Abstract
What is already known about this subject
Polypharmacy has been linked to heightened risk of occurrence of drug-related problems (DRPs) and a detrimental health outcome.
Polypharmacy has been variously defined; in research studies a commonly applied definition has been the concomitant use of five or more drugs.
The value of using a definite number of drugs as a cut-off to describe polypharmacy as a risk factor for the occurrence of DRPs has not been validated.
What this study adds
Nearly half of the patients admitted to general hospitals used five or more drugs; during the hospital stay these patients were prescribed as many new drugs as those admitted with fewer drugs.
The presence of DRPs increased approximately linearly with the number of drugs used, for the range of one to >11 drugs.
To set a strict cut-off to identify polypharmacy and declare that using more than this number of drugs represents a potential risk for occurrence of DRPs, is of limited value in clinical practice.
Aim
To investigate whether polypharmacy defined as a definite number of drugs is a suitable indicator for describing the risk of occurrence of drug-related problems (DRPs) in a hospital setting.
Methods
Patients admitted to six internal medicine and two rheumatology departments in five hospitals were consecutively included and followed during the hospital stay, with particular attention to medication and DRPs. Comparisons were made between patients admitted with five or more drugs and with less than five drugs. Clinical pharmacists assessed DRPs by reviewing medical records and by participating in multidisciplinary team discussions.
Results
Of a total of 827 patients, 391 (47%) used five or more drugs on admission. Patients admitted with five or more and less than five drugs were prescribed the same number of drugs after admission: 4.1 vs. 3.9 drugs [P = 0.4, 95% confidence interval (CI) − 0.57, 0.23], respectively. The proportion of drugs used on admission which was associated with DRPs was similar in the patient group admitted with five or more drugs and in those admitted with less than five drugs. The number of DRPs per patient increased approximately linearly with the increase in number of drugs used; one unit increase in number of drugs yielded a 8.6% increase in the number of DRPs (95% CI 1.07, 1.10).
Conclusion
The number of DRPs per patient was linearly related to the number of drugs used on admission. To set a strict cut-off to identify polypharmacy and declare that using more than this number of drugs represents a potential risk for occurrence of DRPs, is of limited value when assessing DRPs in a clinical setting.
Keywords: drug-related problems, hospitalized patients, polypharmacy
Introduction
The fact that today’s evidence-based guidelines recommend several drugs in the treatment of a single condition makes drug treatment particularly challenging. Further, the population is steadily growing older, meaning that comorbidity is frequently present [1, 2]. Consequently, many patients use a number of medications, a situation often referred to as polypharmacy.
Polypharmacy has been variously defined. It has been defined as the concurrent use of multiple drugs, and some researchers have discriminated between minor (two drugs) and major (more than four drugs) polypharmacy [3–5]. Others have defined it as the use of more drugs than are clinically indicated or too many inappropriate drugs [3, 6], as two or more medications to treat the same condition [7, 8] and as the use of two or more drugs of the same chemical class [7]. Accordingly, even though the term polypharmacy has been used for decades, a clear definition is lacking.
The setting and methods for exploring polypharmacy vary widely. Investigations have been carried out in outpatients/ambulatory patients [6, 9–13], hospitalized patients [14–16], patients in nursing homes [17, 18], elderly patients [11–13, 15, 19, 20] and patients included in prescription or other population databases [5, 21–24]. Studies have also explored polypharmacy in specific disease entities such as psychiatric diseases [8, 16], rheumatic diseases [14], asthma [24] and heart failure [2].
A negative connotation has been linked to the term polypharmacy, undoubtedly due to the observation of frequent hospitalization and negative health outcomes caused by drug-related problems (DRPs), such as adverse drug reactions or poor patient adherence in patients taking many drugs [4, 11, 15, 25]. Therefore, many researchers have focused on how to reduce polypharmacy in patient management [9, 26]. Recognition of the fact that costs associated with inappropriate polypharmacy are high has also paved the way for programmes aiming at better drug therapy along with cost saving. In the USA the introduction of the Medication Therapy Management Program reduced therapy problems in nursing home patients and, in addition, the programme was cost beneficial based solely on drug savings [18, 27].
However, although directing efforts towards reducing polypharmacy is salutary, by being too occupied with this approach one might fail to inform about lack of use of beneficial drugs in some patient groups [28, 29]. Deservedly, some recent studies have investigated undertreatment of various diseases, e.g. heart failure, myocardial infarction and osteoporosis [22, 30–32], thus adding a new dimension to polypharmacy research.
In research on DRPs, polypharmacy is regularly reported as a risk factor for their occurrence, and the presence of polypharmacy has been used as an indicator for the identification of patients who need particular attention. In the expanding literature on DRPs, use of five or more drugs has been widely used to describe polypharmacy. However, the value of using, for example, five drugs as a cut-off to identify polypharmacy has not been validated. We therefore investigated whether polypharmacy, defined as a definite number of drugs, is a suitable indicator for describing the risk of occurrence of DRPs in a hospital setting.
We started with a group of patients admitted to hospital departments and followed these patients prospectively during the hospital stay. Patients admitted to hospital often have many new agents added to their admission drug regimens during hospitalization. Thus, these patients seemed suited to highlighting the challenges of polypharmacy and exploring its relationship to DRPs.
Methods
Design and patients
This prospective multicentre study was approved by The Norwegian Regional Committee for Medical Research Ethics. From May to December 2002, clinical pharmacists recorded the drugs used by patients admitted to eight departments at five general hospitals in Norway. The departments involved were departments of rheumatology with regional referral and acute care departments of respiratory diseases, geriatrics and cardiology. All patients who were in the departments on the days clinical pharmacists attended the multidisciplinary healthcare team were eligible and were included consecutively. These patients (cohort) were followed prospectively during their hospital stay.
The clinical pharmacists usually visited the departments 3–5 days a week (one department 2 days a week), weekends not included. As patients admitted during the weekend were in general hospitalized for longer than the weekend, nearly all hospitalized patients were captured and recruited to the study. In this way selection bias was avoided. Re-admitted patients were excluded.
Age, gender, drugs used on admission, drugs commenced during hospital stay, the reason for hospitalization, relevant medical history and relevant laboratory values were registered for all the patients. Factors assumed to increase the risk of acquiring a DRP – here called clinical/pharmacological risk factors – were also recorded. These factors, which are those most often acknowledged as such in the literature, were: polypharmacy on admission (defined as five or more drugs), reduced renal function (calculated creatinine clearance <50 ml min−1 or serum creatinine above normal range), reduced liver function (aspartate aminotransferase or alanine aminotransferase three times above normal range), confirmed diabetes mellitus, cardiac failure, history of allergy or adverse events to drugs, assumed non-adherence (based on statements from the patient in interviews or on information from the healthcare staff), use of drugs with a narrow therapeutic index and other factors that could affect taking the drugs prescribed.
Patients with polypharmacy
Patients with polypharmacy, i.e. five or more drugs on admission (group 1), were compared with patients with less than five drugs on admission (group 2) with regard to patient characteristics, drug use and occurrence of DRPs. In addition to DRPs recorded on admission, DRPs originating as a consequence of the use of additional agents during hospitalization were also reported for the two groups.
Data collection
Clinical pharmacists registered the data listed above from medical records and from information gathered at the multidisciplinary morning meetings where patients’ diagnoses and therapy were discussed. Physicians, nurses, clinical pharmacists and occasionally physiotherapists and other members of the multidisciplinary healthcare team participated at the meetings. These sources were used by the pharmacist to assess whether the patient had DRPs. The pharmacists brought up these DRPs for discussion at the morning meetings. The DRPs could have been present upon hospital admission, or could have developed after admission.
Classification of drugs
The drugs were classified according to the Anatomical Therapeutic Chemical (ATC) System [33]. The clinical pharmacists recorded whether the drug was taken on a regular basis on admission, as required, or commenced in hospital.
Classification of DRPs
DRPs were defined as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’, in accordance with Pharmaceutical Care Network Europe (PCNE) [34]. Further, the DRPs were classified according to a modified version of Strand et al.[35] described in previous studies of Viktil et al.[36] and Blix et al.[37]. The classification used was: need for additional drug, unnecessary drug, non-optimal drug, non-optimal dose, drug interaction, need for monitoring (e.g. laboratory tests, blood pressure measurements), no further need for the drug, medical chart error (e.g. no specification of the strength of the drug), therapy discussion (e.g. why a particular drug is preferred for the patient), need for patient education (to avoid non-adherence), adverse drug reactions, adherence problems and others.
One medication may cause more than one DRP, some of them dependent on each other. For example, a drug might be connected both to a dosage problem and to the need for monitoring, and as such two ‘medication-related problems’ exist, although this is perceived as only one problem for the patient, which is the medication itself (‘patient-related’ DRP). In this study the frequency of DRPs was specified as the countable number of medication DRPs. Problems regarding adherence to the hospital’s drug formulary were not regarded as DRPs.
Statistical analysis
A database was established and analysed by SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics are shown as means with SD for continuous variables and as frequencies for categorical variables. Tests for differences between the two groups (group 1, admitted with five or more drugs and group 2, admitted with less than five drugs) were performed by Pearson χ2 tests for categorical variables and by independent-samples T-test for continuous variables. Furthermore, a multivariate logistic regression analysis with 95% confidence interval (CI) was performed to assess the influence of gender, age and the different clinical/pharmacological risk factors in the two groups. P< 0.05 was accepted as statistically significant.
Results
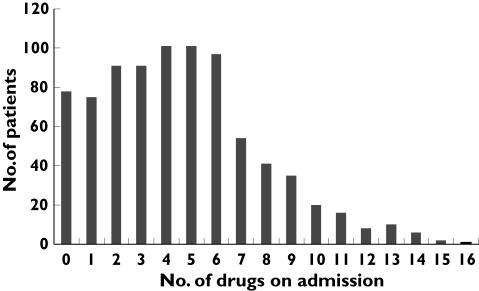
Figure 1 shows the distribution of drug use on admission. Of the 827 patients included in the study, 391 (47.3%) were admitted with five or more drugs (group 1). Table 1 shows descriptive statistics for the two groups. Group 1 had significantly more females, older patients, higher proportions of patients with cardiac failure, diabetes mellitus and reduced renal function, and the patients in this group used more drugs with a narrow therapeutic index than those in group 2. Table 2 shows factors predicting the use of five or more drugs in a logistic regression model. In the multivariate analyses all variables except gender influenced the use of five or more drugs to a lesser extent than in the univariate analyses. For example, the odds ratio (OR) for cardiac failure to predict the use of five or more drugs was reduced from 2.70 in the univariate analysis to 1.68 in the multivariate analysis.
Figure 1.
Distribution of drug use on admission among 827 hospitalized patients
Table 1.
Demographic characteristics in 827 hospitalized patients
| Group 1: ≥ 5 drugs on admission, No. of patients =391 | Group 2: <5 drugs on admission, No. of patients =436 | Difference between groups | ||
|---|---|---|---|---|
| 95% CI* | P-values† | |||
| Gender, no. female (%) | 246 (62.9) | 239 (54.8) | (1, 15) | 0.02 |
| Mean (SD) | Mean (SD) | |||
| [range] | [range] | |||
| Age, years | 75.4 (12.76) | 66.7 (19.41) | (−11.0, −6.5) | <0.01 |
| [21–98] | [15–98] | |||
| No. of drugs per patient used on admission | 7.3 (2.35) | 2.1 (1.42) | (−5.4, −4.9) | <0.01 |
| [5–16] | [0–4] | |||
| No. of clinical/pharmacological risk factors per patient | 2.45 (1.18) | 0.88 (0.96) | (−1.7, −1.4) | <0.01 |
| [1–6] | [1–5] | |||
| Clinical/pharmacological risk factors for having a DRP‡ | % of 391 patients | % of 436 patients | ||
| (no. of patients) | (no. of patients) | |||
| Diabetes mellitus | 15.9 (62) | 7.1 (31) | (4, 13) | <0.01 |
| Cardiac failure | 25.6 (100) | 11.2 (49) | (9, 20) | <0.01 |
| Reduced renal function | 26.3 (103) | 12.2 (53) | (9, 20) | <0.01 |
| Reduced liver function | 1.8 (7) | 2.1 (9) | (−2, 2) | 0.80 |
| History of allergy or adverse reactions to drugs | 16.1 (62) | 14.7 (64) | (−4, 6) | 0.65 |
| Non-adherence | 3.6 (14) | 3.4 (15) | (−2, 3) | 0.91 |
| Use of drugs with narrow therapeutic index | 39.6 (155) | 22.2 (97) | (11, 24) | <0.01 |
| Others | 16.1 (63) | 14.7 (64) | (−3, 6) | 0.58 |
95% confidence interval for the difference between groups, given in percentage for categorical variables and mean difference for continuous variables.
Significant when P< 0.05.
DRP, Drug-related problem.
Table 2.
Factors which predict use of ≥ 5 drugs on admission to hospital among 827 patients
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Gender (female = 1) | 1.40 | (1.06, 1.85) | 1.54 | (1.14, 2.08) |
| Age per year | 1.03 | (1.02, 1.04) | 1.02 | (1.01, 1.03) |
| Diabetes mellitus | 2.46 | (1.56, 3.87) | 2.18 | (1.34, 3.52) |
| Cardiac failure | 2.70 | (1.86, 3.93) | 1.68 | (1.12, 2.51) |
| Reduced renal function | 2.58 | (1.79, 3.71) | 1.72 | (1.16, 2.55) |
| Reduced liver function | 0.88 | (0.32, 2.39) | – | – |
| History of allergy or adverse reactions to drugs | 1.10 | (0.75, 1.60) | – | – |
| Non-adherence | 1.04 | (0.50, 2.19) | – | – |
| Use of drugs with narrow therapeutic index | 2.31 | (1.70, 3.14) | 1.77 | (1.28, 2.46) |
| Others | 1.11 | (0.76, 1.63) | – | – |
OR, Odds ratio; CI, confidence interval. Variables analysed by a logistic regression with≥5 drugs on admission as the dependent variable, and gender, age and the clinical/pharmacological risk factors as independent variables.
There was no difference in the number of drugs commenced during the hospital stay between the group admitted with five or more drugs and the group admitted with fewer drugs: 4.1 (SD = 3.1) and 3.9 (SD = 2.9) drugs per patient, respectively (P= 0.4, 95% CI for difference between groups − 0.6, 0.2).
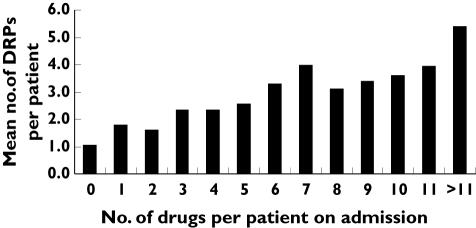
The mean number of DRPs per patient showed an approximately linear relationship to the number of drugs used on admission (Figure 2). However, the distribution of DRPs for each number of drugs is highly skewed. From a linear regression with Ln(DRP) as the dependent variable a one-unit increase in number of drugs yielded a 8.6% increase in the number of DRPs (95% CI 1.07, 1.10). As the figure shows, for patients admitted without any drug, a DRP could still occur during the hospital stay, since it could be associated with drugs commenced during the hospital stay.
Figure 2.
Frequency of drug-related problems (DRPs) per patient in relation to number of drugs used on admission (among 827 patients). DRPs per patient include both DRPs related to drugs on admission and DRPs originating from drugs commenced in hospital
There were no differences between the patients admitted with five or more drugs and those admitted with less than five drugs regarding how often the drugs used on admission within each group were associated with DRPs; this was true for all types of DRPs investigated (Table 3). However, the total number of DRPs related to drugs used on admission was substantially higher in the former group compared with the latter group, respectively, 806 DRPs and 287 DRPs, as a consequence of the use of more drugs. Among the DRPs identified, non-optimal dose was definitely the most frequent DRP in both groups.
Table 3.
Occurrence of different categories of drug-related problems (DRPs) for drugs used on hospital admission in patients using ≥ 5 drugs and patients using <5 drugs on admission
| Type of DRP | Group 1: ≥ 5 drugs on admission, n = 2840 drugs on admission, 391 patients % of drugs causing DRP (no. of DRPs) | Group 2: <5 drugs on admission, n = 934 drugs on admission,436 patients % of drugs causing DRP (no. of DRPs) | Difference between groups | |
|---|---|---|---|---|
| 95% CI * | P-values† | |||
| Need for additional drug | 0.2 (6) | 0.2 (2) | 0 (−0.3, 0.3) | 0.99 |
| Unnecessary drug | 2.4 (69) | 1.9 (18) | 0.5 (−0.5, 1.6) | 0.37 |
| Non-optimal drug | 3.4 (97) | 2.7 (25) | 0.7 (−0.5, 2.0) | 0.27 |
| Non-optimal dose | 7.1 (202) | 7.1 (66) | 0.0 (−1.9, 1.9) | 0.96 |
| Drug interaction | 3.4 (96) | 2.2 (21) | 1.2 (−0.0, 2.3) | 0.08 |
| Need for monitoring | 3.3 (93) | 3.9 (36) | −0.6 (−2.0, 0.8) | 0.40 |
| No further need for drug | 0.7 (20) | 0.8 (7) | −0.1 (−0.7, 0.6) | 0.89 |
| Medical chart error | 2.7 (77) | 3.9 (36) | −1.2 (−2.5, 0.2) | 0.08 |
| Therapy discussion | 1.9 (53) | 2.2 (21) | −0.3 (−1.5, 0.7) | 0.46 |
| Patient adherence | 0.7 (21) | 1.3 (12) | −0.6 (−1.3, 0.2) | 0.12 |
| Patient education | 1.0 (29) | 1.8 (17) | −0.8 (−1.7, 0.1) | 0.054 |
| Adverse drug reaction | 1.3 (37) | 2.0 (19) | −0.7 (−1.7, 0.3) | 0.11 |
95% confidence interval for the difference between groups, given in percentage.
Significant when P < 0.05. Low numbers were found for the DRPs others: 0.2% (6) vs. 0.7% (7) in group 1 and group 2, respectively.
With regard to the frequency of DRPs related to the drugs commenced in hospital, for only one type of DRP, i.e. the DRP drug interaction, was a difference found between the patients admitted with five or more and less than five drugs. In the former group, 2.1% of the drugs commenced in hospital were associated with the DRP drug interaction compared with 1.1% of the drugs in the other group (P = 0.02, 95% CI for difference −1.9%, −0.2%).
The DRPs recorded by the clinical pharmacist, including both DRPs found on admission and additional DRPs observed during the hospital stay and thus also related to drugs commenced during hospital stay, were brought up for discussion in the multidisciplinary morning meetings; 92% of these DRPs were acknowledged as a DRP by the physicians.
A subgroup analysis of patients admitted with heart failure showed that among patients with this condition those patients admitted with five or more drugs commenced taking 5.1 (SD = 3.3) drugs per patient during the hospital stay and those admitted with less than five drugs commenced taking 5.6 (SD = 3.5) drugs per patient (P = 0.4, 95% CI − 0.6, 1.7). However, among the heart failure patients those with less than five drugs on admission received more new drugs in the ATC group B01A (antithrombotic agents) and in the ATC group C (drugs for the cardiovascular system) during the hospital stay than the group admitted with five or more drugs. A similar subgroup analysis of patients with diabetes mellitus showed that these patients also commenced taking the same number of drugs in hospital irrespective of being admitted with five or more or less than five drugs: 4.1 (SD = 3.1) and 4.0 (SD = 2.8) drugs per patient, respectively (P = 0.96, 95% CI −1.3, 1.3). However, in diabetic patients there was no difference between the five or more drugs and the less than five drug groups with regard to drugs in ATC group A10 (drugs used in diabetes) commenced after admission.
Discussion
Polypharmacy has been linked to heightened risk of a detrimental health outcome. However, it is challenging to investigate to what extent polypharmacy creates an increased risk of developing adverse events, as observed symptoms may be related to the disease itself [4].
To our knowledge, this is the first study to investigate the issue of polypharmacy by comparing groups with and without polypharmacy, as commonly defined, with regard to a wide range of DRPs.
We found that nearly half of patients in this study were admitted to hospital with five or more drugs and thus were exposed to polypharmacy according to a common definition. This is a high rate but is representative of patients admitted to general hospitals. Among elderly patients even higher rates have been reported. In a retrospective study of patients admitted to hospital from home care agencies 66% used five or more drugs, 46% used more that seven drugs and 21% used 10 or more drugs [11]. Taking into account that our patients were younger and presumably had less morbidity, our corresponding figures of, respectively, 47%, 23% and 7% are largely in line with the results of that study. In an earlier study we found that 60% of patients hospitalized in rheumatic wards used five or more drugs on admission [14]. In a cross-sectional retrospective study using a self-completed questionnaire, Lawlor et al. reported the use of five or more drugs in 15% of elderly women with falls [19]. Thus, using five or more drugs to define polypharmacy, a considerable proportion of patients are experiencing polypharmacy. Accordingly, the value of this definition could be questioned if the purpose is to identify patients at particular risk of developing DRPs.
The finding that the mean number of DRPs per patient increased approximately linearly with increasing number of drugs used on admission indicates that the risk of having a DRP increases with each additional drug supplied. This linear relationship was present for the whole range of drugs, one to >11 drugs, and there was no levelling off of the effect at any specific number of drugs. Since the presence of DRPs may seriously threaten patients’ health and since more than four-fifths of the DRPs in our study were assessed to be of major or moderate clinical significance [37], it could be argued that the polypharmacy cut-off number of drugs could just as well have been two or three drugs. Thus, our observation demonstrates the arbitrariness of using a cut-off of five drugs to define polypharmacy.
An attempt to relate more accurately the number of drugs used to the risk of unfavourable health outcomes has been undertaken by Flaherty et al.[11]. They found no differences between two home care agency patient groups using five or more or less than five drugs and the likelihood of being admitted to either hospital or to self/family care. Only those using the highest number of drugs, seven or more or ≥ 10 drugs, were more frequently admitted to hospital than to self/family care. This finding is consistent with our observations that the cut-off at five drugs gives poor differentiation. Relevant for this discussion are the findings of Lawlor et al., that the risk of falls in elderly people was associated more with chronic diseases and multiple pathology than with polypharmacy [19].
The relationship between increasing number of drugs used and increased number of DRPs is strong. Hence, whichever level is the basis of the definition, polypharmacy stands out as a marked risk factor for developing DRPs. Our previous analyses, which included various clinical/pharmacological risk factors, indicated similarly, that the total number of risk factors together with the number of drugs used was the strongest risk determinant for the presence of DRPs [37]. Likewise, other researches have pointed to the concomitant use of several drugs as a factor to be looked for in identifying patients who should have selective drug reviews or monitoring [38, 39]. However, the significance of polypharmacy vis-à-vis other risk factors has not been accurately delineated in a hospital setting and would be an interesting research topic.
Instead of searching for undesirable polypharmacy, the focus could be turned around, and a potential for undertreatment, i.e. prescription of too few drugs, could come to the fore, especially among patients using few drugs. The finding that both patients with five or more drugs and less than five drugs on admission commenced taking the same number of drugs during hospital stay does not lend support to the existence of undertreatment among the patients in our study. However, the subanalysis of patients admitted with heart failure revealed that the group admitted with less than five drugs commenced taking slightly more antithrombotic agents and other cardiovascular drugs during the hospital stay than patients admitted with five or more drugs. The explanation for this finding could be some degree of undertreatment of the former group. It is possible that being too occupied with the negative aspect of polypharmacy, and trying to avoid the use of many drugs, may lessen attention to the issue of providing all necessary drugs to an individual patient.
A limitation of our study is the fact that drug discontinuations were not registered systematically as were drugs used on admission and drugs commenced in hospital. However, generic switches were not noted as additional drugs and such switches could not have influenced the reported numbers of new drugs. Furthermore, since one main objective was to catch all DRPs, all drugs and combinations of medications were relevant to this outcome and therefore continuously registered. Thus, it is unlikely that DRPs could have been undetected as a consequence of lack of registration of discontinuations.
There is a fine line between providing an individual patient with appropriate drugs that have the potential to reduce the risk of death or to improve quality of life, and the overuse of drugs. However, to set a strict cut-off and declare that using more than this number of drugs represents a potential risk is not useful in clinical practice. Nor is this an adequate approach for research purposes. It might be as important to follow closely patients using few drugs as those using many.
Acknowledgments
Competing interests: None declared.
We thank the clinical pharmacists Elspeth Walseth, Bodil Jahren Hjemaas, Tine Flindt Vraalsen, Piia Pretsch and Frank Jørgensen, and our colleagues in the multidisciplinary teams of the participating departments. ‘The Norwegian Community Pharmacy Foundation’ and ‘The Norwegian Pharmacy Associations’ Foundation’, both nonprofit organizations, gave financial support to the data collection and establishing the database. K.K.V. is a research fellow at the University of Oslo, which thus has contributed to financing the study.
References
- 1.Buckley BM. Healthy ageing: ageing safely. Eur Heart J. 2001;3(Suppl. N):N6–10. doi: 10.1016/s1520-765x(01)90131-2. [DOI] [PubMed] [Google Scholar]
- 2.Geest SD, Steeman E, Leventhal ME, Mahrer-Imhof R, Hengartner-Kopp B, Conca A, Bernasconi AT, Petry H, Rocca HB-L. Complexity in caring for an ageing heart failure population: concomitant chronic conditions and age related impairments. Eur J Cardiovasc Nurs. 2004;3:263–70. doi: 10.1016/j.ejcnurse.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17:123–32. doi: 10.1111/j.1041-2972.2005.0020.x. [DOI] [PubMed] [Google Scholar]
- 4.Frazier SC. Health outcomes and polypharmacy in elderly individuals: an integrated literature review. J Gerontol Nurs. 2005;31:4–11. doi: 10.3928/0098-9134-20050901-04. [DOI] [PubMed] [Google Scholar]
- 5.Bjerrum L, Rosholm JU, Hallas J, Kragstrup J. Methods for estimating the occurrence of polypharmacy by means of a prescription database. Eur J Clin Pharmacol. 1997;53:7–11. doi: 10.1007/s002280050329. [DOI] [PubMed] [Google Scholar]
- 6.Zarowitz BJ, Stebelsky LA, Muma BK, Romain TM, Peterson EL. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636–45. doi: 10.1592/phco.2005.25.11.1636. [DOI] [PubMed] [Google Scholar]
- 7.Brager R, Sloand E. The spectrum of polypharmacy. Nurse Pract. 2005;30:44–50. doi: 10.1097/00006205-200506000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams ME, Pulliam CC, Hunter R, Johnson TM, Owens JE, Kincaid J, Porter C, Koch G. The short-term effect of interdisciplinary medication review on function and cost in ambulatory elderly people. J Am Geriatr Soc. 2004;52:93–8. doi: 10.1111/j.1532-5415.2004.52016.x. [DOI] [PubMed] [Google Scholar]
- 10.Jameson JP, VanNoord GR. Pharmacotherapy consultation on polypharmacy patients in ambulatory care. Ann Pharmacother. 2001;35:835–40. doi: 10.1345/aph.10259. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty JH, Perry HM, III, Lynchard GS, Morley JE. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55:M554–M559. doi: 10.1093/gerona/55.10.m554. [DOI] [PubMed] [Google Scholar]
- 12.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivela SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55:809–17. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen T, Johansson S, Kennerfalk A, Wallander MA, Svardsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann Pharmacother. 2001;35:1004–9. doi: 10.1345/aph.10351. [DOI] [PubMed] [Google Scholar]
- 14.Viktil KK, Enstad M, Kutschera J, Smedstad LM, Schjott J. Polypharmacy among patients admitted to hospital with rheumatic diseases. Pharmacy World Sci. 2001;23:153–8. doi: 10.1023/a:1011909827909. [DOI] [PubMed] [Google Scholar]
- 15.Alarcon T, Barcena A, Gonzalez-Montalvo JI, Penalosa C, Salgado A. Factors predictive of outcome on admission to an acute geriatric ward. Age Ageing. 1999;28:429–32. doi: 10.1093/ageing/28.5.429. [DOI] [PubMed] [Google Scholar]
- 16.Viola R, Csukonyi K, Doro P, Janka Z, Soos G. Reasons for polypharmacy among psychiatric patients. Pharm World Sci. 2004;26:143–7. doi: 10.1023/b:phar.0000026800.13888.b0. [DOI] [PubMed] [Google Scholar]
- 17.Perri M, III, Menon AM, Deshpande AD, Shinde SB, Jiang R, Cooper JW, Cook CL, Griffin SC, Lorys RA. Adverse outcomes associated with inappropriate drug use in nursing homes. Ann Pharmacother. 2005;39:405–11. doi: 10.1345/aph.1E230. [DOI] [PubMed] [Google Scholar]
- 18.Christensen D, Trygstad T, Sullivan R, Garmise J, Wegner SE. A pharmacy management intervention for optimizing drug therapy for nursing home patients. Am J Geriatr Pharmacother. 2004;2:248–56. doi: 10.1016/j.amjopharm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–7. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jyrkka J, Vartiainen L, Hartikainen S, Sulkava R, Enlund H. Increasing use of medicines in elderly persons: a five-year follow-up of the Kuopio 75+Study. Eur J Clin Pharmacol. 2006;62:151–8. doi: 10.1007/s00228-005-0079-6. [DOI] [PubMed] [Google Scholar]
- 21.Fincke BG, Snyder K, Cantillon C, Gaehde S, Standring P, Fiore L, Brophy M, Gagnon DR. Three complementary definitions of polypharmacy: methods, application and comparison of findings in a large prescription database. Pharmacoepidemiol Drug Safety. 2005;14:121–8. doi: 10.1002/pds.966. [DOI] [PubMed] [Google Scholar]
- 22.Klarin I, Fastbom J, Wimo A. A population-based study of drug use in the very old living in a rural district of Sweden, with focus on cardiovascular drug consumption: comparison with an urban cohort. Pharmacoepidemiol Drug Safety. 2003;12:669–78. doi: 10.1002/pds.878. [DOI] [PubMed] [Google Scholar]
- 23.Kennerfalk A, Ruigomez A, Wallander MA, Wilhelmsen L, Johansson S. Geriatric drug therapy and healthcare utilization in the United kingdom. Ann Pharmacother. 2002;36:797–803. doi: 10.1345/aph.1A226. [DOI] [PubMed] [Google Scholar]
- 24.Ikaheimo P, Hartikainen S, Tuuponen T, Kiuttu J, Klaukka T. Comorbidity and medication load in adult asthmatics. Scand J Prim Health Care. 2005;23:88–94. doi: 10.1080/02813430510018473. [DOI] [PubMed] [Google Scholar]
- 25.Fastbom J. Increased consumption of drugs among the elderly results in greater risk of problems. Lakartidningen. 2001;98:1674–9. [PubMed] [Google Scholar]
- 26.Pitkala KH, Strandberg TE, Tilvis RS. Is it possible to reduce polypharmacy in the elderly? A randomised, controlled trial. Drugs Aging. 2001;18:143–9. doi: 10.2165/00002512-200118020-00007. [DOI] [PubMed] [Google Scholar]
- 27.Trygstad TK, Christensen D, Garmise J, Sullivan R, Wegner SE. Pharmacist response to alerts generated from medicaid pharmacy claims in a long-term care setting: results from the North Carolina Polypharmacy Initiative. J Manag Care Pharm. 2005;11:575–83. doi: 10.18553/jmcp.2005.11.7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurwitz JH. Polypharmacy: a new paradigm for quality drug therapy in the elderly? Arch Intern Med. 2004;164:1957–9. doi: 10.1001/archinte.164.18.1957. [DOI] [PubMed] [Google Scholar]
- 29.Jackson HD, Mangoni AA, Batty GM. Optimization of drug prescribing. Br J Clin Pharmacol. 2004;57:231–6. doi: 10.1011/j.1365-2125.2003.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skrepnek GH, Abarca J, Malone DC, Armstrong EP, Shirazi FM, Woosley RL. Incremental effects of concurrent pharmacotherapeutic regimens for heart failure on hospitalizations and costs. Ann Pharmacother. 2005;39:1785–91. doi: 10.1345/aph.1G124. [DOI] [PubMed] [Google Scholar]
- 31.Sloane PD, Gruber-Baldini AL, Zimmerman S, Roth M, Watson L, Boustani M, Magaziner J, Hebel JR. Medication undertreatment in assisted living settings. Arch Intern Med. 2004;164:2031–7. doi: 10.1001/archinte.164.18.2031. [DOI] [PubMed] [Google Scholar]
- 32.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–20. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 33.WHO Collaboratoring Centre for Drug Statistics Methodology. Oslo: WHO Collaborating Centre; 2002. ATC Classification Index with DDDs. [Google Scholar]
- 34.van Mil F, editor. Proceedings of the International Working Conference on Outcomes Measurements in Pharmaceutical Care. Hilleroed, Denmark: Pharmaceutical Care Network Europe (PCNE); 1999. pp. 9–84. [Google Scholar]
- 35.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24:1093–7. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 36.Viktil KK, Blix HS, Reikvam A, Moger TA, Hjemaas BJ, Walseth EK, Vraalsen TF, Pretsch P, Jorgensen FI. Comparison of drug-related problems in different patient groups. Ann Pharmacother. 2004;38:942–8. doi: 10.1345/aph.1D531. [DOI] [PubMed] [Google Scholar]
- 37.Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P, Vraalsen TF, Walseth EK. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60:651–8. doi: 10.1007/s00228-004-0830-4. [DOI] [PubMed] [Google Scholar]
- 38.Chrischilles EA, Carter BL, Lund BC, Rubenstein LM, Chen-Hardee SS, Voelker MD, Park TR, Kuehl AK. Evaluation of the Iowa Medicaid Pharmaceutical Case Management Program. J Am Pharm Assoc. 2004;44:337–49. doi: 10.1331/154434504323063977. [DOI] [PubMed] [Google Scholar]
- 39.Koecheler JA, Abramowitz PW, Swim SE, Daniels CE. Indicators for the selection of ambulatory patients who warrant pharmacist monitoring. Am J Hosp Pharm. 1989;46:729–32. [PubMed] [Google Scholar]