Abstract
Aims
Previous systematic reviews have found that drug-related morbidity accounts for 4.3% of preventable hospital admissions. None, however, has identified the drugs most commonly responsible for preventable hospital admissions. The aims of this study were to estimate the percentage of preventable drug-related hospital admissions, the most common drug causes of preventable hospital admissions and the most common underlying causes of preventable drug-related admissions.
Methods
Bibliographic databases and reference lists from eligible articles and study authors were the sources for data. Seventeen prospective observational studies reporting the proportion of preventable drug-related hospital admissions, causative drugs and/or the underlying causes of hospital admissions were selected. Included studies used multiple reviewers and/or explicit criteria to assess causality and preventability of hospital admissions. Two investigators abstracted data from all included studies using a purpose-made data extraction form.
Results
From 13 papers the median percentage of preventable drug-related admissions to hospital was 3.7% (range 1.4–15.4). From nine papers the majority (51%) of preventable drug-related admissions involved either antiplatelets (16%), diuretics (16%), nonsteroidal anti-inflammatory drugs (11%) or anticoagulants (8%). From five studies the median proportion of preventable drug-related admissions associated with prescribing problems was 30.6% (range 11.1–41.8), with adherence problems 33.3% (range 20.9–41.7) and with monitoring problems 22.2% (range 0–31.3).
Conclusions
Four groups of drugs account for more than 50% of the drug groups associated with preventable drug-related hospital admissions. Concentrating interventions on these drug groups could reduce appreciably the number of preventable drug-related admissions to hospital from primary care.
Keywords: adverse effects, drug safety, drug therapy, drug toxicity, medication errors, systematic review
Introduction
Drug-related problems are an important cause of morbidity and mortality and a significant burden on healthcare resources [1, 2]. Previous systematic reviews have shown that 4.9–7.7% of admissions are related to adverse drug events [3, 4] and a median of 4.3% of admissions are considered to be drug-related and preventable [3]. These reviews have largely concentrated on the proportion of admissions that are drug related. One review assessed which drugs most commonly contributed to drug-related morbidity, but combined events which had occurred in hospital with those which occurred in a community setting [5]. Little is known about which drugs are most frequently responsible for preventable drug-related hospital admissions, or the most common underlying causes of these admissions.
We undertook a systematic review to address the following questions:
What proportion of hospital admissions are drug related and preventable?
What are the most common drug causes of preventable hospital admissions?
What are the most common underlying causes of preventable drug-related admissions?
Methods
Searching
We sought to identify both published and unpublished studies using a high-sensitivity, low-specificity search of the following databases: the Cochrane controlled trials register, Cochrane database of systematic reviews, Index UK, US Dissertation abstracts, International Pharmaceutical Abstracts, MEDLINE, EMBASE, Cinahl, Pharmline, National Research Register, Psychinfo, Science Citation Index and SIGLE.
In addition, bibliography lists of published reviews were searched for relevant papers [3–5]. Where possible, corresponding authors were contacted for further information. Details of the search strategy are available from the corresponding author.
Selection and abstraction
Two reviewers (S.S. and S.R.) independently screened titles for relevance, resolving disagreements through discussion. The same reviewers independently reviewed the abstracts of articles considered to be relevant. Full papers were retrieved for detailed analysis and assessed according to the inclusion criteria. Two reviewers (S.S. and R.L.H.) abstracted data from papers meeting these criteria and a third reviewer (A.J.A.) verified the data.
Our inclusion criteria were as follows:
Types of studies: studies eligible for inclusion prospectively identified patients admitted with preventable drug-related admissions to hospital using medical record review. Studies reported the number and proportion of preventable drug-related admissions and at least one of the following: types of medication associated with preventable drug-related admissions and underlying causes of preventable drug-related admissions.
Types of participants: patients aged ≥ 16 years.
Types of admissions: admissions to hospital from primary care judged to be drug related and preventable. Admissions caused by adverse drug reactions, under- or overtreatment and problems with patient adherence to medication were included.
Studies excluded from the review were those that: did not use medical record review to identify admissions; focused on specific diseases or treatments, a single drug-related problem, or admissions to a single specialist unit; focused on admissions attributed to drugs of abuse or intentional overdose; focused on hospital readmissions only; focused on events occurring in hospital.
Quality assessment
Papers were assessed as suitable for inclusion if they met the inclusion criteria detailed above. For the purposes of subgroup and sensitivity analyses, we also recorded other methodological and participant characteristics of included studies.
Categorization of drugs into classes and groups
Drugs associated with admissions were categorized into groups using British National Formulary subchapter headings wherever possible [6].
Data synthesis
Summary statistics for our chosen outcomes were calculated from the selected papers using Microsoft Excel (2000) and are reported as follows:
The median (range) percentage of all hospital admissions (as reported by study authors) which were drug related and preventable.
The frequency of drug causes of admissions, reported as the proportion of all drug causes (i.e. greater than the number of admissions, as more than one drug can contribute to a single admission)
The median percentage (range) of all hospital admissions attributed to an underlying cause (as reported by the study authors). Underlying causes included: prescribing problems (admissions which could have been avoided by prescribing an alternative drug or dose of drug), monitoring problems (admissions which could have been avoided by closer monitoring for adverse effects of medication) and adherence problems (admissions which could have been avoided if patients had taken the drugs according to the prescribed directions).
Results
Description of studies
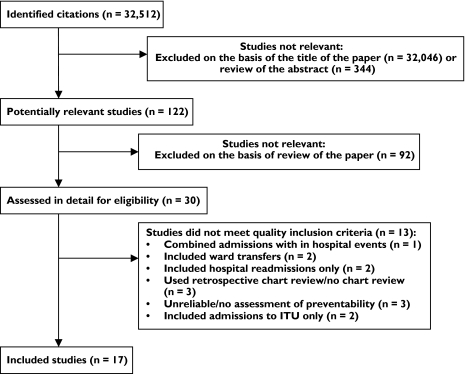
We identified 122 potentially relevant studies, of which 17 satisfied our inclusion criteria. The main reasons for excluding studies are summarized in the QUOROM flow diagram (see Figure 1). A brief description of the studies included in the review is presented in Table 1. Hallas et al. conducted a series of studies covering a range of hospital wards (general medical, cardiac, older people, gastrointestinal and respiratory) [7–11]. Details of the drugs associated with preventable hospital admissions are published in five papers [7–11], whilst a sixth paper summarized the percentage of preventable drug-related admissions for all the units studied [12]. A seventh paper by Hallas et al. reported the results of a survey of admissions following an intervention which had been developed as a result of the earlier work [13].
Figure 1.
Selection process of eligible studies
Table 1.
Details of studies included in the review
| Study | Methodology | Unit studied | Age of patients | Type of admission | Method of assessment of causality and preventability | Types of events classified as DRA | Location of study | Period of data collection | Duration of data collection | Number of admissions | Number (%) DRA | $Number (%) PDRA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bigby 1987 [19] | Prospective medical record review with physician and patientinterview | All wards in a700-bed tertiary teaching hospital | Adults | Unscheduled and emergency admissions | Majority clinical judgement of three reviewers | ADR | Massa-chusetts,USA | 1983–1984 | 1 year | 686 | 66 (9.6) | 36 (5.2) |
| Chan 2001 [21] | Prospective medical record review andpatient and/orrelative interview | Medical wards at a 500-bed public acute care hospital | Adults aged ≥75 years | Acute unplanned admissions | Two reviewers using ‘Hallas criteria’ for causality,severity andpreventability | ADRUnder-treatmentOver-treatmentNon-compliance | Southern Tasmania, Australia | 1998 | 8 weeks | 240 | 45 (18.8) | 37 (15.4) |
| Courtman 1995 [23] | Prospective medical record review by apharmacist | One acute 32-bed medical ward (including20 geriatricbeds) | Patients aged ≥65 years | All admissions | Unknown numberof reviewers used ‘amended Hallas criteria’ for causality and specific criteriafor preventability | ADRUnder-treatmentOver- treatmentNon-compliance | Toronto,Canada | Sep-tember1992 to February 1993 | 19 weeks | 150 | 46 (30.7) | 18 (12.0) |
| Cunningham1997 [14] | Prospective medical record and nursingrecord review by apharmacist and patient interview | Care of the elderly wards in a number of hospitals | Patients aged ≥65 years | Admissions to care of the elderly wards over a 4-week period for eachhospital | Majority judgement of three independentreviewers using ‘Hallas criteria’ for causality and preventability | ADRUnder-treatmentOver-treatment | Tayside,UK | March to December 1992 | 10 months | 1011 | 54 (5.3) | 43 (4.3) |
| Dartnell 1996 [22] | Prospective routine pharmacist review of patients, registrarreview of computer record, and selectedmedical records andpatient interview | Whole hospital | Adults | Admissions via Emergency Department lasting > 24 h | Agreement of two reviewers using modified ‘Karch & Lasagna criteria’ for causality, and specific criteriafor preventability | ADRUnder-treatmentOver-treatmentNon-compliance | Melbourne,Australia | Novemberto December1994 | 30 days | 965 | 55 (5.7) | 36 (3.7) |
| Green 2000 [16] | Prospective medicalrecord review and patient and/or GPinterview, withfollow-up at5 months | Acute medical admissions units in two hospitals | Adults | Random number selection of 200 acute medical admissions | Agreement of two reviewers using ‘Venulet and Ten Hams classification’ and ‘Naranjo’salgorithm’ toassess causality. Assessment of preventabilityunclear | ADR | Liverpool,UK | Unknown | 22 weeks | 200 | 15 (7.5) | 12 (6.0) |
| Hallas 1990 [7] | Prospective medical record review andpatient, relative, general practitioner,or nurse interview | Department of Cardiology | Adults | All admissions | Majorityjudgement of four reviewers using ‘Hallas criteria’ for causality andpreventability | ADRUnder-treatmentOver-treatmentNon-compliance | Odense,Denmark | May to June 1988 | 2 months | NA | NA | NA |
| Hallas 1990 [9] | As above | General medicalward | As above | As above | As above | As above | As above | March to May 1988 | As above | NA | NA | NA |
| Hallas 1992 [10] | As above | Respiratory ward | As above | As above | As above | As above | As above | 1989 | As above | NA | NA | NA |
| Hallas 1991 [11] | As above | Geriatric ward | As above | As above | As above | As above | As above | March toJune 1998 | As above | NA | NA | NA |
| Hallas 1992 [12] | As above | Six medicalwards | As above | As above | As above | As above | As above | March 1988 toMay 1989 | As above | 1999 | 212(10.6) | 67 (3.4) |
| Hallas 1993 [13] | As above | General medicaland geriatricward | As above | Unscheduled admissions to medicalservices fromprimary care | Judgement of one reviewer using ‘Hallas criteria’. Sixty cases randomly selectedfor review bythree reviewers | As above | As above | 1989 | 4 months | 703 | 88 (12.5) | 25 (3.6) |
| Howard 2003 [15] | Prospective routine pharmacist review of patients.Pharmacist review ofselected medicalrecords, and patientand GP interview | Medical admissions ward | Adults | Unscheduled admissions from primary care | Majority judgement of three reviewers used ‘modifiedHallas criteria’ for causality and ‘Hepler criteria’for preventability | ADRUnder-treatmentOver-treatmentNon-compliance | Nottingham,UK | January to June 2001 | 6 months | 4093 | 265 (6.5)178 (4.3) | |
| Lakshmanan1986 [20] | Prospective review of all drug charts,discharge sheets andselected medical records | Medical inpatient services of 769-bed general teaching hospital | Adults | All admissions to medical services | Judgement of one reviewer using guideline criteria for causality and preventability | ADROver-treatment | Ohio, USA | July to August 1984 | 2 months | 834 | 35 (4.2) | 19 (2.3) |
| Lindley 1992 [17] | Prospective medical and nursing recordreview | Acute geriatric, medical and heart care wardsin a 677-bedteaching hospital | Patients aged ≥ 65 years | Emergency and scheduled admissions from primary care(readmissionsexcluded) | Researcher comparison of symptoms with known ADR profile of drugs, and then verification withconsultant,registrar orresearcher | ADR | Manchester,UK | Unknown | 10 weeks | 416 | 26 (6.3) | 13 (3.1) |
| Pirmohamed2004 [18] | Prospective routine pharmacist review ofpatients. Selected medical recordreview and patient, relative or GPinterview byresearch nurse or pharmacist | Medical and surgical wards in two hospitals (excluding obstetrics and gynaecology) | Adults (over 16 years) | All admissions except obstetric and gynaecology patients | Majority judgement of two or three reviewers using ‘Naranjo algorithm’ and ‘Jones method’for causality and ‘Hallas criteria’ for preventability | ADR | Liverpool,UK | November 2001 to April 2002 | 6 months | 18 820 | 975 (5.2) | 687 (3.7) |
| Raschetti 1999 [24] | Prospective nurse review of A&Erecords and follow-upof medical records ofpatients admitted | 700-bed public hospital | Adults (mean age 54.5 years) | All admissions via the emergency department on the first weekof each month | Researcher judgement of causality and preventability | ADRUnder-treatmentOver-treatmentNon-compliance | Milan,Italy | October 1994 to Sep-tember 1995 | 12 weeks | 1833 | 45 (2.5) | 25 (1.4) |
DRA, Drug-related admissions; ADR, adverse drug reaction; PDRA, preventable drug-related admission.
Percentage of admissions that were drug-related and preventable
The search identified 13 papers which met the inclusion criteria. These were conducted between 1983 and 2002. Five studies were conducted in the UK [14–18], two in Denmark [12, 13], two in the USA [19, 20], two in Australia [21, 22], one in Canada [23] and one in Italy [24]. Four studies included adults aged ≥ 65 years [14, 17, 21, 23], whilst the remaining studies included adults of all ages. Four studies included only admissions caused by adverse drug reactions [16–19], whilst the remaining nine studies included a wider definition of admissions due to under- and overtreatment. Seven studies included admissions due to problems with patient adherence to medication [12, 13, 15, 21–24]. Eleven studies used multiple reviewers to assess causality and preventability [12, 14–23] and 10 studies used guidelines or specific criteria to assign causality and preventability [12–18, 21–23]. A meta-analysis of the data reported was deemed inappropriate due to the heterogeneity between studies.
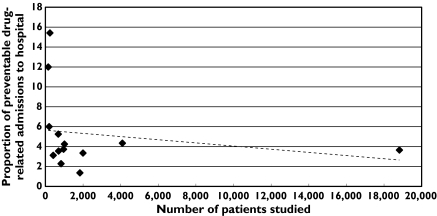
The median (range) percentage of admissions that were preventable and drug-related is 3.73 (1.36–15.42). The scatter plot in Figure 2 shows the relationship between total number of admissions and percentage of preventable drug-related admissions. Two small studies showed a higher than expected proportion of preventable drug-related admissions [21, 23]. These studies included admissions due to problems with patient adherence and, in contrast to the other studies, focused on patients aged ≥ 65 years rather than adults of all ages.
Figure 2.
Scattergram showing the relationship between the number of patients studied and the proportion of preventable drug-related admissions to hospital
Drugs associated with preventable hospital admissions
The search identified 11 papers which met the inclusion criteria [7, 9–11, 13, 15, 16, 18–20, 22]. The studies were conducted between 1983 and 2001 and included adults of all ages. Five studies were conducted in Denmark [7, 9–11, 13], three in the UK [15, 16, 18], two in the USA [19, 20] and one in Australia [22]. The five papers from Denmark [7, 9–11, 13] reported the results of two studies, four papers reported the detailed results for four units [7, 9–11].
Three studies included admissions caused by adverse drug reactions only [16, 18, 20], whilst the remaining studies reported adverse drug reactions, over- and undertreatment and patient adherence problems. To take account of this, the drugs causing admissions have been broken down into three groups: admissions caused by adverse drug reactions and overtreatment; admissions caused by undertreatment; and admissions caused by problems with patient adherence.
The drug groups most frequently associated with all types of preventable drug-related admissions were antiplatelets, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs) and anticoagulants (see Table 2). When preventable drug-related admissions were broken down by type of underlying problem, adverse drug reactions and overtreatment were most commonly associated with antiplatelets, diuretics and NSAIDs, undertreatment problems with antiepileptics and patient adherence problems with diuretics, drugs used in diabetes and antiepileptics (see Table 2).
Table 2.
Drug groups most commonly associated with preventable drug-related admissions relating to adverse drug reactions and overtreatment, undertreatment and problems with patient adherence
| Drug group | All preventable drug-related admissions, no. (%) (n = 1406) | Adverse drug reactions and overtreatment no. (%) (n = 1263) | Patient adherence problems no. (%) (n = 98) | Undertreatment no. (%) (n = 45)$ |
|---|---|---|---|---|
| Antiplatelets (including aspirin when used as an antiplatelet | 225 (16.0)) | 219 (17.3) | 2 (2.0) | 4 (8.9) |
| Diuretics | 223 (15.9) | 202 (16.0) | 20 (20.4) | 3 (2.2) |
| Nonsteroidal anti-inflammatory drugs | 155 (11.0) | 151 (12.0) | 4 (4.1) | 0 |
| Anticoagulants | 117 (8.3) | 113 (8.9) | 4 (4.1) | 0 |
| Opioid analgesics | 69 (4.9) | 68 (5.4) | 1 (1.0) | 0 |
| β-Blockers | 65 (4.6) | 56 (4.4) | 4 (4.1) | 5 (11.1) |
| Drugs affecting the renin–angiotensin system (e.g. angiotensin converting enzyme inhibitors) | 62 (4.4) | 58 (4.6) | 4 (4.1) | 0 |
| Drugs used in diabetes | 49 (3.5) | 40 (3.2) | 9 (9.2) | 0 |
| Positive inotropes | 45 (3.2) | 41 (3.2) | 3 (3.1) | 1 (2.2) |
| Corticosteroids | 44 (3.1) | 41 (3.2) | 2 (2.0) | 1 (2.2) |
| Antidepressant | 42 (3.0) | 41 (3.2) | 1 (1.0) | 0 |
| Calcium channel blockers | 39 (2.8) | 34 (2.7) | 1 (1.0) | 4 (8.9) |
| Antiepileptics | 32 (2.3) | 11 (0.9) | 8 (8.2) | 13 (28.9) |
| Nitrates | 24 (1.7) | 15 (1.2) | 5 (5.1) | 4 (8.9) |
| Inhaled corticosteroids | 8 (0.6) | 0 | 7 (7.1) | 1 (2.2) |
| Potassium channel activators | 7 (0.5) | 1 (0.1) | 2 (2.1) | 4 (8.9) |
| Anti-asthmatics* | 5 (0.4) | 0 | 5 (5.1) | 0 |
| Total | 1211 (86.1) | 1091 (86.4) | 82 (83.7) | 40 (88.9) |
Inhaled and oral bronchodilators and corticosteroids and other antiasthmatic drugs.
Underlying causes of preventable drug-related admissions
The search identified five papers which met the inclusion criteria [12, 15, 19, 22, 23]. The studies were conducted in the USA [19], Canada [23], Australia [22], Denmark [12] and the UK [15] between 1983 and 2001. One study included only adults aged ≥ 65 years [23], whilst the remaining studies included adults of all ages. The underlying causes reported in the papers were prescribing problems (assumed to be a problem if admissions were described as preventable adverse drug reactions and not attributed to monitoring problems), monitoring problems and problems with patient adherence. Other categories were reported in some papers (drug interactions, for example) but could not be applied to all the publications.
Prescribing problems and problems with patient adherence to medication were the most common underlying causes of preventable drug-related admissions [median (range) 30.6% (11.1–41.8) and 33.3% (20.9–41.7), respectively]. Monitoring problems were responsible for a median (range) of 22.2% (0–31.3) preventable drug-related admissions (see Table 3).
Table 3.
Numbers (percentage) of preventable drug-related admissions associated with prescribing problems, monitoring problems and patient adherence problems
| Number (%) of admissions attributed to different underlying causes | ||||||
|---|---|---|---|---|---|---|
| Underlying cause | Bigby 1987 [19] (n = 36) | Courtman 1995 [23] (n = 18) | Dartnell 1996 [22] (n = 36) | Hallas 1992 [12] (n = 67) | Howard 2003 [15] (n = 178) | Median % (range) for all studies |
| Prescribing problem | 4 (11) | 5 (28) | 11 (30) | 28 (42) | 63 (35) | 30.6 (11.1–41.8) |
| Monitoring problem | 12 (33) | 7 (39) | 15 (42) | 14 (21) | 53 (30) | 22.2 (0–31.3) |
| Patient adherence problem | 6 (17) | 8 (22) | 21 (31) | 46 (26) | 33.3 (20.9–41.7) | |
| Unclassified | 14 (39) | 6 (33) | 2 (6) | 4 (6) | 16 (9) | |
| Total | 36 (100) | 18 (100) | 36 (100) | 67 (100) | 178 (100) | |
Discussion
We found that four drug groups accounted for > 50% of preventable drug-related hospital admissions and 12 drug groups accounted for 80% of these admissions. Around one-third of drug-related admissions were associated with prescribing problems, one-third with patient adherence problems and nearly a quarter with inadequate monitoring of medication.
The median of 3.7% of admissions found to be drug related and preventable in our review is slightly lower than that of a previous review [3] because of the inclusion of two recent large UK studies. To our knowledge, only one other review has attempted to detail the drugs most commonly causing patient injury [5]. This review, however, concentrated on adverse drug reactions causing, and occurring during, hospital admission and did not consider whether the admissions were preventable.
We have focused on studies that used prospective medical record review to identify potential drug-related admissions. It is widely accepted that this is the most comprehensive approach to identifying drug-related hospital admissions [25] compared with other methods, such as computer alerts [26] and spontaneous reporting [27]. In addition, the studies included in this review have used either multiple reviewers and/or criteria to assign causality and preventability. The included studies were conducted over a period of 18 years in several different developed countries, mostly in the western hemisphere. Therefore, the results of this study may not be applicable in all countries. Some of the studies used different definitions of causality and preventability and therefore may not be directly comparable. For example, some studies have included admissions considered to be possibly related to drugs and possibly preventable in their assessments of the prevalence of preventable drug-related admissions. In order to avoid falsely inflating the prevalence of preventable drug-related admissions we have, wherever possible, excluded these admissions from the estimates reported in our systematic review.
We have focused on medical admissions to hospital from the general population and have included studies with a broad scope of admission types by excluding studies conducted only on specialist units. However, it is possible that the presence of specialist units in some hospitals may have affected the types of admissions seen.
In all of the cases judged to be preventable in the reviewed studies, the innate toxicity of the drug (or failure to prescribe a drug or sufficient dose) was avoidable in some way. The four drug groups most often causing preventable admissions are commonly used in England [28]. Diuretics account for 5.3% of all primary care prescriptions in England, antiplatelets for 4.0%, NSAIDs for 3.0% and oral anticoagulants for 0.8%. These drug groups have a high innate toxicity, with both diuretics and oral anticoagulants requiring close monitoring for their safe use. In addition, all four drug groups are often used in elderly patients who are more susceptible to adverse effects. The ideal solution to this problem would be to have safer drugs, although no drug is ever likely to be completely without risk. In addition, new drugs take many years to reach the market. In the meantime, there are a number of strategies which can be implemented to help reduce the number of preventable drug-related hospital admissions.
NSAIDs are known to increase the risk of gastrointestinal bleeding and renal dysfunction [29, 30]. Co-prescribing a proton pump inhibitor could reduce the risk of gastrointestinal bleeding (and hospital admission) associated with NSAIDs by between 64% (as secondary prophylaxis) and 78% (as primary prophylaxis) [30]. Other options include using alternative analgesia or prescribing the lowest possible dose of NSAIDs.
Low-dose aspirin also increases the risk of gastrointestinal bleeding [30]. Secondary prophylaxis with Helicobacter pylori eradication, where necessary, and proton pump inhibitors offers a ninefold reduction in the risk of gastric ulcer bleeding [31].
Close monitoring of patients taking potent diuretics could reduce the number of patients admitted with dehydration and/or renal failure. A nurse-led intervention, which included more frequent monitoring of heart failure patients, reduced hospital admissions due to heart failure by 60% and almost halved the number of days spent in hospital [32].
Ensuring adequate monitoring of patients on oral anticoagulants and avoiding coprescription of drugs which increase the risk of bleeding could reduce the number of patients admitted with bleeding events [33]. More effective computer alerts may help to avoid the coprescription of interacting drugs and to alert to the need for increased monitoring [34].
The strategies detailed above concentrate mostly on avoiding adverse drug reactions, but it is also important to remember that preventable patient injury can be caused by undertreatment. Undertreatment can result from prescribing too low a dose, or patients taking less than the prescribed dose of medication. Prescribers should ensure that patients are treated with the minimum effective dose of drug, but not less, especially when prescribing drugs with a narrow therapeutic range, e.g. antiepileptics or digoxin. In addition, it is important to ensure that patients are given adequate information to enable them to take their medication effectively and safely. However, not all cases associated with adherence problems will be avoidable.
While there are a number of studies that suggest ways in which preventable drug-related injuries could be avoided [30–32, 34], it would be helpful to quantify potential benefits and risks using health economic evaluations and, where necessary, further primary research. For example, a health economic evaluation of combining gastro-prophylaxis with low-dose aspirin would help to clarify the patient groups for which prophylaxis might be justified. In addition, there needs to be greater attention to the evidence base which underpins drug monitoring [35].
Also, despite the large number of studies of preventable drug-related admissions, further studies are needed to provide more information on the underlying causes of these admissions. This may help in the development of interventions aimed at improving the safety of prescribing and drug monitoring, and improving adherence to medication.
Antiplatelets, diuretics, NSAIDs and anticoagulants account for more than half of the drug groups associated with preventable drug-related admissions to hospital. Concentrating interventions on these four drug groups could appreciably reduce the number of preventable drug-related admissions to hospital.
Acknowledgments
This systematic review was undertaken with NHS Research & Development funding.
References
- 1.Committee on Quality of Health Care in America and Institute of Medicine. To Err is Human—Building a Safer Health System. Washington DC: National Academy Press; 1999. [Google Scholar]
- 2.Department of Health. An Organisation with a Memory. London: Department of Health; 2000. [Google Scholar]
- 3.Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36:1238–48. doi: 10.1345/aph.1A225. [DOI] [PubMed] [Google Scholar]
- 4.Beijer HJM, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54. doi: 10.1023/a:1015570104121. [DOI] [PubMed] [Google Scholar]
- 5.Wiffen P, Gill M, Edwards J, Moore A. Adverse drug reactions in hospital patients. Bandolier Extra. 2002;June:1–15. [Google Scholar]
- 6.Joint Formulary Committee. British National Formulary. 48. London: British Medical Association and The Royal Pharmaceutical Society of Great Britain; 2004. [Google Scholar]
- 7.Hallas J, Haghfelt T, Gram LF, Grodum E, Damsbo N. Drug related admissions to a cardiology department; frequency and avoidability. J Intern Med. 1990;228:379–84. doi: 10.1111/j.1365-2796.1990.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallas J, Jensen KB, Grodum E, Damsbo N, Gram LF. Drug related admissions to a department of medical gastroenterology. Scand J Gastroenterol. 1991;26:174–80. doi: 10.3109/00365529109025028. [DOI] [PubMed] [Google Scholar]
- 9.Hallas J, Harvald B, Gram LF, Grodum E, Brosen K, Haghfelt T, Damsbo N. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med. 1990;228:83–90. doi: 10.1111/j.1365-2796.1990.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Hallas J, Davidsen O, Grodum E, Damsbo N, Gram LF. Drug-related illness as a cause of admission to a department of respiratory medicine. Respiration. 1992;59:30–4. doi: 10.1159/000196021. [DOI] [PubMed] [Google Scholar]
- 11.Hallas J, Worm J, Beck-Nielsen J, Gram LF, Grodum E, Damsbo N, Brosen K. Drug related events and drug utilization in patients admitted to a geriatric hospital department. Dan Med Bull. 1991;38:417–20. [PubMed] [Google Scholar]
- 12.Hallas J, Gram LF, Grodum E, Damsbo N, Brosen K, Haghfelt T, Harvald B, Beck-Nielsen J, Worm J, Jensen KB, Davidsen O, Frandsen NE, Hagen C, Andersen M, Frolund F, Kromann-Andersen H, Schou J. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol. 1992;33:61–8. doi: 10.1111/j.1365-2125.1992.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallas J, Harvald B, Worm J, Beck-Nielsen J, Gram LF, Grodum E, Damsbo N, Schou J, Kromann-Andersen H, Frolund F. Drug related hospital admissions. Results from an intervention programme. Eur J Clin Pharmacol. 1993;45:199–203. doi: 10.1007/BF00315383. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham G, Dodd TRP, Grant DJ, McMurdo MET, Richards RME. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing. 1997;26:375–82. doi: 10.1093/ageing/26.5.375. [DOI] [PubMed] [Google Scholar]
- 15.Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care. 2003;12:280–5. doi: 10.1136/qhc.12.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Adverse drug reactions as a cause of admission to an acute medical assessment unit: a pilot study. J Clin Pharm Ther. 2000;25:355–61. doi: 10.1046/j.1365-2710.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- 17.Lindley CM, Tully MP, Paramsothy V, Tallis RC. Inappropriate medication is a major cause of adverse drug reactions in elderly patients. Age Ageing. 1992;21:294–300. doi: 10.1093/ageing/21.4.294. [DOI] [PubMed] [Google Scholar]
- 18.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bigby J, Dunn J, Goldman L, Adams JB, Jen P, Landefeld CS, Komaroff AL. Assessing the preventability of emergency hospital admissions. A method for evaluating the quality of medical care in a primary care facility. Am J Med. 1987;83:1031–6. doi: 10.1016/0002-9343(87)90938-7. [DOI] [PubMed] [Google Scholar]
- 20.Lakshmanan MC, Hershey CO, Bresalau D. Hospital admissions caused by iatrogenic disease. Arch Intern Med. 1986;146:1931–4. [PubMed] [Google Scholar]
- 21.Chan M, Nicklason F, Vial JH. Adverse drug events as a cause of hospital admission in the elderly. Intern Med. 2001;31:199–205. doi: 10.1046/j.1445-5994.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 22.Dartnell JG, Anderson RP, Chohan V, Galbraith KJ, Lyon MEH, Nestor PJ, Moulds RFW. Hospitalisation for adverse events related to drug therapy: incidence, avoidability and costs. Med J Aust. 1996;164:659–62. doi: 10.5694/j.1326-5377.1996.tb122235.x. [DOI] [PubMed] [Google Scholar]
- 23.Courtman BJ, Stallings SB. Characterisation of drug-related problems in elderly patients on admissions to a medical ward. Can J Hosp Pharm. 1995;48:161–6. [PubMed] [Google Scholar]
- 24.Raschetti R, Morgutti M, Menniti-Ippolito F, Belisari A, Rossignoli A, Longhini P, La Guidara C. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54:959–63. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- 25.Howard RL, Avery AJ, Morris CJ. Using chart review and clinical databases to study medical error. In: Walshe K, Boaden R, editors. Patient Safety: Research Into Practice. Maidenhead: Open University Press; 2006. pp. 118–29. [Google Scholar]
- 26.Jha AK, Kuperman GJ, Rittenberg E, Teich JM, Bates DW. Identifying hospital admissions due to adverse drug events using a computer-based monitor. Pharmacoepi Drug Saf. 2001;10:113–9. doi: 10.1002/pds.568. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2002;36:1331–6. doi: 10.1345/aph.1A333. [DOI] [PubMed] [Google Scholar]
- 28.Health and Social Care Information Centre. Prescription Cost Analysis: England 2004. Health and Social Care Information Centre; 2005. [11 April 2006]. [Google Scholar]
- 29.Huerta C, Castellsague J, Varas-Lorenzo C, Rodriguez LAG. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45:531–9. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Hawkey CJ, Langman MJS. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-2 inhibitors and proton pump inhibitors. Gut. 2003;52:600–8. doi: 10.1136/gut.52.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai CK, Lam SK, Chu KM, Wong BYC, Hui WM, Hu WHC, Lau GKK, Wong WM, Yuen MF, Chan AOO, Lai CL, Wong J. Lansoprazole for the prevention on recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;26:2033–8. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 32.Blue L, Lang E, McMurray JJV, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, Connolly E, Norrie J, Round CE, Ford I, Morrison CE. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–8. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D’Angelo A, Pengo V, Erba N, Moia M, Ciavarella N, Devoto G, Berrettini M, Musolesi S. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;348:423–8. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 34.Garg AX, Adhikari NKJ, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, Sam J, Haynes RB. Effects of a computerized clinical decisions support systems on practitioner performance and patient outcomes. JAMA. 2005;293:1223–38. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 35.Avery AJ, Savelyich BS, Sheikh A, Cantrill J, Morris CJ, Fernando B, Bainbridge M, Horsfield P, Teasdale S. Identifying and establishing consensus on the most important safety features of GP computer systems: e-Delphi study. Inform Prim Care. 2005;13:3–12. doi: 10.14236/jhi.v13i1.575. [DOI] [PubMed] [Google Scholar]