Abstract
Aims
To determine whether a particular anticonvulsant is more effective or safer than another or placebo in patients with status epilepticus, and to summarize the available evidence from randomized controlled trials, and to highlight areas for future research in status epilepticus.
Methods
Randomized controlled trials of participants with premonitory, early, established or refractory status epilepticus using a truly random or quasi-random allocation of treatments were included.
Results
Eleven studies with 2017 participants met the inclusion criteria. Lorazepam was better than diazepam for reducing risk of seizure continuation [relative risk (RR) 0.64, 95% confidence interval (CI) 0.45, 0.90] and of requirement of a different drug or general anaesthesia (RR 0.63, 95% CI 0.45, 0.88) with no statistically significant difference in the risk of adverse effects. Lorazepam was better than phenytoin for risk of seizure continuation (RR 0.62, 95% CI 0.45, 0.86). Diazepam 30 mg intrarectal gel was better than 20 mg in premonitory status epilepticus for the risk of seizure continuation (RR 0.39, 95% CI 0.18, 0.86).
Conclusions
Lorazepam is better than diazepam or phenytoin alone for cessation of seizures and carries a lower risk of continuation of status epilepticus requiring a different drug or general anaesthesia. Both lorazepam and diazepam are better than placebo for the same outcomes. In the treatment of premonitory seizures, diazepam 30 mg intrarectal gel is better than 20 mg for cessation of seizures without a statistically significant increase in adverse effects. Universally accepted definitions of premonitory, early, established and refractory status epilepticus are required.
Keywords: anticonvulsant therapy, management, meta-analysis, status epilepticus
Introduction
Status epilepticus is defined as a condition in which there is either >30 min of continuous seizure activity, or two or more sequential seizures without recovery of full consciousness between the seizures. Status epilepticus is a medical emergency and is associated with an overall mortality of 8% in children and 30% in adults [1]. About 5–10% of people develop permanent vegetative state or cognitive difficulties. Approximately 12–30% of adults with a new diagnosis of epilepsy present with status epilepticus [2]. Status epilepticus may be convulsive (with limb stiffness and jerking) or nonconvulsive (without limb stiffness and jerking). Though convulsive status epilepticus is associated with a higher mortality and morbidity than nonconvulsive status epilepticus, both require prompt and effective treatment. However,the most effective treatment regimen is not clear from the literature. We conducted a systematic review of all the randomized controlled trials that could be identified to summarize the existing evidence and to highlight areas requiring further research.
In this review we followed Shorvon's classification of status epilepticus, which divides it into early, established and refractory stages [3]. Early status epilepticus consists of the first 30 min of the epileptic state, during which physiological mechanisms compensate for the greatly enhanced metabolic activity. Established status epilepticus is defined as the stage beyond 30 min, where the status continues despite early-stage treatment. It is during this phase that physiological compensation mechanisms begin to fail. If seizures continue for 60–90 min after the initiation of therapy, it is the stage of refractory status. We included trials that recruited people with status epilepticus as well as those that recuited people experiencing a cluster of seizures or a prolonged seizure.
The primary objective of the review was to synthesize the available evidence from randomized controlled trials (RCTs): (i) to determine whether a particular anticonvulsant is more effective or safer in controlling status epilepticus compared with another drug or placebo, and (ii) to highlight areas for future research.
Methods
RCTs using a truly random or quasi-random allocation of treatment were included in this review if they included people with premonitory (cluster of seizures or a prolonged seizure), early, established or refractory status epilepticus. Both convulsive and nonconvulsive status epilepticus were considered. Studies comparing any anticonvulsant drug against placebo or another anticonvulsant drug were included. Our intention was to carry out separate analyses for premonitory stage, early status epilepticus, established and refractory status epilepticus. However, the definitions used in the different studies were both variable and often unclear, which precluded stage-specific analysis.
For published trials the following electronic databases were searched:
Cochrane Epilepsy Group Specialized Register (July 2005).
Cochrane Central Database of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2005).
MEDLINE (1966 to August 2004) (using the highly sensitive search strategy for identifying RCTs [4].
EMBASE (1966 to January 2003).
The search terms used included the following text words: status, epilepticus, anticonvulsant therapy and names of the drugs in combination with any of the above words. The outcome terms were also combined with ‘status’ for searching. All resulting titles and abstracts were scanned and any relevant articles were followed up.
Two review authors independently selected the trials to be included in the review. Any disagreements were resolved by seeking an independent opinion of the third review author. Two review authors assessed the methodological quality of each trial. The trials comparing the same drugs were combined, whereas those comparing different drugs were analysed separately.
RR (relative risk) or RD (risk difference) reductions were calculated by means of the statistical software provided by the Cochrane Collaboration (RevMan version 4.2.7). We tested for heterogeneity between trial results for each outcome using χ2 test. If the test for heterogeneity was statistically nonsignificant, then the results from the different trials were combined to obtain a summary estimate of effect [and the corresponding confidence interval (CI)] using a fixed-effect model. We preferred RR for our analyses, but for some outcomes there were zero events in all the arms of some studies. In such situations RD was used to ensure inclusion of the data in the meta-analysis.
Results
Eleven studies had 2017 participants. Of the 11 studies included in this review, five studied participants with premonitory status [5–9], one established [10], one refractory [11] and two mixed status epilepticus [12, 13]. Two studies did not clearly define the status [14, 15]. Seven studies included only adults [5, 6, 8–10, 12, 13] and four only children [7, 11, 14, 15]. The type of status epilepticus included varied from study to study: four generalized tonic-clonic [5, 9, 10, 14] and four mixed [6, 11–13]. Three studies [7, 8, 15] did not describe the type of status epilepticus.
All studies except three (two intrarectal and one intramuscular midazolam in one arm) used intravenous administration of drugs. Fourteen different comparisons were available, but only three (lorazepam vs. diazepam, both administered intravenously; diazepam plus phenytoin vs. phenobarbital, administered intravenously; diazepam intrarectal gel vs. placebo gel) included more than one study to permit a meta-analysis. The remaining 11 comparisons had only one study.
All participants were followed up only during their hospital stay. No study had postdischarge follow-up. All studies had cessation of status epilepticus and adverse effects as outcomes. Death was an outcome in five comparisons. Other outcomes studied were requirement for ventilatory support (seven comparisons) and continuation of status epilepticus requiring another drug or general anaesthesia (five comparisons). Five studies used similar-looking placebo or comparison drugs. Use of placebo in random sequence with the drug conceals the randomization. In addition, two studies used sealed envelopes to conceal allocation in the randomization process, but whether the envelopes were opaque and serially numbered was unclear from the study reports. The remaining studies did not mention any attempt to conceal randomization. Studies with similar-looking placebo or comparison drug were assumed to be blinded, but six studies did not have blinding of carers or outcome assessors.
Eleven studies included in this review had 2017 study participants. Data extraction was difficult because of heterogeneity in the definition of status epilepticus and the type of data presented. We sought studies with the same types of interventions to combine in a meta-analysis, but such studies were few. We could combine data from seven studies over eight different outcomes. Even here, the definitions used by different authors varied and we assumed that the type of participants were similar. We present the remaining studies separately.
The results are presented according to the comparisons used (Tables 1 and 2).
Table 1.
Summary of comparisons
| Comparison or outcome | Studies | Participants | Statistical method used (fixed model) | Effect size, 95% CI | Statistically nonsignificant trend favouring |
|---|---|---|---|---|---|
| Lorazepam i.v. vs. diazepam i.v. [5,12,14] | |||||
| 01 Risk of seizure continuation | 3 | 264 | RR | 0.64 (0.45, 0.90) | |
| 02 Requirement for ventilatory support | 3 | 264 | RR | 0.73 (0.36, 1.49) | Lorazepam |
| 03 Adverse effects | 3 | 264 | RD | −0.03 (−0.10, 0.03) | Lorazepam |
| 04 Continuation of status requiring a different drug or general anaesthesia | 3 | 264 | RR | 0.63 (0.45, 0.88) | |
| 05 Death | 2 | 203 | RD | 0.02 (−0.04, 0.08) | |
| Lorazepam i.v. vs. placebo i.v. [5] | |||||
| 01 Risk of seizure continuation | 1 | 137 | RR | 0.52 (0.38, 0.71) | |
| 02 Requirement for ventilatory support | 1 | 137 | RR | 0.47 (0.21, 1.07) | Lorazepam |
| 03 Adverse effects | 1 | 137 | RR | 0.47 (0.21, 1.07) | Lorazepam |
| 04 Continuation of status requiring a different drug or general anaesthesia | 1 | 137 | RR | 0.52 (0.38, 0.71) | |
| 05 Death | 1 | 137 | RR | 0.49 (0.18, 1.33) | Lorazepam |
| Lorazepam i.v. vs. diazepam plus phenytal I.v. [9] | |||||
| 01 Risk of seizure continuation | 1 | 192 | RR | 0.79 (0.56, 1.13) | Lorazepam |
| 02 Adverse effects | 1 | 192 | RR | 0.86 (0.63, 1.16) | Lorazepam |
| Lorazepam i.v. vs. phenobarbital i.v. [9] | |||||
| 01 Risk of seizure continuation | 1 | 188 | RR | 0.84 (0.58, 1.21) | |
| 02 Adverse effects | 1 | 188 | RR | 0.86 (0.63, 1.16) | |
| Lorazepam i.v. vs. phenytoin i.v. [9] | |||||
| 01 Risk of seizure continuation | 1 | 198 | RR | 0.62 (0.45, 0.86) | |
| 02 Adverse effects | 1 | 198 | RR | 0.99 (0.72, 1.37) | |
| Midazolam i.v. vs. lorazepam i.v. [15] | |||||
| 01 Risk of seizure continuation | 1 | 27 | RR | 0.20 (0.03, 1.56) | Midazolam |
| 02 Requirement for ventilatory support | 1 | 27 | RR | 0.40 (0.04, 3.90) | Midazolam |
| 03 Respiratory depression | 1 | 27 | RR | 0.40 (0.04, 3.90) | Midazolam |
| 04 Continuation of status requiring a different drug or general anaesthesia | 1 | 27 | RR | 0.20 (0.03, 1.56) | Midazolam |
| Midazolam i.v. vs. diazepam i.v. [11] | |||||
| 01 Risk of seizure continuation | 1 | 40 | RR | 1.36 (0.25, 7.27) | |
| 02 Requirement for ventilatory support | 1 | 40 | RR | 1.11 (0.59, 2.07) | |
| 03 Adverse effects | 1 | 40 | RR | 0.80 (0.39, 1.66) | |
| 04 Death | 1 | 40 | RR | 3.62 (0.87, 14.97) | Diazepam |
Table 2.
Summary of comparisons (continued)
| Comparison or outcome | Studies | Participants | Statistical method used (fixed model) | Effect size, 95% CI | Statistically nonsignificant trend favouring |
|---|---|---|---|---|---|
| Midazolam i.m. vs. diazepam i.v. [7] | |||||
| 01 Risk of seizure continuation | 1 | 24 | RR | 0.85 (0.06, 12.01) | |
| 02 Requirement for ventilatory support | 1 | 24 | RR | 0.85 (0.06, 12.01) | |
| 03 Adverse effects | 1 | 24 | RR | 0.85 (0.06, 12.01) | |
| 04 Continuation of status requiring a different drug or general anaesthesia | 1 | 24 | RR | 0.85 (0.06, 12.01) | |
| Diazepam i.v. vs. placebo i.v. [5] | |||||
| 01 Risk of seizure continuation | 1 | 139 | RR | 0.73 (0.57, 0.92) | |
| 02 Requirement for ventilatory support | 1 | 139 | RR | 0.39 (0.16, 0.94) | |
| 03 Adverse effects | 1 | 139 | RR | 0.46 (0.20, 1.04) | Diazepam |
| 04 Continuation of status requiring a different drug or general anaesthesia | 1 | 139 | RR | 0.73 (0.57, 0.92) | |
| 05 Death | 1 | 139 | RR | 0.28 (0.08, 0.98) | |
| Diazepam gel vs. placebo gel (rectal) [6, 8] | |||||
| 01 Risk of seizure continuation | 2 | 165 | RR | 0.43 (0.30, 0.62) | |
| 02 Adverse effects | 2 | 165 | RR | 1.50 (0.94, 2.37) | Placebo gel |
| Diazepam 30 mg rectal vs. diazepam 20 mg rectal [13] | |||||
| 01 Risk of seizure continuation | 1 | 39 | RR | 0.39 (0.18, 0.86) | |
| 02 Sedation | 1 | 39 | RR | 0.90 (0.53, 1.53) | |
| Diazepam plus phenytoin i.v. vs. phenobarbital i.v. [9, 10] | |||||
| 01 Risk of seizure continuation | 1 | 36 | RR | 4.00 (0.98, 16.30) | Phenobarbital |
| 02 Requirement for ventilatory support | 1 | 36 | RR | 1.00 (0.40, 2.52) | |
| 03 Adverse effects | 2 | 222 | RR | 1.00 (0.77, 1.30) | |
| 04 Death | 1 | 36 | RD | 0.00 (−0.10, 0.10) | |
| Diazepam plus phenytoin i.v. vs. phenobarbital i.v. (premonitory status) [9] | |||||
| 01 Risk of seizure continuation | 1 | 186 | RR | 1.06 (0.76, 1.47) | |
| Diazepam plus phenytoin i.v. vs. phenytoin i.v. (9) | |||||
| 01 Risk of seizure continuation | 1 | 196 | RR | 0.78 (0.59, 1.04) | Diazepam plus phenytoin |
| 02 Adverse effects | 1 | 196 | RR | 1.16 (0.86, 1.56) | |
| Phenobarbital i.v. vs. phenytoin i.v. [9] | |||||
| 01 Risk of seizure continuation | 1 | 186 | RR | 0.78 (0.57, 1.06) | Phenobarbital |
| 02 Adverse effects | 1 | 186 | RR | 1.09 (0.81, 1.47) | |
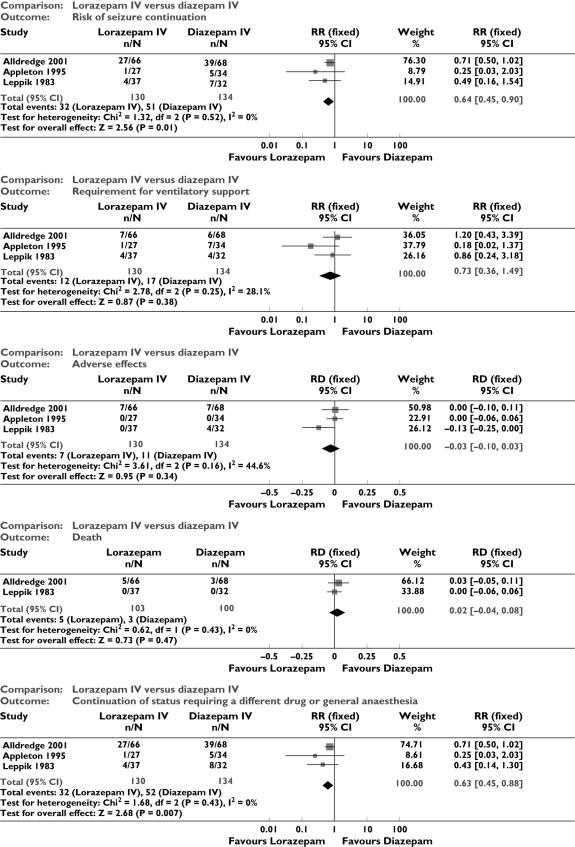
Lorazepam IV vs. diazepam IV (Figure 1)
Figure 1.
Lorazepam vs. diazepam intravenous: outcomes
There were three studies with 289 participants [5, 12, 14]. Data were available for 264 patients and outcome of death was available in two studies ([5, 12]; for 203 participants). There was no statistically significant difference in deaths between the two groups (5/103 vs. 3/100 participants; RD 0.02; 95% CI −0.04, 0.08). Compared with diazepam, lorazepam had a statistically significant lower risk of seizure continuation (32/130 vs. 51/134 participants; RR 0.64, 95% CI 0.45, 0.90) and of continuation of status epilepticus requiring a different drug or general anaesthesia (32/130 participants vs. 52/134; RR 0.63, 95% CI 0.45, 0.88). There was a statistically nonsignificant trend favouring lorazepam for reducing requirement for ventilatory support (12/130 vs. 17/134 participants; RR 0.73; 95% CI 0.36, 1.49) and adverse effects (7/130 vs. 11/134 participants; RD −0.03, 95% CI −0.10, 0.03).
Diazepam gel vs. placebo gel
There were two studies with a total of 165 participants [6, 8]. The risk of seizure continuation was significantly less with diazepam gel compared with placebo gel (24/77 vs. 63/88 participants; RR 0.43, 95% CI 0.30, 0.62). For adverse effects there was a strong but statistically nonsignificant trend towards the placebo gel (29/77 vs. 22/88 participants; RR 1.50, 95% CI 0.94, 2.37).
Diazepam plus phenytoin i.v. vs. phenobarbital i.v
There were two studies with a total of 222 participants [9, 10]. For the outcomes of death and requirement for ventilatory support, data were available in only one study (36 participants). There was no statistically significant difference between the two groups for the following outcomes: requirement for ventilatory support (6/18 vs. 6/18 participants; RR 1.00, 95% CI 0.40, 2.52); adverse effects (57/113 vs. 55/109 participants; RR 1.00, 95% CI 0.77, 1.30) and death (0/18 vs. 0/18 participants; RD 0.00, 95% CI −0.10, 0.10). For risk of seizure continuation, the test for heterogeneity was significant and the type of status epilepticus studied was different, hence the two studies were analysed separately for this outcome. There was a weak statistically nonsignificant trend favouring phenobarbital in one of the studies [10] (8/18 vs. 2/18 participants; RR 4.00, 95% CI 0.98, 16.30). In the other larger study [9], there was no statistically significant difference between the two groups for risk of seizure continuation (42/95 vs. 38/91 participants; RR 1.06, 95% CI 0.76, 1.47).
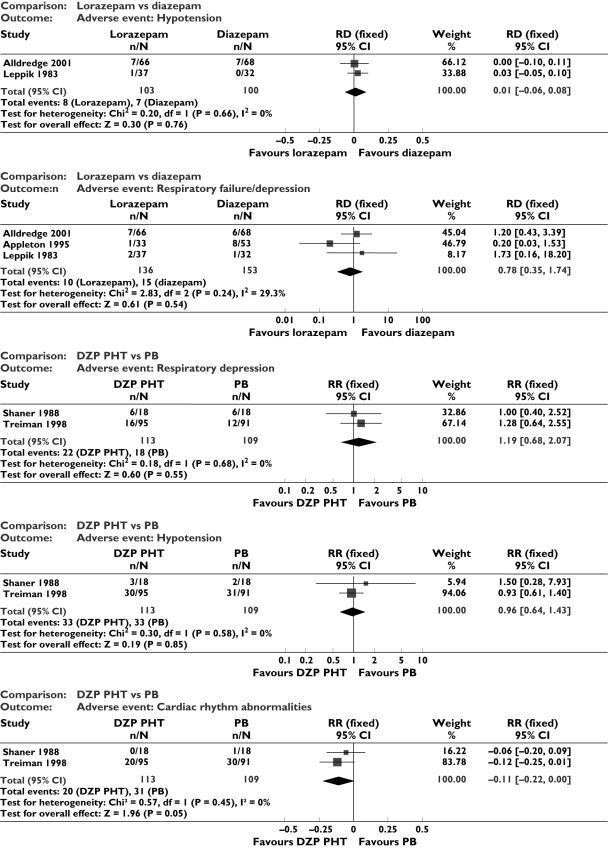
Adverse events (Figure 2)
Figure 2.
Adverse events
For the comparison lorazepam vs. diazepam, three studies [5, 12, 14] could be combined. There was no statistically significant difference between the two drugs for respiratory failure/depression (RR 0.78, 95% CI 0.35, 1.74), or hypotension (RD 0.01, 95% CI −0.06, 0.08). We were able to combine two studies [9, 10] for the comparison diazepam + phenytoin vs. phenobarbital. There was no statistically significant difference between the two interventions for the following adverse events: respiratory depression (RR 1.19, 95% CI 0.68, 2.07); hypotension (RR 0.96, 95% CI 0.64, 1.43) and cardiac rhythm abnormalities (RD −0.11, 95% CI −0.22, 0). The other studies did not have similarity of interventions to allow meaningful meta-analysis. In the study by Singhi et al. [11] comparing midazolam with diazepam, intubation was required in 13/21 with midazolam and 16/19 in diazepam; hypotension was observed in 8/21 in midazolam and 9/19 in diazepam. In the study by Remy [13], the side-effect of sedation and in the study by McCormick [15] the adverse effect of respiratory depression alone were described; data regarding this are shown in Tables 1 and 2. Two studies [6, 8] did not give separate figures for different adverse events (i.e. the heading adverse events included all of them together).
Discussion
Our review demonstrates that there are few reported randomized studies on drugs used in status epilepticus. This is evident from the fact that a search of Medline with the key phrase ‘status epilepticus’ restricted to the last 5 years yielded hundreds of reviews but only a few RCTs. The results are likely to be similar with EMBASE or any other database. It is unlikely that we have missed any randomized trial, because we attempted quite a comprehensive search, including databases such as the Cochrane library, EMBASE and Medline. We speculate that the reason lies in the fact that conducting RCTs in an emergency situation is difficult, particularly when the patient is unconscious, which makes gaining rapid consent to join a trial difficult. The difficulty is not insurmountable, because trials in similar conditions such as stroke and meningitis are being reported in increasing numbers. This review highlights the need to conduct more randomized studies in status epilepticus. Other experts have also noted a lack of RCTs addressing treatment issues in status epilepticus [16, 17].
Even with limited data, we were able to conclude the following: (i) diazepam is better than placebo for cessation of seizures: there is a lower risk of requirement for ventilatory support and continuation of status epilepticus requiring a different drug or general anaesthesia with diazepam; (ii) lorazepam is better than placebo for cessation of seizures and carries a lower risk of continuation of status epilepticus requiring a different drug or general anaesthesia; (iii) lorazepam is better than diazepam for cessation of seizures and has a lower risk of continuation of status epilepticus requiring a different drug or general anaesthesia; (iv) lorazepam is better than phenytoin for cessation of seizures; and (v) diazepam 30 mg intrarectal gel is better than 20 mg in premonitory status epilepticus for cessation of seizures without any statistically significant increase in adverse effects.
The above conclusions favour using lorazepam as the first-line drug in place of more commonly used diazepam. The pharmacokinetic properties of lorazepam also favour its use over diazepam. The anticonvulsant effect of a single dose of diazepam is approximately 20 min, whereas that of lorazepam is >6 h. The shorter duration of the anticonvulsant effect of diazepam in spite of its longer elimination half-life is attributed to its lipid solubility and rapid redistribution to peripheral fat stores. The analysis of adverse events suggests that lorazepam is as safe as diazepam, if not more so. None of the analyses of adverse events shows any significant difference among the various interventions.
This review has demonstrated several areas requiring attention in future research in status epilepticus. A universally acceptable definition of premonitory, early, established and refractory status needs to be agreed upon and used consistently by investigators. Agreement on the definition of outcomes and method of data presentation is also desirable to facilitate meta-analysis. In particular, reports should provide the number of participants having each outcome and the denominator in analyses should be the number of participants rather than the number of episodes of status epilepticus.
Acknowledgments
Competing interests: None declared.
This paper is based on a Cochrane Review published in The Cochrane Library (Prasad K, Al-Roomi K, Krishnan PR, Sequeira R. Anticonvulsant therapy for status epilepticus. Cochrane Database Systematic Reviews 2005; 4: CD003723). Cochrane Reviews are regularly updated as new evidence emerges and in response to feedback, and The Cochrane Library should be consulted for the most recent version of the review. The full text, data tables, results, analyses and reference lists of this article are available in the Cochrane Library.
References
- 1.Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA. 1993;270:854–9. [PubMed] [Google Scholar]
- 2.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 3.Shorvon S. Tonic-clonic status epilepticus. J Neurol Neurosurg Psychiatry. 1993;56:125–34. doi: 10.1136/jnnp.56.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefebvre C, Clarke MJ. Identifying randomised trials. In: Egger M, Smith GD, Altman D, editors. Systematic Reviews in Health Care. Meta-Analysis in Context. 2. London: BMJ Publishing Group; 2001. pp. 69–86. [Google Scholar]
- 5.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O'Neil N, Neuhaus JM, Segal MR, Lowestein DH. A comparison of lorazepam, diazepam and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 6.Cereghino JJ, Cloyd JC, Kuzniecky RI North American Diastat Study Group. Rectal diazepam gel for the treatment of acute repetitive seizures in adults. Arch Neurol. 2002;59:1915–20. doi: 10.1001/archneur.59.12.1915. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain JM, Altieri MA, Futtterman C, Young GM, Ochsenschlanger DW, Waisman Y. A prospective, randomised study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Ped Emerg Care. 1997;13:92–4. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Pellock JM, Mitchell WG, Cloyd JC. Diastat (diazepam rectal gel) in the treatment of acute repetitive seizures in adults. Epilepsia. 1998;39:126. [Google Scholar]
- 9.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, Handforth A, Faught E, Callabresi VP, Uthman BM, Ramsay RE, Mamdani MB. A comparison of four treatments for generalized convulsive status epilepticus. N Engl J Med. 1998;339:792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 10.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–7. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- 11.Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17:106–10. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 12.Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA. 1983;249:1452–4. [PubMed] [Google Scholar]
- 13.Remy C, Jourdil N, Villemain D, Favel P, Genton P. Intrarectal diazepam in epileptic adults. Epilepsia. 1992;33:353–8. doi: 10.1111/j.1528-1157.1992.tb02327.x. [DOI] [PubMed] [Google Scholar]
- 14.Appleton R, Sweeney A, Choonara I, Robson J, Molyneux E. Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol. 1995;37:682–8. doi: 10.1111/j.1469-8749.1995.tb15014.x. [DOI] [PubMed] [Google Scholar]
- 15.McCormick EM, Lieh-Iai M, Knazik S, Negro M. A prospective comparison of midazolam and lorazepam in the initial treatment of status epilepticus in the pediatric patient. Epilepsia. 1999;40:160. [Google Scholar]
- 16.Walker M. Status epilepticus: an evidence based guide. BMJ. 2005;331:673–7. doi: 10.1136/bmj.331.7518.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marik PE, Varon J. The management of status epilepticus. Chest. 2004;126:582–91. doi: 10.1378/chest.126.2.582. [DOI] [PubMed] [Google Scholar]