon helix is well tolerated, a C×A mismatch at the first position is not. It has been shown that the E. coli ribosome can use mitochondrial tRNAs, and it is likely that the mitochondrial ribosome is able to use bacterial tRNAs as well (51, 52), reflecting the inherent similarity between bacterial and mitochondrial ribosomes. Given these various considerations, it is reasonable to envision that the G34A mutation of hmt-tRNAPhe would not appreciably affect decoding of Phe codons in vivo.
Fig. 4.
Decoding of UUU, UUC, and CUU codons by WT and G34A tRNAPhe. (A) fMet-Phe formation is started by mixing 100 nM E. coli ribosomal initiation complexes with 50 nM Phe-tRNAPhe bound by EF-Tu and stopped after 10 min at 25°C. Native, native E. coli tRNAPhe; WT and G34A, WT and mutant forms of in vitro-transcribed E. coli tRNAPhe, respectively; UUU, UUC, and CUU, mRNAs containing UUU, UUC, and CUU codons following the AUG start codon, respectively. (B) Quench-flow experiments to determine the decoding rates. Experiments were performed at 25°C. Data points are the average of three independent experiments. Data were fitted with a single exponential equation.
Table 2.
fMet-Phe dipeptide formation with cognate and near cognate codon–anticodon pairs
| Codons | Native (GAA anticodon) |
WT (GAA anticodon) |
G34A (AAA anticodon) |
|||
|---|---|---|---|---|---|---|
| kapp, s−1 | Final fMet-Phe, nM | kapp, s−1 | Final fMet-Phe, nM | kapp, s−1 | Final fMet-Phe, nM | |
| UUU | 1.8 ± 0.4 | 19 ± 4 | 0.8 ± 0.03 | 19 ± 3 | 0.8 ± 0.2 | 17 ± 1 |
| UUC | 1.5 ± 0.2 | 21 ± 8 | 1.0 ± 0.3 | 20 ± 5 | 0.7 ± 0.4 | 20 ± 2 |
| CUU | 0.05 ± 0.02 | 2.5 ± 0.5 | 0.01 ± 0.01 | 19 ± 6 | ND* | ND* |
The data set using native tRNAPhe was determined in ref. 69. ND, not determined.
*Values are too low to be determined.
G34A hmt-tRNAPhe Does Not Undergo A-to-I Editing.
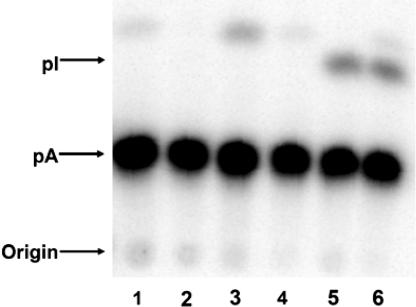
A34 in tRNAs is almost universally edited to inosine in the eukaryotic cytoplasm (53, 54). However, no inosine-containing tRNA has been reported in mitochondria and several studies have shown that A34 remains unchanged in mt-tRNAArgACG (55, 56). Although remote, the possibility remains that the observed defect caused by the G34A mutation in tRNAPhe may be due to A-to-I editing in mitochondria. Inosine at position 34 could lead to misreading of Leu codons as Phe (inosine could pair with UUA by wobbling), causing the mitochondrial defect. To probe whether G34A hmt-tRNAPhe is a substrate for A-to-I editing enzymes, we performed an in vitro A-to-I editing assay. G34A hmt-tRNAPhe was not edited by total human cell extracts or purified Trypanosoma brucei adenosine deaminase acting on tRNA (ADAT) enzymes (Fig. 5). In a control experiment, T. brucei tRNAValAAC underwent A-to-I editing in the presence of either total human cell extracts or T. brucei ADATs. Taken together, these in vitro data imply that the G34A mutant hmt-tRNAPhe is not a substrate for deamination in vivo.
Fig. 5.
G34A tRNAPhe is not a substrate for A-to-I editing enzymes. After incubation with total cell extracts or A-to-I editing enzymes, tRNAs were digested with RNase P1 and separated on cellulose TLC plates. Lanes are as follows: 1, G34A hmt-tRNAPhe without the addition of enzymes; 2, G34A hmt-tRNAPhe with total Hek 293T cell extracts; 3, G34A hmt-tRNAPhe with purified T. brucei ADAT2/ADAT3; 4, T. brucei cytosolic tRNAVal without the addition of enzymes; 5, T. brucei cytosolic tRNAVal with total Hek 293T cell extracts; 6, T. brucei cytosolic tRNAVal with purified T. brucei ADAT2/ADAT3.
Rescue of G34A hmt-tRNAPhe Aminoacylation Defect by hmt-PheRS Variants.
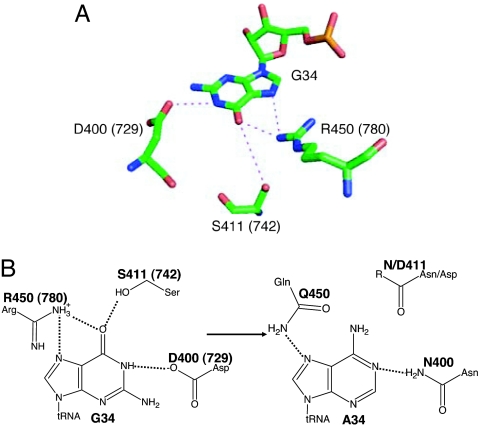
The above data indicated that reduced aminoacylation is the only functional defect of G34A hmt-tRNAPhe, suggesting that the role in translation of this mutant could be rescued via compensatory changes in hmt-PheRS. Mt-PheRS C-terminal regions are homologous to the anticodon binding domain of bacterial enzymes, although mt-PheRSs are monomeric as opposed to their bacterial counterparts, which are heterotetramers. Inspection of the Thermus thermophilus PheRS:tRNAPhe cocrystal structure (Fig. 6A) (57) showed three residues involved in hydrogen bonding the G34 base, D729, S742, and R780, all of which are extremely conserved among bacterial and mitochondrial PheRSs (SI Fig. 7). The equivalent residues in hmt-PheRS are D400, S411, and R450, and the G34A mutation would be predicted to disrupt several hydrogen bonds with these residues. In an effort to restore hydrogen bonding, we first replaced D400 of hmt-PheRS with Asn (Fig. 6B). The resulting D400N PheRS variant showed ≈10-fold improved aminoacylation efficiency toward G34A hmt-tRNAPhe compared with the WT enzyme (Table 3). The D400N substitution also decreased recognition of the WT hmt-tRNAPhe by 3-fold, indicating specific interactions between residue 400 of hmt-PheRS and position 34 of hmt-tRNAPhe. This is also consistent with previous predictions that the C-terminal domain of hmt-PheRS is involved in anticodon recognition (58). In an attempt to further increase the aminoacylation activity of the G34A mutant, we introduced the additional replacements S411 or R450 into hmt-PheRS D400N (Fig. 6B). The double variants showed further reductions in recognition of the WT hmt-tRNAPhe but did not improve aminoacylation of the G34A mutant compared with the D400N variant.
Fig. 6.
Recognition of position 34 of tRNAPhe by PheRS. (A) Recognition of G34 by T. thermophilus PheRS. T. thermophilus residue numbers are shown in parentheses; equivalent residues in hmt-PheRS are D400, S411, and R450. (B) D400N replacement in hmt-PheRS rescues the aminoacylation efficiency of G34A tRNAPhe, likely by restoring a hydrogen bond. S411N, S411D, and R450Q replacements do not further increase the aminoacylation activity of the G34A mutant.
Table 3.
Rescue of hmt-tRNAPhe G34A aminoacylation efficiency with PheRS variants
| Hmt-PheRS | Hmt-tRNAPhe WT |
Hmt-tRNAPhe G34A |
||||||
|---|---|---|---|---|---|---|---|---|
| kcat, min−1 | Km, μM | kcat/Km, μM−1·min−1 | Relative, kcat/Km | kcat, min−1 | Km, μM | kcat/Km, μM−1·min−1 | Relative, kcat/Km | |
| WT | 2.3 ± 0.2 | 0.6 ± 0.1 | 4.1 ± 1.0 | 100 | ND | ND | 0.046 ± 0.005* | 1 |
| D400N | 2.6 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.2 | 39 | 1.8 ± 0.1 | 4.1 ± 0.3 | 0.44 ± 0.04 | 11 |
| D400N/S411D | ND | ND | 0.43 ± 0.06* | 10 | ND | ND | 0.15 ± 0.002* | 4 |
| D400N/S411N | ND | ND | 0.24 ± 0.05* | 6 | ND | ND | 0.21 ± 0.02* | 5 |
| D400N/R450Q | ND | ND | 0.27 ± 0.03* | 7 | ND | ND | 0.39 ± 0.07* | 10 |
The concentrations of PheRS variants were normalized by active site titration. The kcat and Km values are for hmt-tRNAPhe. The kcat/Km is estimated by using subsaturating tRNA concentrations. ND, not determined.
*The Km values are too high to be accurately determined.
Discussion
Mechanism of Pathogenesis of the Human Mitochondrial DNA Mutation G611A.
The majority of hmt-tRNAPhe pathogenic mutations affect the global structure of tRNA, resulting in functional defects such as deficiencies in aminoacylation and EF-Tu binding. These functional defects are likely to be additive, so that a mutation which only mildly affects one aspect of tRNA function, such as aminoacylation, is able to cause severe mitochondrial dysfunction (reviewed in refs. 6, 59). Mutation G34A of hmt-tRNAPhe is one of the few anticodon pathogenic mutations identified to date, and results in a neurological and muscular disease named MERRF (2). Anticodon mutations are rarely found in hmt-tRNAs, because changing the anticodon nucleotides would normally result in severe decoding problems in addition to aminoacylation defects. Such mutations are therefore likely to be lethal for the cell (6). The only other known anticodon pathogenic mutation was found in hmt-tRNAPro, in which G36 is changed to A (43). The resulting mutant contains an anticodon (UGA) reading a Ser codon (UCG) instead of a Pro codon (CCN). It is not clear whether the mutation results in misincorporation of Pro at Ser codons or simply causes reduction of Pro codon decoding efficiency. Hmt-tRNA pathogenic mutations can also have indirect influences on the anticodon. For example, several mutations outside the anticodon loop of hmt-tRNALys and hmt-tRNALeu result in modification defects at U34 (18–20). The unmodified U loses the ability to wobble with G, which in turn leads to decoding deficiencies. In contrast, the above analysis provides evidence that mutation G34A of hmt-tRNAPhe causes a significant defect in aminoacylation but does not affect other steps in protein synthesis or the overall structure of the tRNA. Mitochondrial tRNA processing requires the integrity of the structure as well as certain nucleotides at the 5′ and 3′ ends (13); thus, the G34A mutation would not be expected to impact processing. Taken together, it appears that the sole functional defect of G34A hmt-tRNAPhe is reduced aminoacylation efficiency.
Identity of tRNAPheA34 in Translation.
Adenosines at the tRNA wobble position (A34) are normally edited to inosine by ADAT2/ADAT3 (53, 54). If A-to-I editing occurred at position 34 of the G34A mutant, the aminoacylation defect might be minor as I34 is expected to be well recognized by WT hmt-PheRS. However, inosine at position 34 could lead to the miscoding of Leu codons for Phe. To date, no I34-containing tRNAs have been found in mitochondria (55, 56). It is possible that either A-to-I editing enzymes are not present in mitochondria, or mt-tRNAs are not substrates for these enzymes. Our study showed that G34A hmt-tRNAPhe is not a substrate for purified T. brucei ADAT2/ADAT3, or A-to-I editing enzymes from deamination-competent total human cell extracts. It is thus very unlikely that the G34A hmt-tRNAPhe mutant would undergo an A-to-I conversion at the wobble position, supporting the notion that the pathogenic mutation results in a tRNA with the anticodon AAA rather than the potentially Leu-miscoding IAA. This, in turn, raised the possibility that the G34A mutation could impact ribosomal protein synthesis in addition to aminoacylation. The observation that tRNAPheA34 does not lead to decoding deficiency of the UUC codon is somewhat surprising, but not unprecedented. It has been reported that Mycoplasma capricolum tRNAThrAGU with an unedited A34 decodes the ACC codon in vivo (60). In Salmonella typhimurium, a mutant tRNAProAGG also decodes the CCC codon in vivo, although A34 remains unchanged (61). Additionally, Boren et al. showed that a mutant E. coli tRNAGlyACC reads the GGC codon efficiently in an in vitro translation system (62). It has been proposed that unmodified U34 in hmt-tRNAs is able to pair with A, U, G, or C (63); our studies, together with the previous work described above, now suggest that unmodified A34 may also have an expanded decoding capacity in mitochondria and in mycoplasma species. The observed A×C wobble pairing can potentially be explained by the “two of three” rule, which states that the wobble position is not always used in codon-anticodon recognition (64), consistent with structural studies indicating that the 30S subunit monitors the geometry of the first two base pairs of the codon–anticodon helix much more stringently than the wobble pair (65). It is also possible that the relative flexibility of hmt-tRNAPhe allows the A×C pair to form at the wobble position.
Restoring the Function of a Mutant tRNA That Causes Myoclonic Epilepsy with Ragged Red Fibers (MERFF).
To date, there is no efficient means to treat mitochondrial diseases (30). In an attempt to explore potential routes for gene therapy, we modified the tRNA binding region of nuclear-encoded human mitochondrial PheRS. The resulting enzyme variants showed partially restored aminoacylation efficiency for G34A hmt-tRNAPhe and confirmed predictions that the C terminus of hmt-PheRS is involved in anticodon binding (58, 66). Because the G34A mutation of hmt-tRNAPhe does not affect other aspects of tRNA function, a modified nuclear gene for hmt-PheRS may be able to restore the function of mitochondria containing this mutation via allotopic expression. Considering the threshold effect [a patient with the G34A mutation of hmt-tRNAPhe contains >90% mutant mitochondrial DNA (2)], this partial rescue may at least alleviate, if not fully ablate, the MERRF symptoms associated with the G34A mutation. Validation of such an approach for gene therapy of certain mitochondrial disorders now requires detailed testing of these predictions in a suitable in vivo system.
Materials and Methods
Strains, Plasmids, and General Methods.
E. coli strains BL21 (pArgU218)/PET21c-PheRS expressing C-terminal His6-tagged human mitochondrial PheRS, and BL21 (DE3)/PET21c(+)-Tu expressing His6-tagged bovine mitochondrial EF-Tu were gifts from L. Spremulli (University of North Carolina, Chapel Hill, NC). E. coli JM109/pKECA-Tu producing His6-tagged E. coli EF-Tu was a gift from B. Kraal (Leiden University, Leiden, The Netherlands). The hmt-tRNAPhe gene was cloned into PUC18. PheRS and tRNAPhe mutants were obtained through site-directed mutagenesis as described in ref. 67. The hmt-PheRS variant S411 did not express well in regular LB media, suggesting that this residue might be important for maintaining protein stability or folding. Bovine mitochondrial EF-Tu was purified as described in ref. 68; tRNAs transcripts and other His6-tagged proteins were purified as described in ref. 67. fMet-Phe dipeptide formation assay was performed as described in ref. 69.
Aminoacylation Assay and EF-Tu Protection.
Aminoacylation and Phe-tRNAPhe preparation were performed as described in ref. 70, except that the reactions were done at room temperature. WT and variant PheRS active concentrations were determined in active site titration assays as described in ref. 71. EF-Tu was activated at 37°C for 20 min, in a buffer containing 50 mM Tris·HCl (pH 7.5), 1 mM DTT, 68 mM KCl, 6.7 mM MgCl2, 2.5 mM phosphoenolpyruvate, 0.5 mM GTP, and 30 μg/ml pyruvate kinase. Phe-tRNAPhe hydrolysis was then monitored in the presence and absence of 5 μM bovine mitochondrial EF-Tu in the activation buffer. Aliquots were taken from the reaction at different time points and spotted on 3MM paper discs presoaked with 5% trichloroacetic acid. Discs were washed with 5% trichloroacetic acid and dried, and radioactivity was measured by scintillation counting.
Native and Denaturing Gel Electrophoresis.
Native gels were prepared in 1× TBE (89 mM Tris/89 mM boric acid/2.5 mM EDTA, pH 8.3), 10 mM MgCl2, and 12% acrylamide-bis. The loading buffer for native gels contained 20% sucrose, 0.01% bromophenol blue, and 10 mM MgCl2 in TBE. Fifty picomoles of each tRNA variant were loaded on the gel, respectively. Gels were run at 50 V at 4°C for 11 h. Denaturing gels contained 7 M urea and 12% acrylamide-bis. Denaturing gels were run at 200 V at room temperature for 80 min. Samples were run to approximately the same distances on native and denaturing gels.
A-to-I Editing.
Total human cell extracts were obtained by lysing Hek 293T cells in 10 mM Tris·HCl, pH 8.0. tRNAs were prepared by in vitro transcription in the presence of trace [α-32P]ATP. In vitro A-to-I editing experiments were performed as described in ref. 72.
Supplementary Material
Acknowledgments
We thank Drs. L. Spremulli and B. Kraal for strains and plasmids; S. Yadavalli, K. Dare, S. Walker, and Dr. C. Rappleye for preparation of materials; and C. Hausmann, N. Reynolds, and T. Rogers for critical reading of the manuscript. This work was supported by National Science Foundation Grants 0344002 (to M.I.) and 0620707 (to J.D.A.), National Institutes of Health Grant GM072528 (to K.F.), the United States–Israel Binational Science Foundation (M.I.), and American Heart Association Grant 0565180B (to J.D.A.).
Abbreviations
- hmt
human mitochondrial
- ADAT
adenosine deaminase acting on tRNA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704441104/DC1.
References
- 1.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 2.Mancuso M, Filosto M, Mootha VK, Rocchi A, Pistolesi S, Murri L, DiMauro S, Siciliano G. Neurology. 2004;62:2119–2121. doi: 10.1212/01.wnl.0000127608.48406.f1. [DOI] [PubMed] [Google Scholar]
- 3.Hanna MG, Nelson IP, Morgan-Hughes JA, Wood NW. J Neurol Neurosurg Psychiatr. 1998;65:512–517. doi: 10.1136/jnnp.65.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deschauer M, Swalwell H, Strauss M, Zierz S, Taylor RW. Arch Neurol. 2006;63:902–905. doi: 10.1001/archneur.63.6.902. [DOI] [PubMed] [Google Scholar]
- 5.Wittenhagen LM, Kelley SO. Nat Struct Biol. 2002;9:586–590. doi: 10.1038/nsb820. [DOI] [PubMed] [Google Scholar]
- 6.Wittenhagen LM, Kelley SO. Trends Biochem Sci. 2003;28:605–611. doi: 10.1016/j.tibs.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Kelley SO, Steinberg SV, Schimmel P. Nat Struct Biol. 2000;7:862–865. doi: 10.1038/79612. [DOI] [PubMed] [Google Scholar]
- 8.Tomari Y, Hino N, Nagaike T, Suzuki T, Ueda T. J Biol Chem. 2003;278:16828–16833. doi: 10.1074/jbc.M213216200. [DOI] [PubMed] [Google Scholar]
- 9.Rossmanith W, Karwan RM. FEBS Lett. 1998;433:269–274. doi: 10.1016/s0014-5793(98)00928-4. [DOI] [PubMed] [Google Scholar]
- 10.Levinger L, Giegé R, Florentz C. Nucleic Acids Res. 2003;31:1904–1912. doi: 10.1093/nar/gkg282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga A, Koga Y, Akita Y, Fukiyama R, Ueki I, Yatsuga S, Matsuishi T. Neuromuscul Disord. 2003;13:259–262. doi: 10.1016/s0960-8966(02)00267-5. [DOI] [PubMed] [Google Scholar]
- 12.Levinger L, Jacobs O, James M. Nucleic Acids Res. 2001;29:4334–4340. doi: 10.1093/nar/29.21.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levinger L, Morl M, Florentz C. Nucleic Acids Res. 2004;32:5430–5441. doi: 10.1093/nar/gkh884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helm M, Florentz C, Chomyn A, Attardi G. Nucleic Acids Res. 1999;27:756–763. doi: 10.1093/nar/27.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helm M, Giegé R, Florentz C. Biochemistry. 1999;38:13338–13346. doi: 10.1021/bi991061g. [DOI] [PubMed] [Google Scholar]
- 16.Yasukawa T, Suzuki T, Ishii N, Ueda T, Ohta S, Watanabe K. FEBS Lett. 2000;467:175–178. doi: 10.1016/s0014-5793(00)01145-5. [DOI] [PubMed] [Google Scholar]
- 17.Yasukawa T, Suzuki T, Ueda T, Ohta S, Watanabe K. J Biol Chem. 2000;275:4251–4257. doi: 10.1074/jbc.275.6.4251. [DOI] [PubMed] [Google Scholar]
- 18.Kirino Y, Yasukawa T, Ohta S, Akira S, Ishihara K, Watanabe K, Suzuki T. Proc Natl Acad Sci USA. 2004;101:15070–15075. doi: 10.1073/pnas.0405173101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirino Y, Goto Y, Campos Y, Arenas J, Suzuki T. Proc Natl Acad Sci USA. 2005;102:7127–7132. doi: 10.1073/pnas.0500563102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasukawa T, Suzuki T, Ishii N, Ohta S, Watanabe K. EMBO J. 2001;20:4794–4802. doi: 10.1093/emboj/20.17.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toompuu M, Yasukawa T, Suzuki T, Hakkinen T, Spelbrink JN, Watanabe K, Jacobs HT. J Biol Chem. 2002;277:22240–22250. doi: 10.1074/jbc.M200338200. [DOI] [PubMed] [Google Scholar]
- 22.Sohm B, Frugier M, Brule H, Olszak K, Przykorska A, Florentz C. J Mol Biol. 2003;328:995–1010. doi: 10.1016/s0022-2836(03)00373-5. [DOI] [PubMed] [Google Scholar]
- 23.Park H, Davidson E, King MP. Biochemistry. 2003;42:958–964. doi: 10.1021/bi026882r. [DOI] [PubMed] [Google Scholar]
- 24.Borner GV, Zeviani M, Tiranti V, Carrara F, Hoffmann S, Gerbitz KD, Lochmuller H, Pongratz D, Klopstock T, Melberg A, et al. Hum Mol Genet. 2000;9:467–475. doi: 10.1093/hmg/9.4.467. [DOI] [PubMed] [Google Scholar]
- 25.Degoul F, Brule H, Cepanec C, Helm M, Marsac C, Leroux J, Giegé R, Florentz C. Hum Mol Genet. 1998;7:347–354. doi: 10.1093/hmg/7.3.347. [DOI] [PubMed] [Google Scholar]
- 26.Sissler M, Helm M, Frugier M, Giegé R, Florentz C. RNA. 2004;10:841–853. doi: 10.1261/rna.5267604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomyn A, Martinuzzi A, Yoneda M, Daga A, Hurko O, Johns D, Lai ST, Nonaka I, Angelini C, Attardi G. Proc Natl Acad Sci USA. 1992;89:4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King MP, Koga Y, Davidson M, Schon EA. Mol Cell Biol. 1992;12:480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flierl A, Reichmann H, Seibel P. J Biol Chem. 1997;272:27189–27196. doi: 10.1074/jbc.272.43.27189. [DOI] [PubMed] [Google Scholar]
- 30.DiMauro S, Mancuso M, Naini A. Ann NY Acad Sci. 2004;1011:232–245. doi: 10.1196/annals.1293.023. [DOI] [PubMed] [Google Scholar]
- 31.Smith PM, Ross GF, Taylor RW, Turnbull DM, Lightowlers RN. Biochim Biophys Acta. 2004;1659:232–239. doi: 10.1016/j.bbabio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Taivassalo T, Fu K, Johns T, Arnold D, Karpati G, Shoubridge EA. Hum Mol Genet. 1999;8:1047–1052. doi: 10.1093/hmg/8.6.1047. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Borgeld HJ, Zhang J, Muramatsu S, Gong JS, Yoneda M, Maruyama W, Naoi M, Ibi T, Sahashi K, et al. J Biomed Sci. 2002;9:534–541. doi: 10.1159/000064726. [DOI] [PubMed] [Google Scholar]
- 34.Kolesnikova OA, Entelis NS, Mireau H, Fox TD, Martin RP, Tarassov IA. Science. 2000;289:1931–1933. doi: 10.1126/science.289.5486.1931. [DOI] [PubMed] [Google Scholar]
- 35.Kolesnikova OA, Entelis NS, Jacquin-Becker C, Goltzene F, Chrzanowska-Lightowlers ZM, Lightowlers RN, Martin RP, Tarassov I. Hum Mol Genet. 2004;13:2519–2534. doi: 10.1093/hmg/ddh267. [DOI] [PubMed] [Google Scholar]
- 36.Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S. Science. 2006;314:471–474. doi: 10.1126/science.1129754. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs HT, Turnbull DM. Trends Genet. 2005;21:312–314. doi: 10.1016/j.tig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Moslemi AR, Lindberg C, Toft J, Holme E, Kollberg G, Oldfors A. Neuromuscul Disord. 2004;14:46–50. doi: 10.1016/s0960-8966(03)00168-8. [DOI] [PubMed] [Google Scholar]
- 39.Darin N, Kollberg G, Moslemi AR, Tulinius M, Holme E, Gronlund MA, Andersson S, Oldfors A. Neuromuscul Disord. 2006;16:504–506. doi: 10.1016/j.nmd.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Chinnery PF, Johnson MA, Taylor RW, Lightowlers RN, Turnbull DM. Ann Neurol. 1997;41:408–410. doi: 10.1002/ana.410410319. [DOI] [PubMed] [Google Scholar]
- 41.Tzen CY, Tsai JD, Wu TY, Chen BF, Chen ML, Lin SP, Chen SC. Kidney Int. 2001;59:846–854. doi: 10.1046/j.1523-1755.2001.059003846.x. [DOI] [PubMed] [Google Scholar]
- 42.Kleinle S, Schneider V, Moosmann P, Brandner S, Krahenbuhl S, Liechti-Gallati S. Biochem Biophys Res Commun. 1998;247:112–115. doi: 10.1006/bbrc.1998.8729. [DOI] [PubMed] [Google Scholar]
- 43.Moraes CT, Ciacci F, Bonilla E, Ionasescu V, Schon EA, DiMauro S. Nat Genet. 1993;4:284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- 44.Wakita K, Watanabe Y, Yokogawa T, Kumazawa Y, Nakamura S, Ueda T, Watanabe K, Nishikawa K. Nucleic Acids Res. 1994;22:347–353. doi: 10.1093/nar/22.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson ET, Uhlenbeck OC. Biochemistry. 1992;31:10380–10389. doi: 10.1021/bi00157a028. [DOI] [PubMed] [Google Scholar]
- 46.Sampson JR, Behlen LS, DiRenzo AB, Uhlenbeck OC. Biochemistry. 1992;31:4161–4167. doi: 10.1021/bi00132a002. [DOI] [PubMed] [Google Scholar]
- 47.Rinaldi T, Francisci S, Zennaro E, Frontali L, Bolotin-Fukuhara M. Curr Genet. 1994;25:451–455. doi: 10.1007/BF00351785. [DOI] [PubMed] [Google Scholar]
- 48.Feuermann M, Francisci S, Rinaldi T, De Luca C, Rohou H, Frontali L, Bolotin-Fukuhara M. EMBO Rep. 2003;4:53–58. doi: 10.1038/sj.embor.embor713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woriax VL, Burkhart W, Spremulli LL. Biochim Biophys Acta. 1995;1264:347–356. doi: 10.1016/0167-4781(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 50.Gromadski KB, Rodnina MV. Mol Cell. 2004;13:191–200. doi: 10.1016/s1097-2765(04)00005-x. [DOI] [PubMed] [Google Scholar]
- 51.Kumazawa Y, Schwartzbach CJ, Liao HX, Mizumoto K, Kaziro Y, Miura K, Watanabe K, Spremulli LL. Biochim Biophys Acta. 1991;1090:167–172. doi: 10.1016/0167-4781(91)90097-6. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzbach CJ, Spremulli LL. J Biol Chem. 1989;264:19125–19131. [PubMed] [Google Scholar]
- 53.Gerber AP, Keller W. Science. 1999;286:1146–1149. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- 54.Gerber AP, Keller W. Trends Biochem Sci. 2001;26:376–384. doi: 10.1016/s0968-0004(01)01827-8. [DOI] [PubMed] [Google Scholar]
- 55.Sibler AP, Dirheimer G, Martin RP. FEBS Lett. 1986;194:131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe Y, Tsurui H, Ueda T, Furusihima-Shimogawara R, Takamiya S, Kita K, Nishikawa K, Watanabe K. Biochim Biophys Acta. 1997;1350:119–122. doi: 10.1016/s0167-4781(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 57.Goldgur Y, Mosyak L, Reshetnikova L, Ankilova V, Lavrik O, Khodyreva S, Safro M. Structure (London) 1997;5:59–68. doi: 10.1016/s0969-2126(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 58.Bullard JM, Cai YC, Demeler B, Spremulli LL. J Mol Biol. 1999;288:567–577. doi: 10.1006/jmbi.1999.2708. [DOI] [PubMed] [Google Scholar]
- 59.Florentz C, Sissler M. EMBO Rep. 2001;2:481–486. doi: 10.1093/embo-reports/kve111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inagaki Y, Kojima A, Bessho Y, Hori H, Ohama T, Osawa S. J Mol Biol. 1995;251:486–492. doi: 10.1006/jmbi.1995.0450. [DOI] [PubMed] [Google Scholar]
- 61.Chen P, Qian Q, Zhang S, Isaksson LA, Bjork GR. J Mol Biol. 2002;317:481–492. doi: 10.1006/jmbi.2002.5435. [DOI] [PubMed] [Google Scholar]
- 62.Boren T, Elias P, Samuelsson T, Claesson C, Barciszewska M, Gehrke CW, Kuo KC, Lustig F. J Mol Biol. 1993;230:739–749. doi: 10.1006/jmbi.1993.1196. [DOI] [PubMed] [Google Scholar]
- 63.Agris PF. Nucleic Acids Res. 2004;32:223–238. doi: 10.1093/nar/gkh185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagerkvist U. Proc Natl Acad Sci USA. 1978;75:1759–1762. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogle JM, Ramakrishnan V. Annu Rev Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- 66.Sanni A, Walter P, Boulanger Y, Ebel JP, Fasiolo F. Proc Natl Acad Sci USA. 1991;88:8387–8391. doi: 10.1073/pnas.88.19.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy H, Ling J, Irnov M, Ibba M. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hunter SE, Spremulli LL. Mitochondrion. 2004;4:21–29. doi: 10.1016/j.mito.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Ling J, Yadavalli S, Ibba M. RNA. 2007 doi: 10.1261/rna.684107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling J, Roy H, Ibba M. Proc Natl Acad Sci USA. 2007;104:72–77. doi: 10.1073/pnas.0606272104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibba M, Kast P, Hennecke H. Biochemistry. 1994;33:7107–7112. doi: 10.1021/bi00189a013. [DOI] [PubMed] [Google Scholar]
- 72.Rubio MA, Ragone FL, Gaston KW, Ibba M, Alfonzo JD. J Biol Chem. 2006;281:115–120. doi: 10.1074/jbc.M510136200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.