Abstract
What is already known about this subject?
A previous semiphysiological model has been published, describing the time course of the autoinduction of artemisinin.
The model, which was based on saliva sampling, has been successfully applied in another set of saliva data.
What this study adds?
In this report, we applied the same model to plasma data from six clinical studies involving healthy volunteers and malaria patients.
The model performed well and we suggest that it could be used as a general model in cases of time-dependent pharmacokinetics and drug-drug interactions.
Aims
To describe the time-course of the autoinduction of artemisinin by applying a semiphysiological pharmacokinetic model.
Methods
Plasma concentration-time data from six clinical studies involving oral administration of artemisinin to healthy subjects and malaria patients were included in the analysis. NONMEM was used to apply a semiphysiological model incorporating metabolizing enzymes and a pharmacokinetic model including a separate hepatic compartment.
Results
The model described the data well. The hepatic extraction ratio increased from 0.74 at pre-induced conditions to 0.98 after autoinduction of metabolism.
Conclusions
Our model successfully described the time-course of autoinduction of metabolism of artemisinin in subjects receiving oral artemisinin.
Keywords: artemisinin, autoinduction, pharmacokinetics
Introduction
Several clinical studies involving oral administrations of artemisinin in both healthy subjects and malaria patients have shown remarkable time-dependent pharmacokinetics of the compound [1–4]. A pronounced capacity for autoinduction of drug metabolism is believed to be the cause of this time-dependency. A semiphysiological pharmacokinetic model characterizing the time-dependent pharmacokinetics of artemisinin has previously been developed using saliva concentration-time data from healthy subjects [5]. In the present study, we report the results of the application of this model on plasma data from several clinical studies involving the compound.
Methods
Study design
Artemisinin plasma concentration-time data from six clinical studies involving repeated oral administration to healthy, male subjects (n = 33, between 19 and 45 yearsof age with body weights ranging from 48 to 68 kg) and male patients with uncomplicated falciparum malaria (n = 54, between 15 and 55 years of age with body weights ranging from 37 to 63 kg) were available for the present analysis (Table 1). All studies took place in Vietnam and were approved by the Ministry of Health, Hanoi, Vietnam, the Swedish Medical Products Agency, Uppsala and the Ethics Committee of the Medical Faculty, Uppsala University, Sweden. Written informed consent was obtained from all subjects prior to study enrolment.
Table 1.
Overview of the clinical studies used in the present model
| Study | Type | Artemisinin dosing schedule | Blood sampling times | Concomitant-medication | n | Reference |
|---|---|---|---|---|---|---|
| 1 | Healthy volunteers | Single morning dose (2 × 250 mg) on days 1, 7 and 14, 250 mg twice daily on days 2–6 | Pre-dose, 1, 2, 3, 4, 5, 6, 7, 8 and 10 h after drug intake on days 1, 7 and 14 | 20 mg omeprazole on days −7, 1, 7 and 14 | 9 | [3] |
| 2 | Healthy volunteers | Single morning dose (2 × 250 mg) on days 1 and 38, daily doses of 250 mg on days 29–37 | Pre-dose, 1, 2, 3, 4, 5, 6, 7, 8 and 10 h after drug intake on days 1 and 38 | 500 mg tolbutamide on days 1 and 33, 200 mg mephenytoin on days 1 and 32 | 14 | [9] |
| 3 | Healthy volunteers | Single morning dose (2 × 250 mg) on days 1, 4, and 7, 250 mg twice daily on days 2, 3, 5 and 6 | Pre-dose, 1, 2, 3, 4, 5, 6, 7, 8 and 10 h after drug intake on days 1, 4, 7 and 21 | None | 10 | [1] |
| 4 | Malaria patients | Single morning dose (2 × 250 mg) on days 1 and 5, 250 mg twice daily on days 2–4 | Pre-dose, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 10 h after drug intake on days 1 and 5 | None | 15 | [2] |
| 5 | Malaria patients | Standard group Single dose (2 × 250 mg) on days 1 and 5, 250 mg twice daily on days 2–4 Escalating group Single dose (2 × 50 mg) on day 1, 50 mg twice daily on day 2, 125 mg twice daily on days 3 and 4, and single dose (2 × 250 mg) on day 5 | Pre-dose, 0.5, 1, 2, 3, 4, 5 and 8 h after drug intake on days 1 and 5 in both groups | None | 18 | [4, 10] |
| 6 | Malaria patients | Single morning dose (2 × 250 mg) on days 1 and 5, and 250 mg twice daily on days 2–4 | Pre-dose, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8 and 10 h after drug intake on days 1 and 5 | Nine individuals received one oral multivitamin tablet on days 1–5 | 21 | Unpublished data |
Pharmacokinetic analysis
A previously developed model of enzyme autoinduction [5] was applied to log-transformed plasma data in this study. The model consisted of two parts describing the pharmacokinetics of artemisinin and the time-variant levels of the induced enzyme(s). In this model, artemisinin is introduced to a gut compartment with subsequent absorption into a liver compartment, from which elimination is described by a well-stirred model. The compound is further distributed into a sampling compartment, which represents the entire body except that of the liver. The enzyme part of the model consists of two components, an enzyme pool and a precursor to it. The change in the precursor pool with time is driven by the amount of artemisinin in the liver, increasing the precursor formation rate linearly. Such an increase results in elevated enzyme amounts with subsequent increase in the degree of extraction of the compound. An increased extraction affects both the clearance and the bioavailability of artemisinin. The first-order method without centreing in the program NONMEM, version V (Globomax, MD, USA) was used for modelling. Discriminations between models were based on the objective function value (OFV) provided by NONMEM at a significance level of 0.01 and graphical analysis of residuals and predictions using Xpose, version 3.1 [6].
Several modifications of the original model were tested in the present study. These included a model with one enzyme compartment, no absorption lag-time, linear or saturable effect of the hepatic amounts of artemisinin on the production rate of precursor/enzyme, and linear or saturable effect of enzyme amounts on the intrinsic clearance of the compound. The final structural model was almost identical to the original model by Gordi et al. [5], differing only in no absorption lag-time being estimated.
Exponential variance models were used to account for the interindividual variability in intrinsic clearance and volume of distribution as well as interoccasional variability in artemisinin absorption rate constant. A proportional residual error component was applied in the model. Attempts to run the final model using the FOCE or FOCE INTERACTION options in NONMEM resulted in termination of the process.
Results
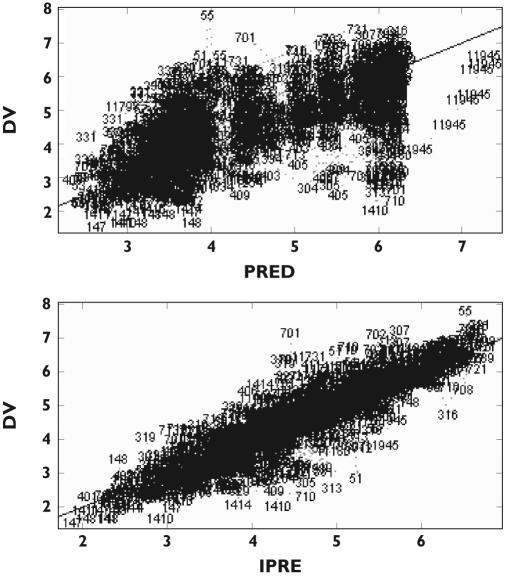
Artemisinin plasma concentration-time profiles were well described by the proposed model (Figure 1). During the model-building process, a model with an interindividual variability term on the slope describing the linear effect of artemisinin concentrations on the rate of production of enzyme precursor, instead of intrinsic clearance (CLint) resulted in a decrease of OFV by 10 units. However, the model with a variability term on CLint was chosen since it was deemed more physiologically relevant. This also resulted in lower CV% values for all estimated parameters, while parameter estimates were essentially identical for the two models. The population estimate of enzyme half-life was 94 h and the intrinsic clearance of artemisinin at low concentrations was estimated to be 1760 l h−1 in the pre-induced state, resulting in a hepatic extraction ratio of 0.74, increasing to 0.98 upon induction (Table 2). High interoccasional variability was found for the absorption rate constant.
Figure 1.
Observations (DV) vs. logarithm of the population prediction (PRED) and individual predictions (IPRED)
Table 2.
Typical pharmacokinetic parameter values for artemisinin and associated interoccasional (IOV) and interindividual (IIV) variability in 33 healthy subjects and 54 malaria patients
| Parameter | Estimate (RSE%) | IOV (RSE%) | IIV (RSE%) |
|---|---|---|---|
| t1/2, ENZ (h) | 94 (27) | NE | NE |
| SIND (1/ng) | 0.045 (32) | NE | NE |
| CLint,0 (l h−1) | 1760 (35) | NE | 0.38 (24) |
| Vp (l) | 26.1 (15) | NE | 1.2 (32) |
| ka (h−1) | 0.09 (13) | 0.64 (23) | NE |
| MIT (h) | 2.0 (43) | NE | NE |
| Km (ng ml−1) | 434 (50) | NE | NE |
| fu | 0.14 (FIXED) | NE | NE |
| Residual error | 0.54 (4.7) | NE | NE |
t1/2, ENZ: enzyme elimination half-life,. SIND: slope of the inducing effect of artemisinin hepatic concentration on the production rate of enzyme precursor, CLint,0: intrinsic clearance in the pre-induced state, Vp: volume of sampling compartment, ka: absorption rate constant, fu: plasma unbound fraction, MIT: mean induction time, Km: hepatic artemisinin concentration resulting in 50% of maximum intrinsic clearance, RSE%: relative standard error in per cent, NE: not estimated.
Discussion
The ability of artemisinin to induce its own metabolism is well-documented in both healthy volunteers [1] and malaria patients [2]. Despite this, little is known about the time-course of the enzyme activity, including the onset and duration of induction. To our knowledge, there is only one pharmacokinetic model describing the autoinduction of artemisinin [5]. It has successfully been applied to another set of data using saliva samples [7]. The present report is an adaption of this model for plasma data.
Simulations of a 5 days administration of artemisinin estimated a hepatic extraction value of 0.74 prior to drug administration, increasing to 0.98 after repeated dosing. This was despite an estimated 16-fold increase in the amount of enzymes in the enzyme compartment. Such a change in the extraction ratio has minor effects on the systemic clearance of artemisinin but results in a 13-fold decrease in its bioavailability. Absence of a corresponding change in half-life indicates that the drug is highly extracted by the liver, with the increased activity of hepatic enzymes mainly affecting its bioavailability but not systemic clearance. Graphs of model parameters vs. disease state (healthy vs. malaria patients) did not reveal any correlation, suggesting similar pharmacokinetics in healthy and malaria patients. This is in accordance with previous findings [1, 2].
The onset of autoinduction was found to be very rapid, with an increased degree of extraction of the compound from 0.74 to 0.90 8 h after the first dose, findings which were similar to the saliva model. This implies that a single dose of artemisinin is capable of enzyme induction and is in accordance with previous findings, where single doses of artemisinin were found to affect the kinetics of the next dose given 1 week later [8]. Despite limited sampling schemes in the studies used in our analysis, the proposed model fitted the plasma data satisfactorily and model estimates were similar to those based on saliva sampling.
The model offers several advantages in describing the time course of any compound exhibiting time-dependent kinetics. When administered orally, liver concentrations are the major driving force of the induction of hepatic enzymes. The model offers the possibility to investigate whether an increase in the systemic clearance or a decrease in bioavailability are the main outcomes of enzyme induction. Another feature is the inclusion of the major components for hepatic elimination (fu, CLint, and QH) allowing the model to describe circumstances other than induction, for example enzyme inhibition or changes in protein binding. Finally, with the incorporation of a precursor compartment the model is able to account for the commonly observed lag-time from the first drug dose until induction is evident. Our proposed model could be of value when studying the changes in the pharmacokinetics of drug combinations. An enzyme pool similar to that of the present model can be used to illustrate the effect of an inducer/inhibitor on the elimination of the second drug. Such a model would allow for estimating the delay, extent, and duration of alterations through simulations.
In conclusion, a previously developed semiphysiological model of artemisinin autoinduction based on saliva sampling was applied to plasma data. The proposed model successfully described the plasma concentration time-course of artemisinin in both healthy subjects and patients with falciparum malaria infections receiving oral doses of artemisinin.
Acknowledgments
The authors would like to thank Dr Michael Ashton and Dr Ulrika Simonsson for providing part of the data.
References
- 1.Ashton M, Hai TN, Sy ND, Huong DX, Van Huong N, Nieu NT, Cong LD. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:25–7. [PubMed] [Google Scholar]
- 2.Ashton M, Nguyen DS, Nguyen VH, Gordi T, Trinh NH, Dinh XH, Nguyen TN, Le DC. Artemisinin kinetics and dynamics during oral and rectal treatment of uncomplicated malaria. Clin Pharmacol Ther. 1998;63:482–93. doi: 10.1016/S0009-9236(98)90044-3. [DOI] [PubMed] [Google Scholar]
- 3.Svensson US, Ashton M, Trinh NH, Bertilsson L, Dinh XH, Nguyen VH, Nguyen TN, Nguyen DS, Lykkesfeldt J, Le DC. Artemisinin induces omeprazole metabolism in human beings. Clin Pharmacol Ther. 1998;64:160–7. doi: 10.1016/S0009-9236(98)90149-7. [DOI] [PubMed] [Google Scholar]
- 4.Gordi T, Huong DX, Hai TN, Nieu NT, Ashton M. Artemisinin pharmacokinetics and efficacy in uncomplicated-malaria patients treated with two different dosage regimens. Antimicrob Agents Chemother. 2002;46:1026–31. doi: 10.1128/AAC.46.4.1026-1031.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordi T, Xie R, Huong NV, Huong DX, Karlsson MO, Ashton M. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br J Clin Pharmacol. 2005;59:189–98. doi: 10.1111/j.1365-2125.2004.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 7.Gordi T, Xie R, Jusko WJ. Semi-mechanistic pharmacokinetic/pharmacodynamic modelling of the antimalarial effect of artemisinin. Br J Clin Pharmacol. 2005;60:594–604. doi: 10.1111/j.1365-2125.2005.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashton M, Gordi T, Trinh NH, Nguyen VH, Nguyen DS, Nguyen TN, Dinh XH, Johansson M, Le DC. Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm Drug Dispos. 1998;19:245–50. doi: 10.1002/(sici)1099-081x(199805)19:4<245::aid-bdd99>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Simonsson US, Jansson B, Hai TN, Huong DX, Tybring G, Ashton M. Artemisinin autoinduction is caused by involvement of cytochrome P450 2B6 but not 2C9. Clin Pharmacol Ther. 2003;74:32–43. doi: 10.1016/S0009-9236(03)00092-4. [DOI] [PubMed] [Google Scholar]
- 10.Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur J Clin Pharmacol. 2000;56:561–6. doi: 10.1007/s002280000179. [DOI] [PubMed] [Google Scholar]