Abstract
What is already known about this subject
Brivaracetam is a new chemical entity structurally related to levetiracetam, displaying a markedly higher affinity for the binding site believed to be primarily involved in the antiepileptic effect of levetiracetam.
Studies to evaluate the pharmacological profile of brivaracetam demonstrate an approximately 10-fold higher potency than levetiracetam as well as a higher efficacy in models of epilepsy.
If translated into therapeutic effects in humans, this would mean a greater decrease in seizure frequency and a higher number of responders and seizure-free patients in refractory epileptic patients as seen with levetiracetam.
What this study adds
This article reports the results of the first in human study with brivaracetam. Its pharmacokinetics and adverse events profile after single administration are evaluated, together with the effect of food on the former.
Aims
The objective of the study was to evaluate the pharmacokinetics (and how they are affected by food), CNS pharmacodynamics and the adverse event profile of brivaracetam after single increasing doses.
Methods
Healthy males (n = 27, divided into three alternating panels of nine subjects) received two different single oral doses of brivaracetam (10–1400 mg) and one dose of placebo during three periods of a randomized, double-blind, placebo-controlled study. The effect of food on its pharmacokinetics was assessed using a standard two-way crossover design in a further eight subjects who received two single oral doses of brivaracetam (150 mg) in the fasting state and after a high fat meal.
Results
Adverse events, none of which were serious, were mostly CNS-related and included somnolence, dizziness, and decreased attention, alertness, and motor control. Their incidence, severity and duration were dose-related. The maximum tolerated dose was established to be 1000 mg. Severe somnolence lasting 1 day occurred in one subject following 1400 mg. Brivaracetam was rapidly absorbed under fasting conditions, with a median tmax of approximately 1 h. Cmax was dose-proportional from 10 to 1400 mg, whereas AUC deviated from dose linearity above 600 mg. A high-fat meal had no effect on AUC (point estimate 0.99, 90%CI: 0.92–1.07) but delayed tmax (3 h) and decreased Cmax (point estimate 0.72, 90%CI: 0.66–0.79).
Conclusions
Brivaracetam was well tolerated after increasing single doses that represent up to several times the expected therapeutic dose. Brivaracetam was found to have desirable pharmacokinetic properties. The most common adverse events were somnolence and dizziness.
Keywords: antiepileptics, brivaracetam, pharmacodynamics, pharmacokinetics, ucb 34714
Introduction
Brivaracetam ((2S)-2-[(4R)-2-oxo-4-propylpyrrolidinyl]butanamide) is a new molecular entity of the acetam family (Figure 1) with potent antiseizure properties in experimental models. Brivaracetam displays a high affinity and high selectivity for the brain specific binding site (synaptic vesicle protein SV2A) identified for levetiracetam [1]. It also reduces sodium currents [2] and reverses inhibition by negative modulators on GABA and glycine receptors. Results in laboratory animals and in vitro have demonstrated activity in experimental models of epilepsy [3, 4], neuropathic pain [5] and essential tremor [6]. The ED50 of brivaracetam was 1.2 mg kg−1 i.p. in corneally kindled mice, 2.4 mg kg−1 i.p. in genetically sound-sensitive mice, and 2.6 mg kg−1 i.p. in genetic absence epilepsy rats from Strasbourg (GAERS) [4]. These corresponded to human doses of 6, 12 and 25 mg day−1 [7]. The present study, the first in humans, was designed to evaluate the pharmacokinetics and adverse effects of brivaracetam and to explore its CNS effects after single oral doses ranging from 10 to 1400 mg in healthy male subjects. In addition, the effect of food on its absorption was assessed after a single oral dose of 150 mg.
Figure 1.

Chemical structure of brivaracetam
Methods
Subjects and study design
Thirty-five healthy men aged 18–55 years, with a body mass index between 19 and 27 kg m−2 were recruited, 27 for the dose-escalation arm of the study (Table 1) and eight for the food-effect arm.
Table 1.
Single increasing dose study design
| Dose step | Week | Dose (mg) | Panel 1 n = 6 active n = 3 placebo | Panel 2 n = 6 active n = 3 placebo | Panel 3 n = 6 active n = 3 placebo |
|---|---|---|---|---|---|
| 1 | 1 | 10 | X | ||
| 2 | 2 | 20 | X | ||
| 3 | 3 | 40 | X | ||
| 4 | 5 | 80 | X | ||
| 5 | 6 | 150 | X | ||
| 6 | 7 | 300 | X | ||
| 7 | 9 | 600 | X | ||
| 8 | 10 | 1000 | X | ||
| 9 | 11 | 1400 | X |
The dose escalation was placebo-controlled in that at each dose six subjects received active drug and the other three placebo. Randomization was allocated such that each subject received active drug at two doses, and one dose of placebo. The food-effect arm was performed according to an open-label two-way-crossover design. The study was approved by The Independent Ethics Committee, Manchester, UK, and was performed at Medeval Ltd (Manchester, UK), in accordance with the latest version of the Declaration of Helsinki.
Hard gelatin capsules were filled with brivaracetam without excipients. The available capsules strengths were 10, 20, 40, 80, 150 and 200 mg. Multiple capsules were utilized to achieve the desired doses. Each batch of capsules reached ≥85% dissolution in water within 15 min. Placebo capsules were filled with microcrystalline cellulose. All capsules were identical in shape, size and colour to allow a double-blind design.
The doses were selected based on the results of animal pharmacological models of epilepsy and of conventional toxicity studies in rodents and nonrodents. Using the data from the preclinical models of epilepsy, the human pharmacologically active dose was calculated to be in the range of 6–25 mg day−1 for safety purposes [7]. The lowest no-adverse-effect level (NOAEL) of brivaracetam in toxicity studies was 15 mg kg−1 day−1 in the dog, corresponding to a maximum recommended starting dose (MRSD) [7] of 50 mg in man. The first dose was set at 10 mg, and was increased using a geometric progression of 2, except for the two highest doses, for which a slower progression rate was applied. Subjects received the treatments after an overnight fast and continued to fast until 4 h after dosing. Blinded adverse effects and pharmacokinetic data were reviewed before each dose escalation.
In the food interaction arm of the study, each subject was randomized to one of two sequences, and received two single oral doses of 150 mg brivaracetam after an overnight fast and following a standard high-fat breakfast, separated by a 7 day wash-out period. The breakfast composition was 15% protein, 25% carbohydrate and 60% fat and contained about 1000 calories.
Subjects were confined in the unit from the evening before until 48 h after each dose and their cardiorespiratory function was monitored for at least 8 h after each dose.
Haematology, serum chemistry and urinalysis data were obtained at predose and 24 h postdose. Twelve-lead electrocardiograms (ECGs) were recorded at the initial and discharge visits and predose, 30 min, 1, 1.5, 2, 3, 4, 6 and 8 h after each administration. The minimal intolerated dose (MID) was defined as the dose at which 50% or more of subjects exhibited dose-limiting toxicity arising from adverse events or exacerbated pharmacodynamic effects or as the dose at which a drug-related, medically unacceptable event would occur in any one subject. The maximum tolerated dose (MTD) was defined as the dose immediately below the MID [8], if attained.
CNS pharmacodynamic markers
The following measurements of CNS function were performed at predose, 1, 4, 8 and 24 h postdose, during each period. The battery of tests consisted of pharmaco-electroencephalographs (pharmaco-EEGs), psychometric tests, motor tests, saccadic eye movements and rating scales. Pharmaco-EEGs were recorded using silver-silver chloride cup electrodes according to the International 10:20 standard positioning system [9]. Throughout the recording, subjects were seated and had their eyes closed. Three main regional zones (frontal, central and occipital) were considered by summing the corresponding channels. The ratio of power in high:low frequencies, calculated as (high alpha + beta):(delta + theta + low alpha) was used as an index of sedation. The psychomotor tests included the Number Pairs Task (NPT) and Choice Reaction Time (CRT) [10] to assess attention, the Tapping Test (TT) to assess motor control, and Saccadic Eye Movements (SEM) [11] to assess sedation. Two rating scales were also used, the Addiction Research Center Inventory (ARCI-49) [12] evaluating mood, sensations, perceptions and vigilance state, and Bond & Lader's Visual Analogue Scale (VAS) [13] for the assessment of alertness, contentedness and calmness.
Determination of brivaracetam concentration in plasma and urine
Blood samples were collected serially into vacutainers containing heparin, and plasma was separated by centrifugation and stored at −20°C. Blood samples were initially obtained up to 24 h postdose at the lower doses (10–150 mg), but the sampling was extended to 48 h at the higher doses (300–1400 mg) and in the food interaction part of the study. All urine was collected in fractions over 24 h and aliquots were stored at −20°C. For the determination of brivaracetam in plasma, aliquots (50 µl) were loaded, together with the internal standard (50 ng ucb 30412), on prewashed (2 ml MeOH and 2 ml 50 mmol l−1 of pH 5.6 phosphate buffer, successively) Bond Elut C18 SPE cartridges. The cartridges were washed with 2 ml of 20% acetonitrile in water and allowed to dry for 3 min. The analytes were then eluted with 1 ml of acetonitrile, which was evaporated to dryness. The extract was taken up with 100 µl of 89.9:10:0.1 water:acetonitrile:trifluoroacetic acid solution (pH 3.0). An aliquot (10 µl) was injected onto the chromatograph. For urine analysis, aliquots (20 µl) were diluted 50-fold with the same water:acetonitrile:trifluoroacetic acid mixture, after addition of internal standard (1 µg ucb 30412) and injected directly onto the chromatograph. The analytical column (Inertsil ODS 3, 5 µm, 50 × 2.1 mm ID) was protected by a guard column (Inertsil ODS 3, 5 µm, 10 × 2.1 mm ID). The mobile phase comprised 0.1% trifluoroacetic acid in 20% water and 80% acetonitrile, adjusted to pH 3.0 with ammonia. Brivaracetam and internal standard were detected by positive ion mass spectrometry with electrospray ionization, using the ions at m/z = 213 and 247, respectively. The instrumentation consisted of a HP1100 liquid chromatograph (Hewlett Packard, Palo Alto, USA) coupled with a Quattro Ultima mass spectrometer (Micromass, Manchester, UK). The lower limit of quantification was 0.05 µg ml−1 in plasma and 0.25 µg ml−1 in urine. The response was linear over the concentration ranges of 0.05–2 µg ml−1 in plasma and 0.25–50 µg ml−1 in urine. Within- and between-day coefficients of variation were ≤5.0% across the range of measurement in plasma and ≤4.7% in urine. The relative error did not exceed 4.4% in plasma and 6.1% in urine. Plasma and urine samples were stable for at least 3 months when stored at −20°C. Sample dilution (up to 1000-fold for plasma samples and 400-fold for urine samples) had no effect on analytical recovery.
Pharmacokinetics and statistical analyses
Pharmacokinetic data were analyzed using standard noncompartmental methods with Kinetica 2000® (version 3.0, Innaphase, Champ sur Marne, France). Maximum concentration in plasma (Cmax) and the corresponding time (tmax) were derived directly from the plasma concentration-time profiles. The area under the plasma concentration-time curve from the time of dosing to the time of the last measurable concentration (AUC(0-t)) was calculated using the linear trapezoidal rule and extrapolated to infinity as AUC(0-t) + Ct/λz, in which λz, the first order rate constant associated with the terminal elimination phase, was estimated by linear regression of time vs. log concentration. The half-life (t1/2) of the terminal elimination phase was calculated from the expression ln(2)/λz. The apparent plasma clearance (CL/F) was obtained from the ratio dose/AUC and the apparent volume of distribution (Vz/F) from (CL/F)/λz. The mean residence time (MRT) was estimated from the ratio AUMC/AUC where AUMC is the area under the first moment curve. Renal clearance (CLR) was calculated from the expression Ae/AUC, where Ae is the total amount of unchanged drug excreted in urine.
Dose proportionality for Cmax and AUC was explored using the random intercept power model [14, 15], with subjects as a random effect, using the equation:
where α is the intercept and β is the slope parameter. Dose proportionality was assumed if β was close to 1 and its 90% confidence interval (CI) was entirely contained within the 80–125% interval corrected for dose range [14, 15]. The dose-independence of λz, CL/F, Vz/F, fraction of the dose excreted in urine (fe), and CLR was assessed using the same model under the condition of β = 0. In the food interaction subgroup, AUC, AUC(0-t) and Cmax were evaluated under fed and fasted conditions according to a univariate model of analysis of variance, adapted to cross-over experimental designs. The factors in the model were the sequences, periods, subjects (nested to the sequence) and treatments. Subjects were random effects. Pharmacodynamic variables were summarized descriptively as mean changes from baseline per dose level. Mean differences from baseline per time point between each dose and placebo were explored for possible effects. Descriptive statistics were also derived for vital signs, ECGs and laboratory measurements. SAS software version 8.1 (SAS Institute, Cary, NC, USA) or StatXact version 4.01 (Cytel Software, Cambridge, MA, USA) was used for the statistical analysis.
Results
Twenty-seven subjects participated in the dose-escalation arm over a period of 12 weeks and each subject was studied three times (two doses of brivaracetam and one of placebo). Six subjects were exposed to brivaracetam at each dose level. The age range was 19.5–54.0 years (median 33.5 years), body weight 62–88 kg (median 74 kg), body mass index 21–27 kg m−2 (median 22.8 kg m−2).
Safety and tolerability
The incidence of adverse events in the dose-escalation protocol is summarized by dose in Table 2. Twenty-five subjects (93%) reported at least one treatment-emergent adverse event following brivaracetam at any dose, compared with five (19%) given placebo. At doses of 300 mg and above, four to six of six subjects reported at least one treatment-emergent adverse event. Most of these affected the CNS, and the most common were dizziness (brivaracetam 15, placebo 0) and somnolence (brivaracetam 11, placebo 1), and were most notable at the higher doses. Both of these adverse events had a rapid onset and resolved within 1 day. Feeling drunk, nasopharyngitis, balance disorder, disturbance of attention, postural dizziness, bradyphrenia and disorientation were reported at brivaracetam doses between 40 and 1000 mg. Three subjects had blurred vision, euphoric mood, fatigue, and complained of abnormal coordination. Of the adverse events that were found in one subject only, the majority occurred at the higher doses. Only subjects in the 1400 mg dose group experienced nausea, arthralgia, dysgeusia and agitation. All the adverse events were mild or moderate in intensity, except the occurrence of severe and dose-limiting somnolence observed in one subject at the highest brivaracetam dose (1400 mg). This effect started 6 min postdose and lasted approximately 11 h. Its maximum severity occurred within the first 10 min and reached such an extent that the subject was unable to keep his eyes open spontaneously, had a constant need for sleep and was unable to walk without support. His Glasgow Coma Scale rating was 14/15. Later, the mood of the subject alternated between euphoria, agitation and somnolence. The subject did not exhibit higher plasma concentrations than the other individuals in the same cohort. In an unsupervised environment the subject would have been exposed to a significant safety risk. This event was therefore assessed as medically unacceptable and 1400 mg was considered as the MID. Therefore the MTD of brivaracetam was set at 1000 mg.
Table 2.
Treatment-emergent adverse events in the single increasing dose protocol
| Brivaracetam dose (mg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 10 | 20 | 40 | 80 | 150 | 300 | 600 | 1000 | 1400 | |
| Number of dosed subjects | 27 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of subjects reporting adverse events | 5 | 2 | 1 | 1 | 3 | 3 | 5 | 6 | 6 | 4 |
| Total number of adverse events | 5 | 2 | 1 | 2 | 5 | 3 | 7 | 11 | 9 | 11 |
| Adverse events (n) | ||||||||||
| Blurred vision | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Dizziness | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 4 | 3 | 2 |
| Somnolence | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 3 | 1 | 2 |
| Psychiatric disorders* | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 |
Agitation, bradyphrenia, disorientation, euphoric mood.
In the food interaction group, six adverse events were recorded in five subjects, and all were classified as mild in intensity. Two (vasovagal attack and fatigue) were recorded after administration of 150 mg brivaracetam following a high-fat meal and four (three subjects experiencing dizziness [excluding vertigo] and one somnolence) after administration of 150 mg brivaracetam under fasted conditions. None of the adverse events led to premature withdrawal from the study.
No clinically significant abnormalities occurred at any dose with respect to the clinical laboratory data, vital signs, physical examination, ECG, and EEG tests.
CNS markers
Taken together, the psychometric tests and rating scales indicated that brivaracetam had a dose-related sedative effect (SEM and ARCI-PCAG), and caused decreased attention (CRT), alertness (Bond and Lader's VAS) and motor control (TT) (Figure 2). These effects appeared clearly from 600 mg upwards. The maximal effect occurred about 1 h postdose, with no residual effects at 24 h postdose. A dose-dependent decrease in peak saccadic velocity (a measure of sedation) was observed, with a rapid onset and maximal impairment at 1 h postdose. At some doses, the effect was still present at 4–8 h postdose, but completely absent at 24 h. Similar results were obtained with the PCAG subscale of the ARCI-49 questionnaire, measuring sedation. No effect was observed in the other four ARCI subscales measuring euphoria, dysphoria, stimulant effects and intellectual capacity. Attention was decreased from 1 to 4 h after administration of 600 and 1400 mg brivaracetam, as indicated by the increased reaction time and decreased percentage of correct responses in the CRT test. No effect was observed on contentedness, but a dose-related decrease of alertness (increased score from baseline) was evident at 1 h and lasted 4–8 h, as indicated by the increase in the VAS scores. Calmness was increased at the highest dose only. A dose-related decrease in motor control (decreased tapping rate) was observed between 1 and 4 h postdose, and lasted until 4–8 h, but no residual effect was observed at 24 h. Regarding the pharmaco-EEGs, no consistent change from baseline was detected with respect to dose or over time.
Figure 2.
Dose-dependency of pharmacodynamic effects (sedation, attention, alertness, motor control) of brivaracetam assessed as mean change from predose (SD) at 1 h postdose (n = 6/dose; n = 27 placebo group). For alertness, an increased VAS score indicates less alertness
Pharmacokinetics
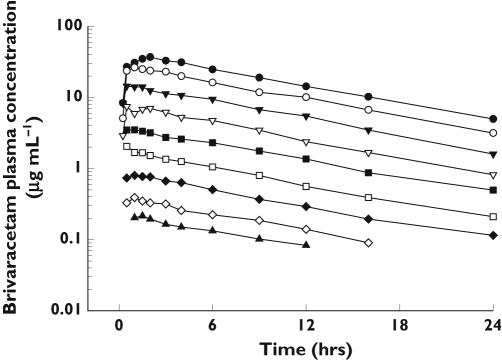
The mean plasma brivaracetam concentration-time profiles after single oral administration of 10 to 1400 mg are shown in Figure 3. Under fasting conditions, brivaracetam was generally rapidly absorbed with a median time to peak plasma concentration (tmax) of 0.5–1 h, and then the drug concentration declined mono-exponentially with an elimination half-life of about 8 h. Brivaracetam was measurable in all plasma samples, except at 16 and 24 h after the 10 mg dose and at 24 h after the 20 mg dose.
Figure 3.
Arithmetic mean plasma concentration vs. time profiles obtained after oral doses of 10 mg (▴), 20 mg (◊), 40 mg (✦), 80 mg (□), 150 mg (▪), 300 mg (▿), 600 mg (▾), 1000 mg (○), and 1400 mg (•) of brivaracetam (n = 6/dose)
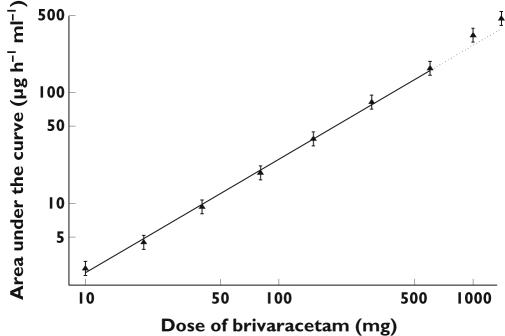
The mean pharmacokinetic parameters for brivaracetam determined in the single-dose study are summarized in Table 3. Dose proportionality was apparent over the dose range of 10–1400 mg for Cmax, but the AUC tended to increase slightly more than proportionally for doses higher than 600 mg (Figure 4). A comparison with the AUC predicted from the regression model using observations up to 600 mg, indicated that the values observed at 1000 and 1400 mg were 28% and 24% higher, respectively. The terminal elimination half-life was not dose dependent, whereas CL/F and Vz/F tended to decrease somewhat at the highest doses. Only a small fraction of the dose was excreted unchanged in the urine over 24 h (3–8%) and no dose-dependent alteration was observed.
Table 3.
Pharmacokinetic parameters for brivaracetam in the single increasing dose subgroup
| Brivaracetam dose (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 10 | 20 | 40 | 80 | 150 | 300 | 600 | 1000 | 1400 |
| Cmax (µg ml−1) | 0.31 (0.12) | 0.43 (0.07) | 0.95 (0.23) | 2.18 (0.37) | 4.24 (0.85) | 8.58 (1.36) | 16.15 (2.02) | 28.46 (4.63) | 41.28 (9.96) |
| tmax (h)* | 0.5 (0.5–2) | 1 (0.5–3) | 1.26 (0.5–2) | 0.5 (0.5–1.5) | 0.75 (0.5–3) | 1 (0.25–2) | 0.75 (0.25–1.5) | 1.25 (0.5–4) | 1.75 (1–4) |
| AUC(0, ∞) (µg ml−1 h) | 2.54 (0.38) | 4.50 (1.48) | 9.74 (1.62) | 19.66 (2.55) | 43.12 (6.01) | 84.25 (15.10) | 170.0 (30.0) | 316.9 (52.0) | 464.6 (87.1) |
| t1/2 (h) | 8.06 (0.87) | 8.18 (1.56) | 8.05 (1.38) | 7.71 (0.96) | 8.04 (1.04) | 7.43 (1.09) | 7.26 (1.02) | 7.41 (1.26) | 7.31 (1.25) |
| CL/F (ml min−1 kg−1) | 0.88 (0.09) | 1.07 (0.32) | 0.95 (0.16) | 0.95 (0.08) | 0.82 (0.09) | 0.86 (0.10) | 0.83 (0.10) | 0.73 (0.06) | 0.70 (0.15) |
| Vz/F (l kg−1) | 0.61 (0.08) | 0.73 (0.11) | 0.65 (0.02) | 0.63 (0.07) | 0.57 (0.06) | 0.54 (0.03) | 0.52 (0.06) | 0.46 (0.06) | 0.44 (0.09) |
| MRT (h) | 11.6 (1.71) | 11.9 (2.23) | 11.9 (1.85) | 11.1 (1.52) | 11.9 (1.37) | 11.8 (1.38) | 10.7 (1.45) | 11.1 (1.70) | 11.3 (1.47) |
| fe (%) | 3.22 (1.70) | 3.96 (0.87) | 5.73 (1.26) | 5.47 (1.75) | 6.12 (1.77) | 8.00 (4.14) | 6.91 (2.03) | 5.79 (1.82) | 7.46 (1.45) |
| CLR (ml min−1 kg−1) | 0.070 (0.055) | 0.052 (0.023) | 0.063 (0.016) | 0.059 (0.022) | 0.057 (0.012) | 0.081 (0.048) | 0.066 (0.022) | 0.049 (0.016) | 0.061 (0.023) |
Values are arithmetic mean (SD)
median (range), n = 6 subjects/dose.
Figure 4.
The relationship between the geometric mean (90% CI; n = 6) AUC of brivaracetam vs. dose. The regression line was calculated over the dose range from 10 to 600 mg
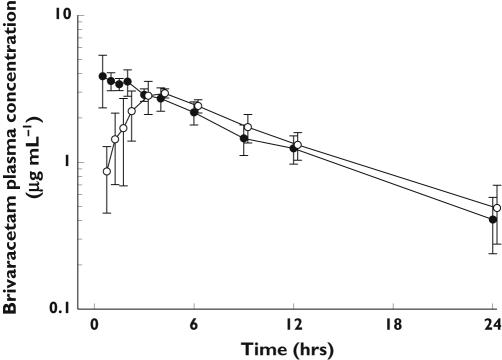
The mean plasma profiles of brivaracetam following a single administration of 150 mg oral doses in the fasted state and after a high fat meal are illustrated in Figure 5. Food had no effect on the extent of absorption of brivaracetam, but decreased its rate of absorption. The fed:fasted geometric mean ratio was 0.99 for AUC (90% CI 0.92, 1.07) and 0.72 for Cmax (90% CI 0.66, 0.79). The median time to peak plasma concentration was delayed from 0.5 to 3.5 h. Food had no effect on the other pharmacokinetic parameters measured (Table 4).
Figure 5.
Arithmetic mean plasma concentration vs. time profiles obtained after oral administration of single 150 mg doses of brivaracetam under fasted conditions (•) or after a high fat meal (○) (n = 8)
Table 4.
Pharmacokinetic parameters for brivaracetam (150 mg) in the food interaction subgroup
| Parameter | Treatment Fasted | Fed |
|---|---|---|
| Cmax (µg ml−1) | 4.41 (0.64) | 3.16 (0.15) |
| tmax (h)* | 0.51 (0.5–2) | 3.50 (1–6) |
| AUC(0, ∞) (µg ml−1 h) | 41.7 (8.6) | 41.4 (9.2) |
| t1/2 (h) | 7.61 (1.62) | 7.98 (1.81) |
| CL/F (ml min−1 kg−1) | 0.79 (0.19) | 0.80 (0.22) |
| Vz/F (l kg−1) | 0.50 (0.07) | 0.53 (0.06) |
| MRT (h) | 10.9 (2.30) | 12.4 (2.40) |
| fe (%) | 6.80 (2.19) | 6.55 (1.22) |
| CLR (ml min−1 kg−1) | 0.054 (0.018) | 0.053 (0.017) |
Values are arithmetic mean (SD)
median (range), n = 8 subjects/treatment.
Discussion
The purpose of this study was to assess the pharmacokinetics and CNS pharmacodynamics and the adverse effects of single oral doses of brivaracetam in healthy males. An alternating panel design was used, which is known to be more efficient than conventional sequential panel designs, while achieving the same precision [16, 17].
Brivaracetam was generally well tolerated and the adverse events seen were mainly those expected for a drug with CNS activity. The proportion of subjects reporting adverse events appeared to be comparable between placebo and the lower doses (10, 20 and 40 mg) of brivaracetam. In contrast, a dose-dependent increase in the percentage of subjects reporting adverse events was observed at doses ≥80 mg. The most commonly reported effects were CNS related, consisted mainly of somnolence and dizziness, and were more frequently observed at the higher doses. Both events had a rapid onset, and had resolved within 1 day. The majority of adverse events were mild or moderate, and only one subject had a severe effect (somnolence) after the intake of the highest dose of 1400 mg. Since the plasma concentrations in this subject were similar to those observed in the other subjects of the same cohort, the adverse event was probably related to individual sensitivity to the drug. This adverse event was considered unacceptable by the investigator, and the MTD was subsequently set at 1000 mg for healthy subjects after a single intake. No clinically significant individual abnormalities occurred at any dose with respect to the results of the clinical laboratory tests, vital signs, physical examination, ECG, and the standard clinical EEG. Food did not appear to change the pattern or intensity of the adverse events.
The results of the psychometric tests were consistent with the adverse event data. Brivaracetam had a sedative effect and resulted in decreased attention, alertness and motor control. These effects were dose-related, occurring at 600 mg and higher. The maximum effect was achieved approximately 1 h postdose and decreased rapidly. For some of the tests and at high doses, the effects were still present at 8 h, but had subsided at 24 h postdose. The pharmaco-EEG did not show any conclusive results.
Under fasting conditions, brivaracetam was rapidly absorbed. Cmax increased proportionally with the dose from 10 to 1400 mg, whereas increase in AUC was higher than expected at doses above 600 mg. The low value of Vz/F suggests that the drug is not highly concentrated in the major organs. The low apparent clearance of brivaracetam, compared with hepatic blood flow, suggests limited extraction by the liver. Less than 10% of the dose was excreted as parent drug in the urine. The renal clearance of brivaracetam was much lower than creatinine clearance, suggesting extensive tubular reabsorption, since the extent of protein binding of the drug is small ( < 18%, unpublished data).
Our results are consistent with a high-fat meal decreasing the rate, but not the extent, of absorption of brivaracetam by delaying gastric emptying, and suggest that the drug can be dosed without regard to meals. Nevertheless, a further study with a formulated product will be needed to confirm the absence of a food interaction.
In conclusion, this study has indicated that single oral doses of 10–1000 mg brivaracetam are well tolerated and that its clinical development is supported by the favourable pharmacokinetic characteristics of the drug. Pending confirmation by therapeutic studies, the MTD appears to be well above the therapeutic dose range predicted in humans, based on animal models of epilepsy. Acute adverse events include dose-related somnolence and dizziness. Based on the adverse event profile, the rapid absorption and half-life of brivaracetam, a twice daily dosing regimen appears to be optimal for decreasing the peak to trough fluctuations. From the results of the current study, it is estimated that steady state would be achieved within 2 days with an accumulation factor of about 50%. Considering the pharmacokinetic linearity of brivaracetam and the safety margin from the single dose MTD, a maximum dose of 400 mg twice daily is contemplated in multiple dosing studies. Food was shown to affect the rate but not the extent of the oral absorption of brivaracetam.
Acknowledgments
Competing interests: PR was a director, shareholder and employee of the company conducting the research study for UCB in a commercial basis. MLS-M, AS and J-MR are permanent employees of UCB SA. DT and EP have been employed by UCB SA in the past five years.
References
- 1.Lynch BA, Lambeng N, Nocka K, Kensel-Hammels P, Bajjalieh SM, Matagne AC, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Nat Acad Sci. 2004;101:9861–6. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zona C, Pieri M, Klitgaard H, Margineanu D-G. ucb 34714, a new pyrrolidone derivative, inhibits Na+ currents in rat cortical neurons in culture. Epilepsia. 2004;45(Suppl 7):146. [Google Scholar]
- 3.Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, Frycia AM, Moureau FG, Klitgaard HV, Gillard MR, Fuks B, Michel P. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–49. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- 4.Matagne A, Kenda B, Michel P, Klitgaard H. ucb 34714, a new pyrrolidone derivative, suppresses seizures epileptogenesis in animal models of chronic epilepsy in vivo. Epilepsia. 2003;44(Suppl 8):53–4. [Google Scholar]
- 5.Lamberty Y, Ardid D, Eschalier A, Alloui A, Matagne A, Kenda B, Michel P, Klitgaard H. A new pyrrolidone derivative, ucb 34714, is effective in neuropathic pain models in rats: comparison with gabapentin. J Pain. 2003;4(Suppl 1):53. [Google Scholar]
- 6.De Ryck M, Matagne A, Kenda B, Michel P, Klitgaard H. Contrasting effects of ucb 34714 and drugs for essential tremor on harmaline-induced elicited versus spontaneous tremor and sedation in rats. Mov Disord. 2004;19(Suppl 9):S443. [Google Scholar]
- 7.FDA Guidance for Industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. [Google Scholar]
- 8.Cutler NL, Sramek JJ, Greenblatt DJ, Chaikin P, Collins J. Defining the maximum tolerated dose: an update. J Clin Pharmacol. 2000;40:1183–204. doi: 10.1002/j.1552-4604.1997.tb05624.x. [DOI] [PubMed] [Google Scholar]
- 9.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–5. [PubMed] [Google Scholar]
- 10.Parkin C, Kerr JS, Hindmarch I. The effects of practice on choice reaction time and critical flicker fusion threshold. Hum Psychopharmacol. 1997;12:65–70. [Google Scholar]
- 11.Griffiths AN, Marshall RW, Richens A. Saccadic eye movement analysis as a measure of drug effects on human psychomotor performance. Br J Clin Pharmacol. 1984;18:73S–82S. doi: 10.1111/j.1365-2125.1984.tb02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1970;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 13.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–8. [Google Scholar]
- 14.Gouch K, Hutchinson M, Keene O, Byron B, Ellis S, Lacey L, McKellar J. Assessment of dose proportionality: report from the statisticians in the pharmaceutical industry/pharmacokinetics UK joint Working Party. Drug Inf J. 1995;29:1039–48. [Google Scholar]
- 15.Smith BP, Vandenhende FR, Desarte KA, Farid NA, Welch PA, Callaghan JT, Forghe ST. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278–83. doi: 10.1023/a:1026451721686. [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, Chen C. Trial design optimization for studying dose proportionality. Clin Pharmacol Ther. 2000;67:159. [Google Scholar]
- 17.Yin Y, Chen C. Optimizing first-time-in-human trial design for studying dose proportionality. Drug Inf J. 2001;35:1065–78. [Google Scholar]