Abstract
Aims
The aims of the study were a) to determine if there is evidence of saturable protein binding of cefazolin in plasma across the range of concentrations achieved clinically (between patient variability) and b) to investigate whether saturable protein binding is also evident from trough and peak concentrations in the same patient (within patient variability).
Methods
Unbound and total plasma concentrations were measured in patients who were treated with cefazolin intravenously by continuous infusion or intermittent injection. In study (i) single random samples were taken from one series of patients. In study (ii) paired samples (troughs and peaks) were taken from a second series of patients.
Results
Thirty-one patients were included in study (i). Linear regression analysis of the percentage unbound vs. unbound plasma concentrations revealed a slope significantly different from zero, suggesting saturable protein binding. Mean values for percentage unbound ranged from 9% at low concentrations (8.5 mg l−1) to 51% at high concentrations (140 mg l−1). Twelve patients were investigated in study (ii). Values for protein binding ranged from 85% at low concentrations (2.7 mg l−1) to 52% at high concentrations (200.3 mg l−1). The percentage unbound was significantly higher (P < 0.0001) at high (peak) concentrations than at lower (trough) concentrations, confirming saturable protein binding.
Conclusions
The protein binding of cefazolin is saturable in vivo in humans, both between and within patients.
Keywords: antibiotics, cefazolin, cephalosporins, protein binding
Introduction
Cefazolin is a first generation intravenous cephalosporin used widely in the treatment of Gram positive infections in both hospital inpatients and outpatients [1]. It is well tolerated, stable, and has a relatively narrow spectrum of activity [2, 3].
The protein binding of cefazolin is usually quoted as 80–90% [4–6]. The site of binding on albumin appears to be either the warfarin (site I) site or the bilirubin site [7] or both, and cefazolin is subject to displacement by endogenous substances such as bilirubin and free fatty acids [8], and also by other acidic drugs, such as furosemide, piretanide, clofibrate, phenylbutazone, valproic acid, salicylic acid and sulfamethoxazole [8, 9]. The protein binding of cefazolin is lower when albumin concentrations are low, such as in cirrhosis [10] and renal disease [6], and is affected by haemodialysis [11]. Various studies in animals and humans support theconcept that the protein binding of cefazolin is concentration-dependent (saturable) within the range of concentrations likely to be encountered clinically [8, 11–14]. However these studies were conducted using human plasma in vitro [8, 11] or in animals in vivo [12–14].
Protein binding is generally unimportant in therapeutics except in the interpretation of total (bound + unbound) concentrations [15]. However, it may be important for antibiotic therapy, as it is the unbound drug that is active against microorganisms [16]. Occasionally it is useful to measure antibiotic concentrations to determine whether these are above the minimum inhibitory bacteria concentrations (MICs). We have shown previously that plasma unbound cefazolin concentrations correlate with tissue unbound concentrations [17]. Without a knowledge of the extent of protein binding, it is difficult to predict unbound drug concentrations from total measured concentrations and therefore doses of antibiotic required for maximal activity.
This study was designed to characterize the in vivo protein binding of cefazolin, both between and within patients who were being treated with the drug.
Methods
Ethics Committee approval
The study was reviewed and approved by the regional ethics committee. No part of patient care was influenced by participation in the study. Written informed consent was obtained for those patients who were part of the initial and final parts of this study. Patients undergoing intermittent dosing (see below) had blood samples taken as part of their routine care. The regional ethics committee confirmed that consent from these patients was not required, but verbal consent was obtained.
Patient selection
For study (i) patients were recruited who were being treated with cefazolin either within the home antibiotic program or as in-patients at Christchurch Hospital, Christchurch, New Zealand. For study (ii) inpatients at Christchurch Hospital who were given intermittent cefazolin for the measurement of within patient trough and peak concentrations were recruited.
Drug administration
In study (i) all home antibiotic patients received cefazolin (Eli Lilly, Auckland, New Zealand) by continuous intravenous infusion. The hospital inpatients received cefazolin by either intermittent or constant infusions. Doses ranged from 2 to 6 g 24 h−1 based on a standard dose of 3 g 24 h−1 adjusted at the discretion of the attending physician for body size, severity of infection, and renal function. For the home antibiotic patients, the cefazolin was administered via a Homepump ECLIPSE® C-Series (5 ml h−1) (I-flow corporation, Lakefront, CA, USA) in 120 ml normal saline through a peripherally inserted central catheter (PICC line) (Arrow International Inc, Reading, USA). The inpatients on intermittent infusion received their daily drug in three divided doses in 50–100 ml of normal saline infused over 10–15 min. The inpatients receiving continuous infusions had their total daily dose added to normal saline and infused at a constant rate over 24 h. In study (ii) the intermittent doses of cefazolin were either 1 g or 2 g in 10 ml or 20 ml water for injection, respectively, and were given by i.v. bolus.
Blood/plasma sampling
In study (i) a blood sample (5 ml) was taken any time in the 24 h infusion period from patients receiving constant infusions. In patients receiving intermittent boluses, samples were taken at the end of the dosing interval (trough concentration). In study (ii) paired blood samples (5 ml) were taken immediately (within 5 min) prior to a dose (trough) and 15–20 min after a dose (peak). Samples were centrifuged immediately to separate the plasma, transported to the laboratory (5 min) and then stored at −30°C prior to analysis, which in all cases was performed within 4 weeks. Ultracentrifugation was carried out after thawing to separate unbound from bound drug. The stability of cefazolin at −30°C was confirmed by measuring concentrations before freezing, and after four freeze-thaw cycles. The mean concentrations before freezing and after freeze-thawing deviated by < 8%.
Drug analysis
The total and unbound drug concentrations were determined using the HPLC method described by Howard et al. [17]. All cefazolin concentrations were measured in duplicate. For determination of total cefazolin in plasma, 0.7 ml of acetonitrile was added to 0.3 ml of plasma to precipitate the proteins. The mixture was vortexed for 30 s and centrifuged at 15 000 g for 10 min. The clear supernatant (50 µl) was injected into the HPLC system. Unbound drug was separated from total drug by ultrafiltration (2600 g for 30 min at 37°C) using a Diaflo® ultrafiltration membrane, YMT DISCS, 30K NMWL, 14 mm (Amicon Inc, Beverly, USA). The filtrate (50 µl) was injected onto the HPLC system. HPLC analysis was performed using the Agilent 1100 Series system equipped with a quaternary pump, a variable wavelength detector set at 272 nm and an autosampler (Hewlett-Packard, Waldbronn, Germany). An Aqua C18 5 µm, 4 × 3.0 mm internal diameter guard column and an Aqua C18 5 µm, 75 × 4.6 mm internal diameter analytical column (Phenomenex, Torrance, CA, USA) were used for separation. Data were collected and analyzed using the Agilent ChemStation (Hewlett-Packard, Waldbronn, Germany). The mobile phase was a mixture of 0.01 m phosphate buffer pH 6.5 and acetonitrile (90:10 v:v) and the flow rate was 1 ml min−1. Under these conditions the retention time of cefazolin was about 8 min. Standard curves for total and unbound cefazolin were linear (r2 > 0.99) over the concentration ranges 1.5–200 mg l−1 and 0.04–20 mg l−1, respectively. Samples which were above the upper limit of this range were initially measured, then diluted and measured again. The results are those of the diluted samples. The lower limits of quantification for total and unbound cefazolin were 1.5 mg l−1 and 0.04 mg l−1, respectively. The absolute recoveries of total cefazolin at concentrations of 4.0, 40.0 and 160 mg l−1 were >95%, whereas recoveries during ultrafiltration at concentrations of 0.4, 4.0 and 16 mg l−1 were >90%. Intra- and interday coefficients of variation (CV%) for total cefazolin were < 7% and < 5.5%, respectively (concentration range 4.0–160 mg l−1). For unbound cefazolin the intra- and interday coefficients of variation were < 1% and < 2%, respectively (concentration range 0.4–16 mg l−1). The bias was < 7% for total cefazolin (at concentrations of 4.0, 40.0 and 160 mg l−1) and < 2% for unbound cefazolin (at concentrations of 0.4, 4.0 and 16 mg l−1).
Statistical analysis
The data were analyzed by linear regression and the paired Student's t-test.
Results
Demographics
Thirty-one patients (24 males and seven females, median age 60 (range 25–91) years completed study (i). Twelve patients (seven males and five females, median age 54 (range 19–75) years completed study (ii).
Cefazolin concentrations
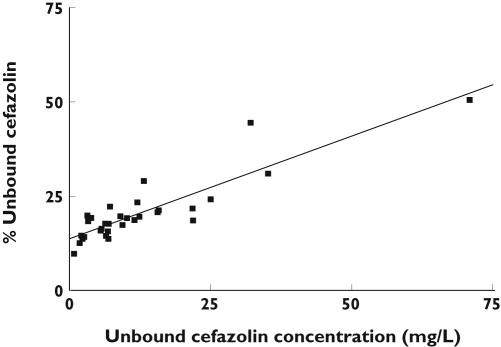
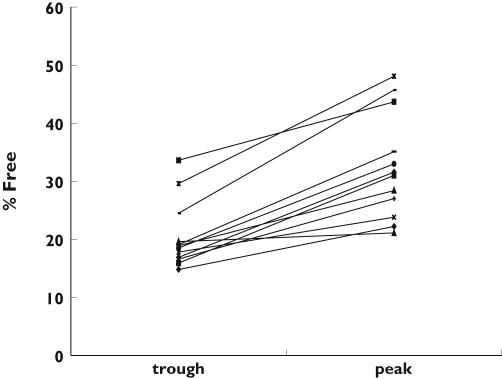
In study (i) median total and unbound plasma cefazolin concentrations, and the percentage unbound were 45.3 mg l−1 (range 8.5–140), 7.2 mg l−1 (range 0.83–70.9) and 18.6% (range 9.8–50.6), respectively. Linear regression analysis of the percentage unbound vs. unbound plasma concentrations revealed a r2 value of 0.79 and a slope significantly different from zero (P < 0.0001) (Figure 1), suggesting saturable protein binding. In study (ii) the trough and peak unbound and total plasma concentrations, the percentage unbound, and the peak minus trough difference in percentage unbound are presented in Table 1. There was a significant difference in the percentage unbound between the trough and peak concentrations, with the largest difference being 21.2% (trough 24.5%, peak 45.7%) and the smallest 1.5% (trough 19.6%, peak 21.1%) (P < 0.0001) (95% CI 8.5, 15.6). (Figure 2). Linear regression analysis of the differences between peak and trough samples in percentage unbound vs. unbound concentration revealed a r2 value of 0.72 and a slope significantly different from zero (P < 0.0005).
Figure 1.
The relationship between percentage unbound cefazolin vs. unbound cefazolin concentrations (r2 = 0.79) between patients
Table 1.
Total, unbound and percentage unbound cefazolin concentrations within patients (n = 12)
| Trough total concentration (mg l−1) | Peak total concentration (mg l−1) | Trough unbound concentration (mg l−1) | Peak unbound concentration (mg l−1) | Trough (% unbound) | Peak (% unbound) | Difference in % unbound (peak–trough) | Albumin (g l−1) (normal 35–50 g l−1) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.3 | 101.6 | 0.4 | 21.4 | 19.6 | 21.1 | +1.5 | 41 |
| 2 | 4.5 | 141.0 | 0.8 | 33.5 | 17.8 | 23.8 | +6.0 | 44 |
| 3 | 2.7 | 185.0 | 0.4 | 41.0 | 14.8 | 22.2 | +7.4 | 42 |
| 4 | 5.3 | 152.4 | 1.0 | 43.3 | 18.9 | 28.4 | +9.5 | 37 |
| 5 | 3.6 | 150.8 | 1.2 | 65.9 | 33.6 | 43.7 | +10.1 | – |
| 6 | 7.8 | 140.2 | 1.3 | 37.8 | 16.6 | 27.0 | +10.4 | – |
| 7 | 5.4 | 141.7 | 1.0 | 46.8 | 18.5 | 33.0 | +14.5 | 45 |
| 8 | 16.0 | 229.8 | 2.7 | 72.7 | 16.9 | 31.6 | +14.7 | 41 |
| 9 | 6.3 | 157.0 | 1.0 | 48.6 | 15.9 | 31.0 | +15.1 | 39 |
| 10 | 24.6 | 197.4 | 4.7 | 69.4 | 19.1 | 35.1 | +16.0 | 33 |
| 11 | 69.5 | 200.3 | 20.6 | 96.3 | 29.6 | 48.1 | +18.5 | 29 |
| 12 | 9.8 | 161.9 | 2.4 | 74.0 | 24.5 | 45.7 | +21.2 | 37 |
| Median | 5.8 | 159.4 | 1.1 | 47.7 | 18.7 | 31.3 | 12.4 |
Figure 2.
The percentage of cefazolin unbound at trough and peak concentrations (n = 12)
Discussion
This study has demonstrated saturable protein binding of cefazolin in vivo and variability in this parameter both between patients and within patients at therapeutic doses.
These results are consistent with those reported in vitro in humans [8, 11], and in vivo in animals [12–14]. In guinea pigs the extent of protein binding was 86% at a total concentration of cefazolin of 5 mg l−1, and 79% at 173 mg l−1[12]. In rabbits the protein binding values of 95% at total cefazolin concentrations up to 100 mg l−1, 89% at 200 mg l−1, and 69% at 400 mg l−1 have been reported [13]. In rats the extent of protein binding was approximately 85% at low total concentrations of cefazolin (25 mg l−1), decreasing to 75% at approximately 100 mg l−1[14]. In contrast, a study in dogs failed to demonstrate saturable protein binding [18]. However, the maximum total concentration of cefazolin was only 65 mg l−1, at which saturation may not have become evident.
Two humans in vitro studies have been performed in which increasing concentrations of antibiotic were added to human plasma [8, 11]. In one of these the extent of protein binding was 92% at total concentrations of cefazolin up to 100 mg l−1, decreasing to 85% at 200 mg l−1[8]. The authors predicted that at 400 mg l−1 the extent of protein binding would be about 65%. In the second study, the extent of protein binding was 80% at 5 mg l−1, decreasing to 70% at 150 mg l−1[11]. In our in vivo study (ii), median protein binding decreased from 81.3% at low (trough) concentrations to 68.7% at high (peak) concentrations When the differences in the percentage unbound between peak and trough samples were compared with the corresponding differences in unbound concentration using linear regression analysis, the slope was significantly different from zero, further supporting the presence of concentration-dependent protein binding of cefazolin.
Other factors that may influence protein binding include human serum albumin concentration and the co-administration of other drugs. However, our results, particularly those in study (ii), demonstrated saturable protein binding ‘within’ patients suggesting that saturation is a concentration related phenomenon. When molar concentrations were calculated for the higher concentrations of cefazolin in this study (200 mg l−1) and compared with those of albumin (40 g l−1) there were approximately twice as many molecules of cephazolin than of albumin. In addition there are reported to be two to five binding sites for cephazolin on each albumin molecule [7]. This means that in patients with albumin concentrations of 40 g l−1 and high cefazolin concentrations (200 mg l−1) there are between four to 10 fold more albumin binding sites than cephazolin molecules. The number of sites will clearly diminish with lower albumin concentrations. As expected the percentage of unbound cefazolin was affected by albumin concentration. However our second study demonstrated differences in the peak and trough percentages of unbound drug within patients, i.e. at a constant albumin concentration.
The practical significance of saturable protein binding may be small in routine clinical practice. However, drug concentrations might occasionally be measured to perform serum inhibitory dilutions, or to compare against MIC values of the infecting organism. Since drug concentration measurement usually involves the estimation of total drug (bound and unbound) the presence of saturable protein binding would be important in the interpretation of the data. The other area of the practical importance of saturable protein binding would be in pharmacokinetic studies. Apparent volume of distribution and clearance values for total drug are known to vary with dose as a result of saturable protein binding.
In summary, this study has demonstrated saturable protein binding of cefazolin in vivo both between and within patients over a wide range of concentrations associated with normal therapeutic doses.
References
- 1.Steckelberg JM, Rouse MS, Tallan BM, Osmon DR, Henry NK, Wilson WR. Relative efficacies of broad-spectrum cephalosporins for treatment of methicillin-susceptible Staphylococcus aureus experimental infective endocarditis. Antimicrob Agents Ch. 1993;37:554–8. doi: 10.1128/aac.37.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grayson ML, Silvers J, Turnbridge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Australia. 1995;162:249–53. [PubMed] [Google Scholar]
- 3.Corwin P, Toop L, Mcgeoch G, Than M, Wynn-Thomas S, Wells JE, Dawson R, Abernethy P, Pithie A, Chambers S, Fletcher L, Richards D. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. Br Med J. 2005;330:129–32. doi: 10.1136/bmj.38309.447975.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirby WMM, Regamey C. Pharmacokinetics of cefazolin compared with other cephalosporins. J Infect Dis. 1973;128:S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- 5.Nightingale CH, Greene DS, Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975;64:1900–27. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- 6.Craig WA, Welling PG, Jackson TC, Kunin CM. Pharmacology of cefazolin and other cephalosporins in patients with renal insufficiency. J Infect Dis. 1973;128(Suppl):s347–s353. doi: 10.1093/infdis/128.supplement_2.s347. [DOI] [PubMed] [Google Scholar]
- 7.Nerli B, Romanini D, Pico G. Structural specificity requirements in the binding of beta lactam antibiotics to human serum albumin. Chem Biol Int. 1997;104:179–202. doi: 10.1016/s0009-2797(97)00024-0. [DOI] [PubMed] [Google Scholar]
- 8.Decroix MO, Zini R, Chaumeil JC, Tillement JP. Cefazolin serum protein binding and its inhibition by bilirubin, fatty acids and other drugs. Biochem Pharmacol. 1988;37:2807–14. doi: 10.1016/0006-2952(88)90044-5. [DOI] [PubMed] [Google Scholar]
- 9.Morgant C, Contrepois A, Chau NP, Romaru A, Fourtillan J, Carbon C. Effects of furosemide, piretanide, and water loading on urinary excretion of cefazolin in humans. Antimicrob Agents Chemother. 1984;25:618–21. doi: 10.1128/aac.25.5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi K, Tsunoo M, Tsuneoka K. Pharmacokinetics and protein binding of cefazolin and cephalothin in patients with cirrhosis. J Antimicrob Chemoth. 1986;17:347–51. doi: 10.1093/jac/17.3.347. [DOI] [PubMed] [Google Scholar]
- 11.Greene DS, Tice AD. Effect of haemodialysis on cefazolin protein binding. (letter) J Pharm Sci. 1977;66:1508–10. doi: 10.1002/jps.2600661050. [DOI] [PubMed] [Google Scholar]
- 12.Fritz PE, Hurst WJ, White WJ, Lang CM. Pharmacokinetics of cefazolin in guinea pigs. Laboratory Anim Sci. 1987;37:646–51. [PubMed] [Google Scholar]
- 13.Matsui H, Okuda T. Penetration of cefpiramide and cefazolin into peritoneal capsular fluid in rabbits. Antimicrob Agents Chemother. 1988;32:33–6. doi: 10.1128/aac.32.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadai M, Hasegawa T, Kato K, Wang L, Nabeshima T, Kato N. Alterations in pharmacokinetics and protein binding behaviour of cefazolin in endotoxemic rats. Antimicrob Agents Chemother. 1993;37:1781–5. doi: 10.1128/aac.37.9.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clinl Pharmacol Ther. 2002;71:115–21. doi: 10.1067/mcp.2002.121829. [DOI] [PubMed] [Google Scholar]
- 16.Craig WA. Does the dose matter? Clin Infect Dis. 2001;33(Suppl 3):S233–7. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 17.Howard GW, Begg EJ, Chambers ST, Vella-Brincat J, Zhang M, Kirkpatrick CMJ. Free and total plasma and interstitial fluid concentrations at steady state during continuous infusion. J Antimicrob Chemother. 2002;50:429–43. doi: 10.1093/jac/dkf129. [DOI] [PubMed] [Google Scholar]
- 18.Waterman NG, Raff MJ, Scharfenberger L, Barnwell PA. Protein binding and concentrations of cephaloridine and cefazolin in serum and interstitial fluid of dogs. J Infect Dis. 1976;133:642–7. doi: 10.1093/infdis/133.6.642. [DOI] [PubMed] [Google Scholar]