Abstract
What is already known about this subject
Isoprostanes are the product of free radical oxidation of arachidonic acid bound to phospholipids.
Their hydrolysis from phospholipids is presumably catalysed by phospholipases A2.
Atorvastatin reduces protein concentrations of secretory PLA2s and concentrations of LDL, with which PAF-AH (group VII phospholipase) is associated.
What this study adds
Atorvastatin affects PAF-AH activity and this effect is strongly associated with its lipid-lowering effect, but it has no effect on groups IIA and V PLA2s' activity.
Thus, PAF-AH is no independent risk factor of cardiovascular diseases.
Moreover, a role of PAF-AH in the liberation of 15-F2t-isoP from phospholipids is excluded.
Aims
Isoprostanes are the product of free radical oxidation of arachidonic acid, whose hydrolysis from phospholipids is presumably catalysed by phospholipases A2 (PLA2s) such as group IIA or V PLA2s, or group VII PLA2 [platelet-activating factor acetylhydrolase (PAF-AH), lipoprotein-associated phospholipase]. Atorvastatin reduces concentrations of low-density lipoprotein (LDL), with which PAF-AH is associated, and PLA2s' protein concentrations. We investigated the effect of atorvastatin on PLA2s and PAF-AH activity and the urinary excretion of 15-F2trans-isoprostane (15-F2t-IsoP, 8-iso-PGF2α, iPF2α-III).
Methods
Twenty-four hypercholesterolaemic individuals naive to lipid-lowering therapy were randomized to atorvastatin 40 mg or placebo for 6 weeks. The 15-F2t-isoP urinary excretion (gas chromatography/mass spectrometry), PAF-AH and group IIA and V PLA2 activities (photometry) were assessed at baseline and end-point.
Results
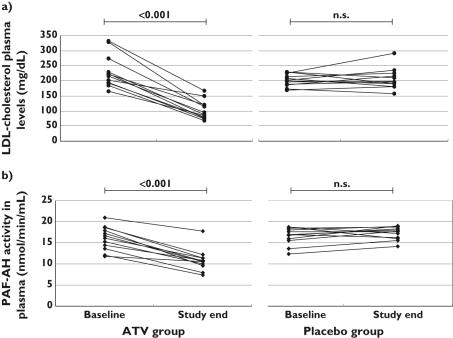
At end-point, 15-F2t-isoP urinary excretion concentrations as well as PLA2s' activity were unchanged under atorvastatin (mean change 0.21 ± 1.79 ng h−1, 95% confidence interval −0.92, 1.35 and 0.33 ± 0.94 nmol min−1 ml−1, −0.27, 0.93) and under placebo (mean change 0.69 ± 1.69 ng h−1, −0.52, 1.90 and 1.29 ± 2.16 nmol min−1 ml−1, −0.25, 2.84). Atorvastatin treatment decreased total (P < 0.001) and LDL-cholesterol (P < 0.001) but had no effect on high-density lipoprotein. PAF-AH activity was lowered in the atorvastatin group (mean change − 5.27± 1.96 nmol min−1 ml−1, −6.51, −4.03, P < 0.001) but not in the placebo group (mean change 1.02 ± 1.64 nmol min−1 ml−1, 0.15, 2.20), and the change in PAF-AH activity was correlated with that in total (P = 0.03) and LDL-cholesterol (P = 0.03).
Conclusion
Our results show a lowering effect of atorvastatin on PAF-AH activity associated with its lipid-lowering effect and exclude a key role of PAF-AH in the liberation of 15-F2t-isoP from phospholipids.
Keywords: isoprostanes, lipoprotein-associated phospholipase, oxidative stress, secretory phospholipases
Introduction
Isoprostanes are stable end-products of lipid peroxidation [1], which exert biological effects such as vasoconstriction [2]. They have been shown to increase in patients suffering from coronary heart disease (CHD) [3, 4]. Likewise, conditions such as diabetes mellitus [5], hypercholesterolaemia [6], obesity [7] and pulmonary hypertension [8] have been associated with an increase in isoprostanes. F2-isoprostanes have also been found in atherosclerotic lesions [9]. This isoprostane family in general and the 15-F2trans-isoprostane (15-F2t-IsoP, 8-iso-PGF2α, iPF2α-III) in particular correlated with the number of cardiovascular risk factors and are therefore considered a reliable marker of CHD [4].
Because they hydrolyse oxidized phospholipids at the sn-2 position, generating lysophospholipids and oxidized fatty acids, phospholipases have been hypothesized to be responsible for the release of isoprostanes in the blood stream [10, 11]. One of the enzymes that would come into question is the platelet-activating factor acetylhydrolase (PAF-AH), also known as lipoprotein-associated phospholipase A2 or group VIIA phospholipase A2. In plasma, 70% of PAF-AH circulates with low-density lipoprotein (LDL), in which it exerts a longer half-life than in high-density lipoprotein (HDL) [12, 13]. In fact, some studies have demonstrated concurrent decreases in PAF-AH protein concentrations [14–16] and activity [14] in plasma and LDL-cholesterol in response to different lipid-lowering drugs. Like F2-isoprostanes, PAF-AH is also expressed by macrophages in human atherosclerotic lesions [17]. Among others, PAF-AH catabolizes PAF, a phospholipid which binds the PAF-receptor, thereby causing increased vascular permeability and activating platelets and leucocytes [12], a function that should confer PAF-AH anti-inflammatory properties. On the contrary, a considerable number of clinical and experimental reports support a role of PAF-AH as a proinflammatory molecule and risk factor for CHD [18–24].
Other phopholipases' A2 (PLA2s) involvement in the release of isoprostanes must be considered, such as group IIA and group V PLA2. Group IIA PLA2 participates in immediate and delayed phases of cellular arachidonic acid release and is increased in inflammatory states [12]. In one clinical study, treatment with both atorvastatin and simvastatin led to a reduction in group IIA PLA2 protein concentrations [25]. Group V PLA2 promotes atherosclerotic lesions by modifying LDL particles [26]. Group IIA and V PLA2s have been found to act jointly in inflammatory states [27].
We investigated if a statin treatment (atorvastatin 40 mg day−1 for 6 weeks) in hypercholesterolaemic patients naive to lipid-lowering therapy would lead to a lowering in PAF-AH and/or PLA2s activity and in 15-F2t-isoP urinary excretion, thereby indicating which enzyme(s) is involved in the release of the latter.
Methods
Patients and study protocol
Twenty-four participants aged between 35 and 60 years were included in this study. Hypercholesterolaemia was defined as LDL-cholesterol concentrations ≥160 mg dl−1 (4.2 mmol l−1). All hypercholesterolaemic participants were naive to statins or other lipid-lowering medications. Exclusion criteria were: history of alcoholism or drug abuse; pregnancy or breastfeeding status; liver disease or liver insufficiency [serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >1.5-fold above the upper limit of the normal range, 10–35 U l−1 for women, 10–50 U l−1 for men]; advanced kidney disease (creatinine clearance < 30 ml min−1), nephrotic syndrome or dysproteinaemia; diabetes mellitus. We assessed eligibility and obtained written informed consent as stipulated in the study protocol approved by the local Review Board for Studies in Humans.
All participants were invited to the study centre on the morning of day 1 and a 24-h urine sample collection was started. Urine was collected into a container prepared with 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (4-hydroxy-TEMPO) and ethylinediamine tetraacetic acid as antioxidants. Twenty-four hours later, patients returned to the study centre in the morning. A fasting blood sample was drawn, blood samples were centrifuged (2000 g, 20 min, 4°C) immediately and plasma was divided into aliquots and stored at −20°C until analysis. Urine samples were collected, divided into aliquots and kept frozen at −20°C until analysis. Participants were given the study medication or placebo in a neutral packaging, instructed about the intake scheme and dismissed. Two weeks later, they returned to the study centre to have their biochemical parameters controlled in order to detect any intolerance reaction. On day 42 of the study, participants returned to the study centre and underwent an investigation identical to that on the first day.
Biochemical analyses
The urinary concentration of 15-F2t-isoP was determined by gas chromatography/mass spectrometry (GC-MS) as described previously [28]. Briefly, urine samples were thawed and a labelled internal standard of 15-F2t-isoP was added at 1 ng ml−1. Physiological and labelled 15-F2t-isoP were extracted by immunoaffinity and derivatized. Samples were subsequently analysed by GC-MS: physiological 15-F2t-isoP was detected at m/z 569 and the internal standard at m/z 573. Activities of plasma group IIA and V PLA2s and PAF-AH were assessed using commercially available assay kits (Cayman Chemicals, Ann Arbor, MI, USA), following sample concentration with Amicon Ultra Centrifugation Filter Devices (Millipore, Billerica, MA, USA). Ultra-sensitive C-reactive protein (hsCRP) was measured on a Dade Behring BN II nephelometer (Dade Behring GmbH, Eschborn, Germany) with polystyrene microbeads coated with monoclonal mouse antibodies [29]. Plasma total cholesterol, LDL and HDL concentrations as well as plasma and urinary creatinine concentrations were determined by standard laboratory methods using certified assays in the local clinical laboratory.
Calculations and statistical methods
All data were tested for normal distribution with the Shapiro–Wilk test. The distribution of 15-F2t-isoP and hsCRP was skewed, as reported previously [4, 7, 30]. Differences between groups are given as mean (SD) except for parameters not normally distributed (median and interquartile range). Comparisons between study end and baseline involving parameters not normally distributed were performed with the Wilcoxon test. All other comparisons were performed by T-test. Correlation coefficients are Pearson's. P < 0.05 was accepted for statistical significance. For statistical analyses, SPSS version 13.0 was used (SPSS Inc., Chicago, IL, USA).
Results
Participants' characteristics at baseline are presented in Table 1. There were two smokers in the placebo group and none in the treatment group. The PROCAM (Prospective Cardiovascular Münster) score was 8.95 [interquartile range (IQR) 3.65–29.7] in the placebo group and 6.00 (IQR 3.65–23.7) in the atorvastatin group (P = 0.630).
Table 1.
Participants' characteristics at baseline and study end
| Baseline | Placebo Baseline | Study end | P vs. baseline | ATV Baseline | Study end | P vs. baseline | P placebo vs. ATV at baseline |
|---|---|---|---|---|---|---|---|
| N | 12 | 12 | – | 12 | 12 | – | – |
| Men, n (%) | 6 (50) | 6 (50) | – | 6 (50) | 6 (50) | – | – |
| Age (SD) | 58.3 (5.0) | 58.3 (5.0) | – | 58.9 (7.3) | 58.9 (7.3) | – | 0.821 |
| Body mass index, kg m−2 (SD) | 25.3 (1.8) | 25.3 (1.8) | – | 24.2 (4.1) | 24.2 (4.1) | – | 0.405 |
| Total cholesterol, mg dl−1 (SD) | 284 (30) | 289 (35) | 0.844 | 320 (61) | 182 (32) | < 0.001 | 0.078 |
| Low-density lipoprotein, mg dl−1 (SD) | 202 (21) | 206 (34) | 0.469 | 231 (54) | 103 (31) | < 0.001 | 0.102 |
| High-density lipoprotein, mg dl−1 (SD) | 50.6 (13.1) | 51.4 (11.5) | 0.625 | 59.1 (11.6) | 62.3 (11.8) | 0.101 | 0.103 |
| 15-F2t-isoP urinary excretion, ng h−1 (IQR) | 8.0 (6.4–11.1) | 8.9 (7.4–12.3) | 0.721 | 9.8 (6.6–12.5) | 8.4 (6.9–13.3) | 0.875 | 0.763 |
| PLA2s activity, nmol min−1 ml−1 (SD) | 4.35 (0.61) | 5.25 (2.06) | 0.172 | 4.12 (1.14) | 4.45 (0.83) | 0.247 | 0.599 |
| PAF-AH activity, nmol min−1 ml−1 (SD) | 16.7 (2.0) | 17.3 (1.5) | 0.196 | 16.1 (2.8) | 10.9 (2.6) | < 0.001 | 0.552 |
| hsCRP, mg l−1 (IQR) | 1.50 (0.70–2.37) | 1.60 (0.70–2.00) | 0.507 | 1.35 (0.83–1.80) | 0.85 (0.70–2.17) | 0.553 | 0.932 |
PLA2s, Phospholipases A2; PAF-AH, platelet-activating factor acetylhydrolase; hsCRP, ultra-sensitive C-reactive protein.
At the end of the study, none of the biochemical parameters (total, LDL- and HDL-cholesterol plasma concentrations, 15-F2t-isoP urinary excretion, PAF-AH and PLA2s activity, CRP) was modified in the placebo group (Table 1). The mean change in 15-F2t-isoP urinary excretion was +0.69 ng h−1 [95% confidence interval (CI) −0.52, 1.90].
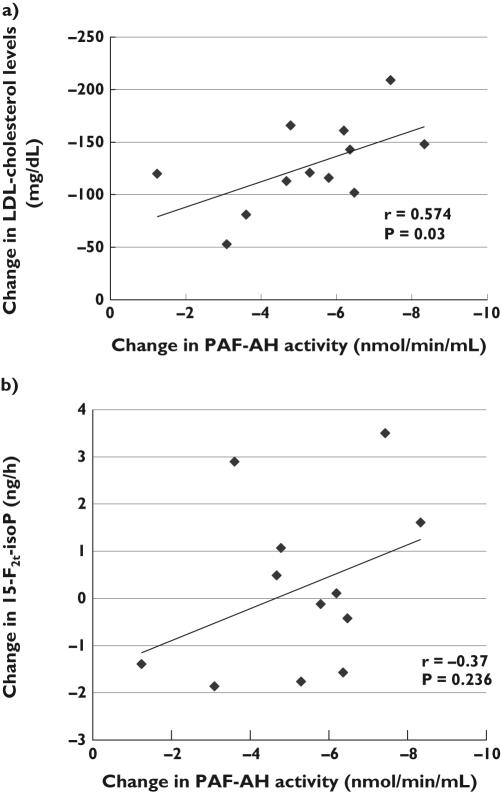
In the atorvastatin group, a nonsignificant reduction in 15-F2t-isoP urinary excretion was observed, whereas PLA2s activity remained virtually unaffected. Total and LDL-cholesterol were significantly lowered, which was paralleled by a decrease of PAF-AH activity in all patients with active treatment (Figure 1a,b). The change in PAF-AH activity was correlated with the change in LDL-cholesterol concentrations (r = 0.574, P = 0.03) (Figure 2a) and with that in total cholesterol (r = 0.562, P = 0.03), but not with the change in 15-F2t-isoP urinary excretion (Figure 2b). The mean change in 15-F2t-isoP urinary excretion was +0.21 ng h−1 (95% CI −0.92, 1.35). No episode of intolerance was recorded in this group, although liver enzyme ALT was slightly increased (43.5 U l−1, SD 15.6 vs. 32.6 U l−1, SD 12.6; P = 0.004). Creatine kinase (155.7 U l−1, SD 97.5 vs. 175.2 U l−1, SD 185.3 at baseline; P = 0.659) and AST (33.8 U l−1, SD 9.6 vs. 30.1 U l−1, SD 9.6 at baseline; P = 0.147) were not modified, nor was the inflammatory marker hsCRP (0.85 mg l−1, IQR 0.70–2.17 vs. 1.35 mg l−1, IQR 0.83–1.80 at baseline, P = 0.553).
Figure 1.
Individual change in (a) low-density lipoprotein (LDL)-cholesterol plasma concentrations and (b) platelet-activating factor acetylhydrolase (PAF-AH) activity in the ATV-treated group. ATV, Atorvastatin; NS, not significant
Figure 2.
Correlation between the change in platelet-activating factor acetylhydrolase (PAF-AH) activity and the change in (a) low-density lipoprotein (LDL)-cholesterol levels and (b) 15-F2t-isoP urinary excretion, in participants treated with atorvastatin
Discussion
We report reduced PAF-AH activity secondary to cholesterol lowering after 6 weeks' treatment with atorvastatin 40 mg. The treatment did not induce any change in other secretory phospholipase activity, nor in 15-F2t-isoP urinary excretion.
The biological functions of PAF-AH appear paradoxical and their pro- or anti-inflammatory and atherogenic effects are still a matter of debate. PAF-AH catabolizes the proinflammatory PAF [12] and, in vitro, its activity in HDL parallels HDL's ability to prevent LDL oxidation [31]. A genetic deficiency in PAF-AH studied in Japanese subjects was significantly associated with asthma, stroke, myocardial infarction, brain haemorrhage and nonfamilial cardiomyopathy [32, 33]. On the other hand, PAF-AH can transform lyso-PAF back into the biologically active PAF and its analogues. This reaction may occur in the atherogenic small dense LDL particles and may confer them with higher proinflammatory potential in atherosclerosis-prone areas [34]. The lysophospholipids and oxidized fatty acids generated by PAF-AH from highly oxidized LDL are proinflammatory, upregulating adhesion molecules and cytokine production, thereby having a deleterious effect on the arterial wall [22–24, 34]. PAF-AH has been postulated to be an independent risk factor for cardiovascular disease in healthy middle-aged men [35] and women [19] as well as in men with a history of coronary events [18]. One other possible mechanism for the proinflammatory effect of PAF-AH could be an involvement in the liberation of isoprostanes into the blood stream. Indeed, isoprostanes are not mere markers, but also mediators of oxidative stress: they are vasoconstrictors in several vascular beds [36], activate platelets [37] and stimulate monocyte adhesion to endothelial cells [38]. However, the results of the present study, especially the correlation between the change in PAF-AH activity and that in LDL-cholesterol concentrations in such a small group, argue against an independent role of PAF-AH in cardiovascular disease and, in accordance with recent reports [39, 40], rather suggest that these observations were actually closely connected to LDL-cholesterol concentrations. Moreover, contrary to recent observations that plasma samples from PAF-AH-deficient subjects do not release F2-isoprostanes from esterified precursors ex vivo and that PAF-AH transgenic mice have a higher capacity to release F2-isoprostanes compared with nontransgenic littermates [41], the unchanged urinary excretion of 15-F2t-isoP despite the marked atorvastatin-induced lowering in PAF-AH activity rather shows that the involvement of plasma PAF-AH in 15-F2t-isoP in vivo in hypercholesterolaemic patients is marginal to nil. This, in turn, is compatible with the observation from Stafforini et al. that the catabolism rate of PAF-AH was much slower for isoprostanes than for its other substrates, a hint that this enzyme is not the main one responsible for isoprostane liberation.
The intake of atorvastatin 40 mg over 6 weeks significantly lowered PAF-AH activity, but as the exact role of PAF-AH remains controversial, the potential benefits of this reduction are also uncertain. According to the literature, overly increased PAF-AH expression and/or activity are associated with pathological states [22–24, 34]. Thus, although the decrease in the inflammatory marker hsCRP was not significant (Table 1), we hypothesize that in such pathological states as hypercholesterolaemia, a lowering in PAF-AH activity to normal physiological levels lowers atherogenesis and inflammatory potential and is beneficial. However, since overly decreased PAF-AH expression and/or activity is associated with pathological states also [11, 32, 33], these should not be excessively lowered either. Indeed, PAF-AH is secreted in response to inflammatory stimuli [42], yet it is unclear if this happens in response to or as part of an inflammatory cascade. If proven to be a primarily anti-inflammatory and -atherogenic enzyme, PAF-AH's reported proinflammatory and pro-atherogenic properties could be a mere imbalance or reversal of its functions brought about by unknown pathophysiological conditions.
The reason why we did not see a significant decrease in 15-F2t-isoP is not clear. The choice of the statin used does not explain it. Indeed, a few studies have reported a decrease in 15-F2t-isoP after atorvastatin treatment [43, 44]. Sugiyama et al. [44] observed a significant decrease in 15-F2t-isoP as soon as 4 weeks of a 10-mg daily intake of atorvastatin. An insufficient lowering of cholesterol concentrations, which has been associated in some studies [45] with that in 15-F2t-isoP urinary excretion, is not the explanation either. Indeed, in a therapy scheme intended to produce a 20% reduction of total cholesterol concentrations after 60 days, as soon as after 1 month simvastatin induced a significant reduction in 15-F2t-isoP urinary excretion concentrations [45]. Despite a >40% lowering in total cholesterol, we could not reproduce this result for atorvastatin in our setting. Since liver and muscle enzymes were not significantly increased, with the exception of ALT, which did not reach 1.5-fold of the upper limit of the normal range, the hypothesis of oxidation injury in the muscles or liver to explain the lack of change in 15-F2t-isoP excretion [46] also seems irrelevant. Of course, the explanation could be that 15-F2t-isoP is in fact liberated by PLA2s (groups IIA and V). Nevertheless, at least one study has suggested a link between 15-F2-isoprostane and group IIA and V PLA2s [47].
Wiklund et al. [25] have reported a significant reduction in group IIA PLA2 plasma protein concentrations after a 6-week daily intake of atorvastatin 40 mg. Thus, a likely argument for the lack of change in PLA2s' activity in our study could be the fact that we measured enzyme activity where others measured plasma protein concentrations. Indeed, protein quantification does not give information about the catalytic activity of PLA2 present in the sample, and reaction rates can remain unchanged or even rise with comparable protein levels [47]. Since PLA2s are involved in inflammatory processes, a reason for the lack of change in their activity could be that atorvastatin, as reflected by the hsCRP (Table 1), did not significantly affect the inflammatory status of treated participants, although Sugiyama et al. [44] have reported a significant decrease in hsCRP after a 4-week 10 mg day−1 intake of atorvastatin. Taken together, these data and ours suggest that the possible association between PLA2s and 15-F2t-isoP should be further investigated.
A limitation of our study is that the biochemical parameters were not measured in the same biological compartment: all parameters except 15-F2t-isoP were quantified in plasma. Nevertheless, there is an excellent correlation between plasma and urine 15-F2t-isoP [48]. Since artefactual isoprostane formation through lipid auto-oxidation is less of an issue in urine samples than in plasma [49], 15-F2t-isoP quantification in urine is more reliable and was favoured. We cannot rule out the possibility that a larger number of participants would have allowed the drawing of more definite conclusions regarding the parameters possibly linked to inflammation, e.g. hsCRP, PLA2s and 15-F2t-isoP. However, in a previous study including 12 individuals with similar baseline 15-F2t-isoP concentrations [50], we were able to detect a significant 24% decrease in urinary 15-F2t-isoP excretion, i.e. ≈2 ng h−1. Finally, it is undeniable that PAF-AH plasma protein concentrations instead of activity could have led to different conclusions, first, because, similar to PLA2s, there is no direct correlation between protein concentrations and activity [51], suggesting that a fraction of the protein can become inactive; and second, because activity seems more closely related to LDL-cholesterol than protein concentrations [52]. However, since we were interested in PAF-AH involvement in the enzymatic liberation process of isoprostanes, its activity was more relevant than its protein concentrations.
In conclusion, although an association between group IIA and V phospholipase and 15-F2t-isoP must be further investigated, our data strongly suggest that the previously reported increased cardiovascular risk associated with PAF-AH was connected with LDL-cholesterol. Furthermore, we have eliminated a key role of the enzyme PAF-AH in the release of 15-F2t-isoP.
References
- 1.Morrow JD, Roberts LJ., II The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 2.Cracowski JL, Devillier P, Durand T, Stanke-Labesque F, Bessard G. Vascular biology of the isoprostanes. J Vasc Res. 2001;38:93–103. doi: 10.1159/000051036. [DOI] [PubMed] [Google Scholar]
- 3.Vassalle C, Botto N, Andreassi MG, Berti S, Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron Artery Dis. 2003;14:213–8. doi: 10.1097/01.mca.0000063504.13456.c3. [DOI] [PubMed] [Google Scholar]
- 4.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, Gutzki FM, Berger J, Frölich JC, Böger RH. Urinary 8-iso-Prostaglandin F2α as a risk marker in patients with coronary heart disease; a matched case–control study. Circulation. 2004;109:843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 5.Devaraj S, Hirany SV, Burk RF, Jialal I. Divergence between LDL oxidative susceptibility and urinary F2-isoprostanes as measures of oxidative stress in type 2 diabetes mellitus. Clin Chem. 2001;47:1974–9. [PubMed] [Google Scholar]
- 6.Davi G, Alessandrini P, Mezzetti A, Minotti G, Bucciarelli T, Costantini F, Cipollone F, Bon GB, Ciabattoni G, Patrono C. In vivo formation of 8-epi-prostaglandin F2α is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:3230–5. doi: 10.1161/01.atv.17.11.3230. [DOI] [PubMed] [Google Scholar]
- 7.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ Framingham Study. Obesity and systemic oxidative stress; clinical correlates of oxidative stress in the Framingham Study. Arterioscl Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 8.Cracowski JL, Cracowski C, Bessard G, Pepin JL, Bessard J, Schwebel C, Stanke-Labesque F, Pison C. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:1038–42. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- 9.Waddington EI, Croft KD, Sienuarine K, Latham B, Puddey IB. Fatty acid oxidation products in human atherosclerotic plaque; an analysis of clinical and histopathological correlates. Atherosclerosis. 2003;167:111–20. doi: 10.1016/s0021-9150(02)00391-x. [DOI] [PubMed] [Google Scholar]
- 10.Sametz W, Hummer K, Butter M, Wintersteiger R, Juan H. Formation of 8-iso-PGF (2alpha) and thromboxane A(2) by stimulation with several activators of phospholipase A(2) in the isolated human umbilical vein. Br J Pharmacol. 2000;131:145–51. doi: 10.1038/sj.bjp.0703547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tselepis AD, Chapman MJ. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl. 2002;3:57–68. doi: 10.1016/s1567-5688(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 12.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 13.Stafforini DM, Carter ME, Zimmerman GA, McIntyre TM, Prescott SM. Lipoproteins alter the catalytic behavior of the platelet-activating factor acetylhydrolase in human plasma. Proc Natl Acad Sci USA. 1989;86:2393–7. doi: 10.1073/pnas.86.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisaf M, Tselepis AD. Effect of hypolipidemic drugs on lipoprotein-associated platelet activating factor acetylhydrolase. Implication for atherosclerosis. Biochem Pharmacol. 2003;66:2069–73. doi: 10.1016/s0006-2952(03)00559-8. [DOI] [PubMed] [Google Scholar]
- 15.Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586–93. doi: 10.1161/01.ATV.0000222983.73369.c8. [DOI] [PubMed] [Google Scholar]
- 16.Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM. A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol. 2001;38:1302–6. doi: 10.1016/s0735-1097(01)01554-6. [DOI] [PubMed] [Google Scholar]
- 17.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K, Yla-Herttuala S. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–17. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 18.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–55. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 19.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–42. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 20.Blankenberg S, Stengel D, Rupprecht HJ, Bickel C, Meyer J, Cambien F, Tiret L, Ninio E. Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res. 2003;44:1381–6. doi: 10.1194/jlr.M300086-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Tsoukatos DC, Liapikos TA, Tselepis AD, Chapman MJ, Ninio E. Platelet-activating factor acetylhydrolase and transacetylase activities in human plasma low-density lipoprotein. Biochem J. 2001;357:457–64. doi: 10.1042/0264-6021:3570457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPhee CH, Moores KE, Boyd HF, Dhanak D, Ife RJ, Leach CA, Leake DS, Milliner KJ, Patterson RA, Suckling KE, Tew DG, Hickey DM. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–87. [PMC free article] [PubMed] [Google Scholar]
- 23.Macphee CH. Lipoprotein-associated phospholipase A2: a potential new risk factor for coronary artery disease and a therapeutic target. Curr Opin Pharmacol. 2001;1:121–5. doi: 10.1016/s1471-4892(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 24.Macphee CH, Suckling KE. Lipoprotein-associated phospholipase A(2): a target directed at the atherosclerotic plaque. Expert Opin Ther Targets. 2002;6:309–14. doi: 10.1517/14728222.6.3.309. [DOI] [PubMed] [Google Scholar]
- 25.Wiklund O, Mattsson-Hulten L, Hurt-Camejo E, Oscarsson J. Effects of simvastatin and atorvastatin on inflammation markers in plasma. J Intern Med. 2002;251:338–47. doi: 10.1046/j.1365-2796.2002.00966.x. [DOI] [PubMed] [Google Scholar]
- 26.Wooton-Kee CR, Boyanovsky BB, Nasser MS, de Villiers WJ, Webb NR. Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation. Arterioscler Thromb Vasc Biol. 2004;24:762–7. doi: 10.1161/01.ATV.0000122363.02961.c1. [DOI] [PubMed] [Google Scholar]
- 27.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–98. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 28.Tsikas D, Schwedhelm E, Suchy MT, Niemann J, Gutzki FM, Erpenbeck VJ, Hohlfeld JM, Surdacki A, Frölich JC. Divergence in urinary 8-iso-PGF (2alpha) (iPF (2alpha)-III, 15-F(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B Anal Technol Biomed Life Sci. 2003;794:237–55. doi: 10.1016/s1570-0232(03)00457-4. [DOI] [PubMed] [Google Scholar]
- 29.Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of a particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann Clin Biochem. 1998;35:745–53. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- 30.Koenig W, Sund M, Fröhlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–42. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 31.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tjoelker LW, Stafforini DM. Platelet-activating factor acetylhydrolases in health and disease. Biochim Biophys Acta. 2000;1488:102–23. doi: 10.1016/s1388-1981(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 33.Hiramoto M, Yoshida H, Imaizumi T, Yoshimizu N, Satoh K. A mutation in plasma platelet-activating factor acetylhydrolase (Val279–>Phe) is a genetic risk factor for stroke. Stroke. 1997;28:2417–20. doi: 10.1161/01.str.28.12.2417. [DOI] [PubMed] [Google Scholar]
- 34.Macphee CH, Milliner K, Moores K, Tew DG. The involvement of LDL-associated phospholipase A2 in atherogenesis. Pharmacol Rev Commun. 1996;8:309–15. [Google Scholar]
- 35.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–8. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 36.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 37.Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 1997;17:2309–15. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 38.Leitinger N, Huber J, Rizza C, Mechtcheriakova D, Bochkov V, Koshelnick Y, Berliner JA, Binder BR. The isoprostane 8-iso-PGF(2alpha) stimulates endothelial cells to bind monocytes: differences from thromboxane-mediated endothelial activation. FASEB J. 2001;15:1254–6. doi: 10.1096/fj.00-0498fje. [DOI] [PubMed] [Google Scholar]
- 39.Kardys I, Oei HH, van der Meer IM, Hofman A, Breteler MM, Witteman JC. Lipoprotein-associated phospholipase A2 and measures of extracoronary atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2006;26:631–6. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 40.Albert MA, Glynn RJ, Wolfert RL, Ridker PM. The effect of statin therapy on lipoprotein associated phospholipase A2 levels. Atherosclerosis. 2005;182:193–8. doi: 10.1016/j.atherosclerosis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, Bonventre JV, Prescott SM, Roberts LJ. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281:4616–23. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 42.Castro Faria Neto HC, Stafforini DM, Prescott SM, Zimmerman GA. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem Inst Oswaldo Cruz. 2005;100(Suppl. 1):83–91. doi: 10.1590/s0074-02762005000900014. [DOI] [PubMed] [Google Scholar]
- 43.Sinzinger H, Oguogho A. Variable influence of statins on isoprostanes in hyperlipidemia. Adv Exp Med Biol. 2003;525:209–12. doi: 10.1007/978-1-4419-9194-2_45. [DOI] [PubMed] [Google Scholar]
- 44.Sugiyama M, Ohashi M, Takase H, Sato K, Ueda R, Dohi Y. Effects of atorvastatin on inflammation and oxidative stress. Heart Vessels. 2005;20:133–6. doi: 10.1007/s00380-005-0833-9. [DOI] [PubMed] [Google Scholar]
- 45.De Caterina R, Cipollone F, Filardo FP, Zimarino M, Bernini W, Lazzerini G, Bucciarelli T, Falco A, Marchesani P, Muraro R, Mezzetti A, Ciabattoni G. Low-density lipoprotein level reduction by the 3-hydroxy-3-methylglutaryl coenzyme A inhibitor simvastatin is accompanied by a related reduction of F2-isoprostane formation in hypercholesterolemic subjects: no further effect of vitamin E. Circulation. 2002;106:2543–9. doi: 10.1161/01.cir.0000038500.43292.d7. [DOI] [PubMed] [Google Scholar]
- 46.Sinzinger H, Lupattelli G, Chehne F, Oguogho A, Furberg CD. Isoprostane 8-epi-PGF2alpha is frequently increased in patients with muscle pain and/or CK-elevation after HMG-Co-enzyme-A-reductase inhibitor therapy. J Clin Pharm Ther. 2001;26:303–10. doi: 10.1046/j.1365-2710.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 47.Staff AC, Ranheim T, Halvorsen B. Augmented PLA2 activity in pre-eclamptic decidual tissue – a key player in the pathophysiology of ‘acute atherosis’ in pre-eclampsia? Placenta. 2003;24:965–73. doi: 10.1016/s0143-4004(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 48.Oguogho A, Mehrabi M, Sinzinger H. Increased plasma, serum and urinary 8-epi-prostaglandin F2 alpha in heterozygous hypercholesterolemia. Wien Klin Wochenschr. 1999;111:113–8. [PubMed] [Google Scholar]
- 49.Schwedhelm E, Böger RH. Application of gas chromatography-mass spectrometry for analysis of isoprostanes: their role in cardiovascular disease. Clin Chem Lab Med. 2003;41:1552–61. doi: 10.1515/CCLM.2003.238. [DOI] [PubMed] [Google Scholar]
- 50.Troost R, Schwedhelm E, Rojczyk S, Tsikas D, Frölich JC. Nebivolol decreases systemic oxidative stress in healthy volunteers. Br J Clin Pharmacol. 2000;50:377–9. doi: 10.1046/j.1365-2125.2000.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Donoghue M, Morrow DA, Sabatine MS, Murphy SA, McCabe CH, Cannon CP, Braunwald E. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation. 2006;113:1745–52. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 52.Iribarren C, Gross MD, Darbinian JA, Jacobs DR, Jr, Sidney S, Loria CM. Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: the CARDIA study. Arterioscler Thromb Vasc Biol. 2005;25:216–21. doi: 10.1161/01.ATV.0000148322.89911.44. [DOI] [PubMed] [Google Scholar]