Abstract
Aims
To characterize the absorption kinetics and bioavailability of an inhaled hydrophilic solute deposited at various sites within the airways.
Methods
Nine healthy nonsmokers received one intravenous, one oropharyngeal and two pulmonary doses of technetium-99 m-labelled diethylene triamine pentaacetic acid (99mTc-DTPA) in an open and crossover fashion. Pulmonary doses were administered as nebulized large and fine droplet-sized aerosols by Pari and UltraVent nebulizers at fairly rapid and slow inhalation flows, respectively. Plasma concentration-time profiles and 24 h urinary excretion of radioactivity were determined. One dose of 99mTc-labelled Nanocoll, as a marker of mucociliary clearance (MCC), was also administered by Pari for similar lung deposition as the 99mTc-DTPA and followed by repeated chest γ-imaging.
Results
Intrapulmonary deposition patterns of 99mTc-DTPA differed significantly (the mean ratio of penetration index (Pari:UltraVent) was 76% with 95% CI 63%, 91%). However, no differences in rate or extent of 99mTc-DTPA absorption were detected. Mean absorption time was 1.8 h (mean difference (Pari–UltraVent): −0.1 h with 95% CI −0.6 h, 0.3 h) and the bioavailability was 70% (mean ratio (Pari:UltraVent): 101% with 95% CI 90%, 115%). The pulmonary elimination half-life of 99mTc-Nanocoll (8 h and 45 min) was significantly longer than that of 99mTc-DTPA (less than 2 h). The oral bioavailability of 99mTc-DTPA was estimated to be 3.1%.
Conclusions
The main elimination pathway of the inhaled hydrophilic solute 99mTc-DTPA from the lungs is trans-epithelial absorption. Despite different intrapulmonary radioaerosol deposition patterns, as verified by gamma scintigraphy, no differences in 99mTc-DTPA absorption kinetics or bioavailability were detected.
Keywords: aerosol, drug absorption, lung deposition, mucociliary clearance, pharmacokinetics, technetium-99 m
Introduction
In order to maximize absorption of inhaled, systemically acting pharmaceuticals, alveolar deposition is generally attempted. Selective alveolar deposition of therapeutic drug aerosols is never achieved in clinical practice and a portion of the inhaled dose is always deposited in the throat or larger airways. Thus, to characterize the pharmacokinetics of such drugs adequately, deposition outside the alveolar region should be considered.
Because the respiratory tract is recognized as a potential alternative route of administration for systemicallyacting drugs with poor oral bioavailability, a more widespread appreciation of pulmonary drug absorption has emerged [1]. Drug transfer across the alveolocapillary barriers has been widely studied, but there is less information on drug absorption in the tracheobronchial airways and throat.
Inhaled drug aerosols are removed from the lungs via one or both of two major pathways, namely the vascular route (involving trans-epithelial absorption into the blood) and the mucociliary route (involving mucociliary clearance along the surface of the airways). The predominant elimination pathway and the overall disappearance rate are likely to depend on the initial site of aerosol deposition within the lungs [2, 3].
Much existing knowledge on the rate and extent of pulmonary absorption of inhaled drug aerosols has been acquired using experimental pharmaceuticals. Previous studies have shown striking similarities in the absorption kinetics and bioavailability of inhaled technetium-99 m-labelled diethylene triamine pentaacetic acid (99mTc-DTPA, MW 492) and the hydrophilic drug terbutaline (MW 225) and insulin (MW 5786). For example, the fate of 99mTc-DTPA was found to vary with lung volume and breathing pattern [4–7]. The effect of lung expansion on the rate of 99mTc-DTPA pulmonary disappearance was similar to the increased rate of absorption of inhaled terbutaline observed during physical exercise in normal nonsmokers [8] as well as the increased rate of absorption of insulin delivered during deep as opposed to shallow inhalation [9]. Furthermore, the effect of smoking on the rate of 99mTc-DTPA pulmonary disappearance [10, 11] was similar to the increased rates of absorption of terbutaline and insulin observed in smokers compared with nonsmokers [12, 13].
Pulmonary absorption of 99mTc-DTPA can be determined using gamma scintigraphic imaging, provided the radiolabel is not removed from the lungs by a mechanism other than trans-epithelial transport [14]. In the alveolar tract, trans-epithelial absorption may be the only elimination pathway, but in the conducting airways the radiolabel is probably also removed by MCC. Thus, to estimate accurately the rate and extent of absorption of 99mTc-DTPA independently of the initial aerosol deposition site within the airways, a method involving the measurement of plasma radioactivity concentrations should be considered [15, 16]. Alternatively, to estimate the extent of absorption, a noninvasive method involving the measurement of urinary excretion of radioactivity could be used [17]. Any contribution to the systemically available radioactivity by the fraction of aerosol filtered off in the throat and swallowed should be negligible because the gastrointestinal absorption of 99mTc-DTPA is poor [15, 17–20].
The aim of this work was to compare the rate and extent of absorption of a hydrophilic solute in the oral cavity and the conducting airways and the alveolar tract of the lungs, preferentially.
Methods
Materials
The following materials were used: DTPA (TechneScan DTPA, Mallinckrodt Medical BV, Petten, the Netherlands), human serum albumin nanocolloidal particles (Solco Nanocoll Kit, Nycomed Amersham Sorin, Saluggia, Italy), molybdenum-99 (99Mo) technetium-99 m generator (Ultra-TechneKow FM, Mallincrodt Medical BV, Petten, the Netherlands), spray pump (VP7/100 S, Valois, Marly-le-Roi, France), Pari nebulizer (Pari LL, Pari GmbH, Starnberg, Germany), UltraVent nebulizer (UltraVent Radioaerosol Delivery System, Mallinckrodt Medical Inc, St. Louis, Missouri, USA), pulsing device (Spira Dosimeter Electro 2, Spira Respiratory Care Center Ltd, Hämeenlinna, Finland), silica gel coated glass fibre sheets (ITLC/SG, Gelman Siences, Ann Arbor, Michigan, USA), dose calibrator (Capintec CRC-15R, Capintec Inc, Ramsey, New Jersey, USA), gamma counter (Wallac 1480 Wizard 3″, Wallac OY, Turku, Finland), and gamma camera (Toshiba GCA-901A, Toshiba Medical Systems, Tochigi, Japan).
Subjects
This study was carried out in nine healthy, nonsmoking men with a mean age of 35 years (range: 24–57 years), a body weight of 82 kg (70–110 kg) and a height of 183 cm (174–192 cm). At inclusion, their mean forced expiratory volume in 1 s (FEV1) was 96% (75–118%) and vital capacity 96% (83–116%) of predicted normal values [21]. Subjects with recent symptoms of an upper or lower respiratory tract infection were excluded. The study was performed in accordance with the principles stated in the Declaration of Helsinki. Approvals were obtained from the Research Ethics Committee at the University of Lund/Malmö, Sweden, and the Radiation Protection Committee at Malmö University Hospital, Sweden. Subjects were informed about the purpose of the study and gave their written infomed consent before inclusion.
Dosing regimens
Each subject received a single dose of about 5 MBq 99mTc on five different occasions at intervals of at least 2 days. On day 1, an intravenous (i.v.) dose of 99mTc-DTPA was administered, followed by four aerosol doses in an open and crossover manner. The aerosol administrations consisted of one dose of 99mTc-DTPA delivered by a spray pump, one dose of 99mTc-DTPA delivered by a Pari nebulizer, one dose of 99mTc-DTPA delivered by an UltraVent nebulizer, and one dose of 99mTc-Nanocoll delivered by the Pari nebulizer. Subjects were studied in groups of three. Each group was randomly assigned to one of three different administration regimes. The isotope was administered from 08.00 h to 10.00 h, and the procedures were all managed and supervised by the same member of the study team. One subject did not comply with the instructions given and was withdrawn from the study after having received two doses (the i.v. dose of 99mTc-DTPA and the nebulized dose of 99mTc-Nanocoll, on separate occasions). The other subjects all completed the study.
Radiopharmaceuticals
On the morning prior to administration, commercially available kits of DTPA and human serum albumin nanocolloidal particles (Nanocoll, with at least 95% of particles having a diameter ≤80 nm) were labelled with the gamma ray emitting radionuclide 99mTc-sodium pertechnetate in physiological saline eluted from a 99Mo/99mTc generator. Procedures followed the instructions in the package inserts by the respective manufacturer. Depending on the mode of administration, 99mTc-DTPA solutions of various concentrations of radioactivity were prepared and administered. For i.v. administration, 1 ml of 99mTc-DTPA of about 5 MBq ml−1 (at the time of administration) was injected. The syringe was measured for radioactivity before and after dosing using a dose calibrator. For the oropharyngeal administration, 100 µl of 99mTc-DTPA of about 50 MBq ml−1 was administered as one single puff using a spray pump. For the pulmonary administrations, 2 ml of 99mTc-DTPA of about 500 MBq ml−1 (Pari) or 1000 MBq ml−1 (UltraVent) was added to the nebulizer. Likewise, 2 ml of a 99mTc-Nanocoll-suspension of about 1000 MBq ml−1 was added to the Pari nebulizer. While seated in an erect position in front of the gamma camera, subjects inhaled the nebulized aerosol until a count rate of about 350 s−1 over the chest was reached.
Quality control of radiopharmaceuticals
In accordance with recommendations given by the respective manufacturer, procedures used to label DTPA and Nanocoll with 99mTc were verified using silica gel coated glass fibre sheets. About 4 h after labelling of DTPA, 0.1% was present as reduced hydrolyzed technetium and 0.3% as free pertechnetate. About 4 h after labelling of Nanocoll, 1.0% was present as free pertechnetate.
Administration of radioaerosols
The oropharyngeal dose was administered after actuation of 10 priming doses from the lead-shielded spray pump. When the subject was holding his breath, a single puff of aerosol was sprayed onto the dorsal wall of the pharynx. The pulmonary doses were administered using the lead-shielded nebulizer driven by compressed medical air at a pressure of about 400 kPa. The nebulizer was connected in series to a pulsing device allowing aerosol to be delivered for 300 ms at the beginning of each inhalation (after an initial 100 ml of air had been inhaled). The UltraVent mouth piece was also used with the Pari nebulizer to minimize any contamination of ambient air with radioactivity. A visual indicator assisted subjects in maintaining an inhalation flow of about 0.8 l s−1 (Pari) or 0.5 l s−1 (UltraVent). In an attempt to compensate for the different nebulizer output rates obtained in experiments before the study, different concentrations of radioaerosol solutions/suspensions were used as described above. However, for the same target count rate to be reached, administration by UltraVent still required a larger number of inhalations than administration by Pari. For each dose, the mean inhalation flow and volume as well as the total number of inhalations triggering aerosol delivery were recorded (Table 1) and actual pulmonary doses (Table 2) assessed scintigraphically, as described below.
Table 1.
Mean (range) inhalation flow (l s−1), volume (l), and number of aerosol inhalations
| Radioaerosol | Nebulizer | Inhalation flow | Inhaled volume | Number of inhalations |
|---|---|---|---|---|
| 99mTc-DTPA | Pari | 0.8 (0.6–0.8) | 1.2 (0.5–1.8) | 6 (3–12) |
| 99mTc-DTPA | UltraVent | 0.5 (0.5–0.5) | 0.8 (0.3–1.5) | 23 (11–35) |
| 99mTc-Nanocoll | Pari | 0.8 (0.8–0.8) | 1.3 (0.6–2.0) | 4 (2–9) |
Table 2.
Mean (CV) penetration index (PI) and deposition of radioactivity in the lungs, trachea/oesophagus, and oral cavity immediately after nebulization and subsequent mouth rinsing
| Deposition of radioactivity | ||||||
|---|---|---|---|---|---|---|
| Radioaerosol | Nebulizer | PI | Lungs (MBq 99mTc) | Trachea/oesophagus (MBq 99mTc) | Oral cavity(MBq 99mTc) | Trachea/oesophagus andoral cavity (% of total) |
| 99mTc-DTPA | Pari | 1.8 (24%) | 7.5 (14%) | 0.4 (52%) | 0.4 (59%) | 10 |
| 99mTc-DTPA | UltraVent | 2.4 (11%) | 5.5 (26%) | 0.1 (38%) | < 0.1 n.a. | < 4 |
| 99mTc-Nanocoll | Pari | 1.7 (21%) | 12.9 (34%) | 0.5 (87%) | 0.7 (77%) | 9 |
n.a. = not applicable.
Immediately after nebulization, thorough mouth rinsing, repeated 4–6 times using a total of 250 ml of water, was performed to decrease aerosol deposition in the oral cavity, which could interfere with the scintigraphic measurements of the intrapulmonary aerosol deposition pattern.
At the end of the study, the amount of aerosol delivered by the spray pump was measured by weighing replicates of 1, 5, and 10 doses. The aerosol of 99mTc-DTPA generated by the Pari nebulizer was characterized in terms of mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). A five-stage multistage liquid impinger [22], refrigerated to prevent aerosol evaporation [23], was used. Assessments of droplet size distribution were based on dose to impinger [24]. The MMAD (GSD) of the aerosol of 99mTc-DTPA generated by the Pari was found to be 2.9 (2.3) µm.
Measurement of radioactivity after 99mTc-DTPA administration
After each dose of 99mTc-DTPA, the radioactivity in plasma and urine was measured. Venous blood was sampled before administration and at 5, 10, 20, 40, and 60 min and at 2, 3, 4, 5, and 7 h postdose. The radioactivity in 1 ml of plasma was monitored for 5 min using a gamma counter. Count errors ranged from 0.4% to 7.6%, from 1.2% to 25.8%, and from 0.5% to 2.6%, in samples taken after i.v., oropharyngeal, and pulmonary administration, respectively. Urine was collected for 24 h in three consecutive fractions: 0–6, 6–12, and 12–24 h post administration. For each fraction, the weight of urine was determined and the volume calculated using a density factor of 1.02. Radioactivity was measured in 1 ml during a period of 5 min using a gamma counter. The total number of counts collected per subject and administration resulted in count errors ranging from 0.2% to 1.4%. Radioactivity was corrected for background radiation and for the physical decay of 99mTc (t1/2 = 6.02 h) to the time of administration.
Pharmacokinetic parameters were computed using standard noncompartmental methods. The area under the plasma concentration vs. time curve, AUC, was calculated using the trapezoidal rule to the last measured plasma concentration and the remaining area was extrapolated to infinity, AUCextrapolated. The latter was calculated using the terminal elimination rate estimated for i.v. administration. The i.v. dose, Dosei.v., was determined by measuring the radioactivity in the syringe radioactivity before and after administration. The oropharyngeal dose, Doseoral, was determined from the radioactivity concentration of the radioaerosol solution administered and, assuming its density to be 1 g ml−1, the mean weight of solution delivered per actuation of the spray pump. The pulmonary dose, Doselung, was determined from transmission and aerosol emission scintigrams, as described below. The fraction of radioactivity excreted in urine, fe, was calculated as the amount excreted, Ae, relative to administered radioactivity (Dosei.v., Doseoral, or Doselung, as appropriate). Bioavailability was calculated both as the dose-normalized AUC ratio (FAUC) and as the fe ratio (Ffe) between a non i.v. and the i.v. route.
Measurement of radioactivity after 99mTc-DTPA and 99mTc-Nanocoll administration
For each pulmonary dose of 99mTc-DTPA or 99mTc-Nanocoll, chest and head deposition of radioactivity was measured using planar gamma scintigraphy. All images were acquired with the subject in the sitting position in front of a single-headed gamma camera fitted with a low-energy high resolution collimator and with a field of view 40 × 52 cm. Quality control of the gamma camera was performed in accordance with the manufacturer's recommendations. A 15% energy window was centred over the 140 keV photo peak. Gamma camera sensitivity was calibrated using a thin 99mTc source.
On one occasion, prior to administration of 99mTc-DTPA or 99mTc-Nanocoll, a transmission image was measured in the posterior view, using a flood source of 57Co. The transmission scintigram was used to correct for tissue attenuation of gamma rays using a previously described method [25], to provide lung contours of each subject, and to delineate regions of interest in the aerosol emission scintigrams.
Immediately after aerosol administration, anterior and posterior images of the chest and head were acquired for 2 min in each view. On average, the anterior chest image was acquired 2 min post aerosol administration and the posterior chest image 4 min later. Deposition of radioactivity in regions of interest (i.e. the oral cavity, the tracheal/oesophageal region, the central lung zone mainly containing the conducting airways, and the peripheral lung zone mainly containing the alveoli) was calculated as the geometric mean of anterior and posterior counts corrected for area-normalized background radioactivity (obtained using a region drawn over the shoulders), for tissue attenuation of gamma rays and for physical decay of 99mTc back to the time of aerosol administration. The intrapulmonary aerosol deposition pattern of radioactivity within the right lung was established as a penetration index (PI) defined as the ratio of peripheral to central lung zone deposition, calculated on the basis of a previously described method [26]. Total pulmonary deposition (Doselung) was calculated as radioactivity in the right lung multiplied by 1.9, as described previously [27].
For each pulmonary dose of 99mTc-DTPA or 99mTc-Nanocoll administered using the Pari nebulizer, the amount of radioactivity in the lungs was assessed hourly up to 4 h postdose to allow the pulmonary elimination half-life to be estimated. Thus, in addition to the images obtained immediately after aerosol administration, a posterior 2 min chest image was also acquired at 1, 2, 3, and 4 h post administration. Radioactivity-time curves were obtained for each lung. The amount of radioactivity in the whole lung and peripheral lung zone was calculated for each time-point as the posterior count corrected for the area-normalized background radioactivity, for tissue attenuation of gamma rays and for physical decay of 99mTc back to the time of aerosol administration.
Statistical analysis
Comparisons between administration modes were made using multiplicative or additive analysis of variance (anova) models with subject and mode of administration as fixed factors. A difference between two administration modes was expressed as a mean ratio or difference of estimates with 95% confidence intervals (CI) on the ratio or difference. Elimination half-lives were computed by weighted linear regression after converting the individual values to means for each scan time. Separate determinations were made for each mode of administration. Variables derived from data obtained from more than one administration mode were correlated using linear mixed effects modelling. Normal least square regression was used to correlate variables obtained within a mode of administration. All hypothesis testing was done using two-sided alternative hypotheses. A P value of less than 5% was considered statistically significant.
Results
The spray pump used delivered a mean (SD) of 100.1 (2.9) mg of 99mTc-DTPA solution per actuation. The mean (SD) concentration of radioactivity used in the spray pump was 54.6 (4.3) MBq ml−1. The oral bioavailability of 99mTc-DTPA was estimated to be 3.1% (Table 3). The estimate derived from the fraction of dose excreted in urine (Ffe) was considered more reliable than the one calculated from plasma data (FAUC) for the following reasons: plasma radioactivity was low after oropharyngeal administration, the urinary recovery of radioactivity after i.v. administration was complete and urine was collected for a longer period (24 h) than plasma (7 h).
Table 3.
Doses given and pharmacokinetic parameters for 99mTc-DTPA administered to the oropharynx or to the lung (Pari or UltraVent nebulizers), and comparisons calculated as the ratio (Pari:UltraVent) or difference (Pari − UltraVent)
| Oropharynx | Lung (Pari) | Lung (UltraVent) | Comparison (Pari:UltraVent) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Meana | Range | Meana | Range | Meana | Range | Ratio (%) | 95% CI | P value | |
| Dose (MBq) | 5.5 | (4.8–6.1) | 7.5 | (6.1–9.0) | 5.5 | (3.7–8.4) | 137 | (109, 171) | 0.013 |
| Cmax (kBq l−1)b | 15 | (2–57) | 163 | (92–371) | 93 | (55–153) | 175 | (106, 289) | 0.034 |
| Cmax/Dose ((kBq l−1) MBq−1) | 3 | (1–11) | 22 | (15–54) | 17 | (12–26) | 128 | (82, 200) | 0.236 |
| AUC (kBq l−1 h)b | 103 | (16–444) | 813 | (609–1237) | 551 | (377–823) | 147 | (112, 194) | 0.012 |
| FAUC (%) | 13 | (2–49) | 73 | (56–95) | 68 | (57–92) | 108 | (98, 119) | 0.110 |
| Ae (MBq)b | 0.2 | (0–0.5) | 5.0 | (4.2–5.9) | 3.6 | (2.4–5.2) | 138 | (107, 179) | 0.020 |
| Ffe (%) | 3.1 | (1–10) | 67 | (57–83) | 66 | (52–83) | 101 | (90, 115) | 0.815 |
| Difference | 95% CI | P value | |||||||
| tmax (h) | 2.9 | (2.0–4.0) | 1.2 | (0.1–2.0) | 1.8 | (1.0–4.0) | −0.6 | (−1.7, 0.5) | 0.229 |
| MAT (h) | 3.0 | (2.1–3.3) | 1.7 | (0.7–2.1) | 1.8 | (0.9–2.5) | −0.1 | (−0.6, 0.3) | 0.518 |
geometric mean except for tmax and MAT (arithmetic means),
not corrected for differences in dose.
MAT mean absorption time, calculated as MRTnon-i.v. – MRTi.v..
The amount of radioactivity (99mTc) found in the lungs, trachea/oesophagus, and oral cavity immediately after nebulization and the subsequent mouth rinsing is shown in Table 2. Using UltraVent, the target lung dose of 5 MBq was reasonably well achieved, but with Pari it was exceeded, probably because the target count rate (which was used as a surrogate measure of lung dose) had been set based on experience from clinical procedures involving the use of only UltraVent. Doses to the lung were calculated as the right lung deposition multiplied by 1.9. However, they could equally well have been derived from total deposition in the right and left lungs (data not shown), based on the small ( < 5%) difference in mean dose estimated by the two methods. Radioactivity in the tracheal/oesophageal and oral cavity regions did not exceed 10% (Pari) and 4% (UltraVent) of the total amount of radioactivity deposited.
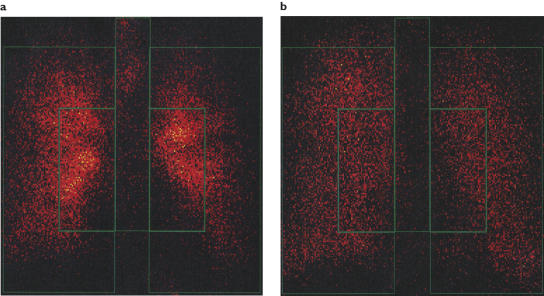
For nebulized 99mTc-DTPA, a more peripheral pulmonary deposition pattern was obtained after administration by UltraVent than when using Pari (Figure 1). The mean CV penetration index (PI) was 2.4 (11%) for UltraVent and 1.8 (24%) for Pari and the PI ratio (Pari:UltraVent) was 76% with a 95% CI of 63% to 91% (P = 0.005). For 99mTc-Nanocoll, also delivered by Pari, the PI was 1.7 (21%).
Figure 1.
Pulmonary deposition of radioactivity in a representative subject after administration of 99mTc-DTPA using Pari (a) or UltraVent (b) nebulizers
The pulmonary elimination half-lives of 99mTc-DTPA and 99mTc-Nanocoll delivered by the Pari nebulizer are shown in Table 4. There was a five-fold difference in half-life, measured in the whole lung region, between the two radioaerosols (525 min for 99mTc-Nanocoll and 104 min for 99mTc-DTPA). The difference in the half-lives measured in peripheral lung regions was increased to about six-fold (659 min for 99mTc-Nanocoll and 102 min for 99mTc-DTPA). The half-life of 99mTc-DTPA delivered by the UltraVent nebulizer was 109 min (whole lung) and 106 min (peripheral lung zone), values that were similar to those after Pari nebulization.
Table 4.
Mean elimination half-life in the whole organ and in the peripheral zone of each lung after administration of 99mTc-Nanocoll and 99mTc-DTPA, respectively, using the Pari nebulizer
| Elimination half-life (min) | |||||
|---|---|---|---|---|---|
| 99mTc-Nanocoll | 99mTc-DTPA | Difference | SEM | 95% CI | |
| Right lung, whole | 513 | 99 | 414 | 53 | (311, 517) |
| Left lung, whole | 537 | 108 | 429 | 49 | (332, 526) |
| Right lung, peripheral | 715 | 94 | 621 | 140 | (347, 894) |
| Left lung, peripheral | 603 | 111 | 492 | 83 | (329, 654) |
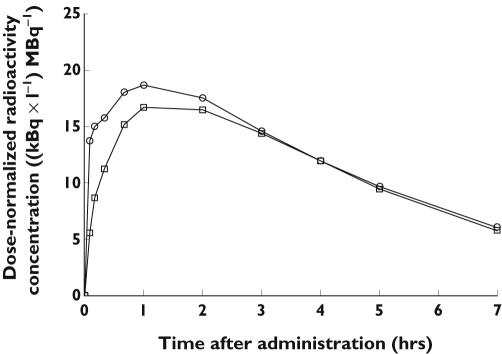
Pharmacokinetic parameters for 99mTc-DTPA are given in Tables 3 and 5, and plasma radioactivity vs. time curves for the pulmonary administrations of 99mTc-DTPA are shown in Figure 2. Vss found after i.v. administration was 17 l, indicating distribution in the extracellular fluid only. The mean AUCextrapolated was about 27% for the oropharyngeal and about 18% for each of the pulmonary administrations. A significantly larger (+47%) mean AUC was found with Pari than with UltraVent (P = 0.012). As suggested by the similar pulmonary bioavailability (FAUC) estimates of 73% and 68% of lung dose for Pari and UltraVent, respectively (P = 0.110), the observed difference in AUC mainly resulted from the difference in lung dose between nebulizers. By adjusting for dose, the differences in Cmax and Ae also disappeared. Neither tmax nor mean absorption time was found to differ between the two modes of pulmonary administration, but both parameters appeared to be longer after oropharyngeal than after pulmonary administration.
Table 5.
Dose given and pharmacokinetic parameters for intravenously administered 99mTc-DTPA
| Meana | Range | |
|---|---|---|
| Dosei.v. (MBq) | 5.2 | (4.9–5.6) |
| AUC (kBq l−1 h) | 762 | (561–902) |
| AUCextrapolated (%) | 8.1 | (3.0–16.4) |
| t1/2 (h) | 2.1 | (1.5–3.3) |
| CL (l h−1) | 6.8 | (5.6–9.0) |
| Vss (l) | 17 | (14–20) |
| MRT (h) | 2.5 | (1.7–3.6) |
| Ae (MBq) | 5.3 | (4.9–5.8) |
| fe (%) | 101.6 | (96.7–109.3) |
geometric mean except for AUCextrapolated and MRT (arithmetic means).
Vss volume of distribution under steady-state conditions, calculated as CL × MRTi.v.
MRT mean residence time, calculated as AUMC/AUC where AUMC is the area under the curve of first moment of the plasma concentration vs. time.
Figure 2.
Mean plasma concentration of dose-normalized radioactivity vs. time after pulmonary administration of 99mTc-DTPA nebulized using Pari (○) or UltraVent (□) nebulizers
The maximal contribution to the systemically available amount of radioactivity in the trachea/oesophagus and oral cavity was estimated from the oral bioavailability (Ffe) of 3.1%. It corresponded to less than 1% of the systemically available radioactivity.
Discussion
The different regions of healthy lungs can be targeted by altering one or more factors related to the characteristics of an aerosol (e.g. droplet size or bolus volume), or by altering the pattern of breathing during aerosol inhalation (e.g. inhalation flow, level of lung inflation during aerosol penetration into the respiratory tract, subsequent volume of air inhaled, exhalation flow, or breath holding time) [3, 28–30]. In the present study, a significant change in intrapulmonary deposition pattern was achieved by varying aerosol droplet size and the flow at which the subjects inhaled the aerosol. Thus a larger aerosol delivered by the Pari nebulizer inhaled fairly rapidly (at an inhalation flow of about 0.8 l min−1) targeted the central lung, and a smaller aerosol delivered by the UltraVent nebulizer inhaled more slowly (at 0.5 l min−1) targeted the peripheral lung. The different intrapulmonary deposition patterns were confirmed by gamma scintigraphy. Based on the PI data, it was concluded that pulmonary deposition was more peripheral after administration using UltraVent than when using Pari. In addition to this difference in intrapulmonary aerosol deposition patterns between nebulizers, the relative amount of radioactivity retained in the tracheal/oesophageal region was larger for the Pari than for the UltraVent nebulizer. This suggests that, although aerosol deposition patterns within the lungs overlapped, different regions of the lungs were targeted by the two modes of administration.
Irrespective of the intrapulmonary aerosol deposition pattern generated, a short MAT of about 1.8 h and a high F of about 70% were observed, which indicate that 99mTc-DTPA is rapidly and extensively absorbed across epithelia throughout the lower airways. Since 99mTc-DTPA is physiologically inert and distributes almost exclusively to extracellular fluid (confirmed by a value of 17 l obtained for the volume of distribution at steady state), a majority of the remaining 30% not systemically absorbed during 24 h is likely to be removed from the lungs by mucociliary transport. The pulmonary disappearance half-life of the MCC marker 99mTc-Nanocoll (8 h and 45 min) produced by the Pari nebulizer was significantly longer than that of 99mTc-DTPA (1 h and 44 min with the Pari and 1 h and 49 min with the UltraVent nebulizer). Thus, the MCC of 99mTc-DTPA should be less important than trans-epithelial absorption. The similarities in the effect of lung expansion or cigarette smoking on the absorption pharmacokinetics of inhaled 99mTc-DTPA, terbutaline, and insulin [4–13] suggest that hydrophilic substances are absorbed in a similar manner across epithelia throughout the lower airways.
After administration by the Pari nebulizer, 99mTc-DTPA disappeared from the lungs several times faster than 99mTc-Nanocoll. Any differences in DTPA and albumin, with respect to their mucus binding properties or their propensities for distribution in airway lining fluid, between the periciliary sol layer and the mucus layer, may have affected the ability of 99mTc-Nanocoll to reflect accurately the mucociliary transport of 99mTc-DTPA. To circumvent any such limitations of the methodology in their evaluation of the relative contribution of MCC to the pulmonary elimination of 99mTc-DTPA in central airways, Chinet and coworkers measured the change in background-corrected tracheal/oesophageal radioactivity after administration of 99mTc-DTPA [31]. Although central lung radioactivity decreased during the initial 10 min by approximately 13%, no increase in tracheal/oesophageal radioactivity was detected, which seemed to indicate that MCC does not significantly contribute to the pulmonary disappearance of 99mTc-DTPA. Values of 104 and 109 min obtained for the pulmonary elimination half-life of 99mTc-DTPA in our study were in agreement with those previously reported for healthy subjects (52–107 min) [2, 10, 15, 32–34]. Although the rate of pulmonary elimination of 99mTc-labelled human serum albumin in healthy subjects has been determined previously, differences in aerosol formulations and administration techniques prevent direct comparisons between studies. For instance, the 99mTc-Nanocoll pulmonary elimination half-life of 525 min obtained in our study with particulate human serum albumin was several times longer than the value of 80 min observed with human serum albumin in solution [2].
For an inhaled hydrophilic drug solute to reach the systemic circulation from the airway lumen, it has to pass through the epithelial lining fluid (ELF) and diffuse across the epithelium. Given the differences in ELF composition and thickness, epithelial cell layer and blood flow between the tracheobronchial and alveolar regions, drug absorption should vary depending on the initial site of aerosol deposition within the lungs. However, in the present study, in which no differences in 99mTc-DTPA absorption were detected between adminstration by the Pari and UltraVent nebulizers, 99mTc-DTPA appeared to be absorbed across epithelia throughout the lower airways.
The 24 h urinary recovery of 99mTc-DTPA after oropharyngeal administration (3.1%) was in agreement with values of 2.7%, 2.8% and 4.3% found by others [18–20]. The fraction excreted in urine should also reflect the oral bioavailability of 99mTc-DTPA because the compound is physiologically inert and eliminated completely via the kidneys [35], a finding confirmed by our i.v. study. The estimate of oral bioavailability derived from urine data was preferred to that from plasma data because the concentration of radioactivity in many of the plasma samples taken after oropharyngeal administration was low. Using the observed oral bioavailability (Ffe) of 3.1%, the amount of radioactivity in the oesophagus and oral cavity that could have contributed to the systemically available amount of nebulized 99mTc-DTPA was estimated to be less than 1%. Stomach radioactivity was not measured in the present study and, thus, any contribution from swallowed, absorbed radioactivity is unknown. However, the degree to which radioactivity in the oral cavity or gastrointestinal tract might have contributed to the systemic radioactivity absorbed from the lungs, was estimated, assuming a nebulizer delivery efficiency of 99mTc-DTPA as high as that of budesonide in a previous study [36]. In that study, a Pari nebulizer identical to the one in the present study and an aerosol administration technique aiming at maximal pulmonary delivery were used. About 36% of the dose delivered minus the amount of the drug lost due to exhalation and mouth rinsing reached the lungs. Assuming this value for lung deposition, no more than about 6% of radioactivity could have originated from the oral cavity and gastrointestinal tract.
In conclusion, our findings suggest that the main pathway of elimination from the lungs of the inhaled hydrophilic solute 99mTc-DTPA is trans-epithelial absorption. Despite different intrapulmonary radioaerosol deposition patterns, no differences in 99mTc-DTPA absorption kinetics or bioavailability were detected.
Acknowledgments
We acknowledge the Swedish Research Council and the Faculty of Medicine at Lund University for full financial support of this study and the biomedical laboratory technologists Birgit Andersson, Eva Jeremiasson, Anita Kvist, and Åsa Wickman for skilful contributions to its performance.
References
- 1.Tronde A. Dissertation presented at Uppsala University. Uppsala: Tryck & Medier; 2002. Pulmonary drug absorption. In vitro and in vivo investigations of drug absorption across the lung barrier and its relation to drug physicochemical properties. [Google Scholar]
- 2.Bennett WD, Ilowite JS. Dual pathway clearance of 99mTc-DTPA from the bronchial mucosa. Am Rev Respir Dis. 1989;139:1132–8. doi: 10.1164/ajrccm/139.5.1132. [DOI] [PubMed] [Google Scholar]
- 3.Groth S, Hermansen F, Rossing N. Pulmonary clearance of inhaled 99Tcm-DTPA: significance of site of aerosol deposition. Clin Physiol. 1990;10:85–98. doi: 10.1111/j.1475-097x.1990.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 4.Woolman PS, Jones DK, Barber RW, Higenbottam TW. The rapid reversibility of effects of changing lung volume on the clearance rate of inhaled 99Tcm-DTPA in man. Nucl Med Commun. 1987;8:881–7. doi: 10.1097/00006231-198711000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lorino AM, Meignan M, Bouissou P, Atlan G. Effects of sustained exercise on pulmonary clearance of aerosolized 99mTc-DTPA. J Appl Physiol. 1989;67:2055–9. doi: 10.1152/jappl.1989.67.5.2055. [DOI] [PubMed] [Google Scholar]
- 6.Evander E, Wollmer P, Jonson B. Pulmonary clearance of inhaled [99Tcm]DTPA. effects of ventilation pattern. Clin Physiol. 1990;10:189–99. doi: 10.1111/j.1475-097x.1990.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanel B, Law I, Mortensen J. Maximal rowing has an acute effect on the blood-gas barrier in elite athletes. J Appl Physiol. 2003;95:1076–82. doi: 10.1152/japplphysiol.00082.2002. [DOI] [PubMed] [Google Scholar]
- 8.Schmekel B, Borgström L, Wollmer P. Exercise increases the rate of pulmonary absorption of inhaled terbutaline. Chest. 1992;101:742–5. doi: 10.1378/chest.101.3.742. [DOI] [PubMed] [Google Scholar]
- 9.Farr SJ, McElduff A, Mather LE, Okikawa J, Ward ME, Gonda I, Licko V, Rubsamen RM. Pulmonary insulin administration using the AERx® system: Physiological and physicochemical factors influencing insulin effectiveness in healthy fasting subjects. Diabetes Technol Ther. 2000;2:185–97. doi: 10.1089/15209150050025131. [DOI] [PubMed] [Google Scholar]
- 10.Jones JG, Lawler P, Crawley JCW, Minty BD, Hulands G, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–8. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 11.Wollmer P, Evander E. Biphasic pulmonary clearance of 99mTc-DTPA in smokers. Clin Physiol. 1994;14:547–59. doi: 10.1111/j.1475-097x.1994.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmekel B, Borgström L, Wollmer P. Difference in pulmonary absorption of inhaled terbutaline in healthy smokers and non-smokers. Thorax. 1991;46:225–8. doi: 10.1136/thx.46.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelmann A, Jendle J, Mellén A, Petersen AH, Dahl UL, Wollmer P. The impact of smoking on inhaled insulin. Diabetes Care. 2003;26:677–82. doi: 10.2337/diacare.26.3.677. [DOI] [PubMed] [Google Scholar]
- 14.Dolovich MB, Jordana M, Newhouse MT. Methodologic considerations in mucociliary clearance and lung epithelial absorption measurements. Eur J Nucl Med. 1987;13:S45–S52. doi: 10.1007/BF00253291. [DOI] [PubMed] [Google Scholar]
- 15.Groth S, Lassen NA, Rossing N. Determination of the mean transit time for the transport of aerosolized 99mTc-DTPA across the pulmonary epithelial membrane. A plasma sample method. Clin Physiol. 1988;8:93–103. doi: 10.1111/j.1475-097x.1988.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 16.Groth S, Kristjansen PEG, Mortensen J, Merrild D. Pulmonary absorption of instilled and inhaled DTPA in smokers. Clin Physiol. 1990;10:231–43. doi: 10.1111/j.1475-097x.1990.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 17.Köhn H, König B, Klech H, Pohl W, Mostbeck A. Urine excretion of inhaled technetium-99m-DTPA: an alternative method to assess lung epithelial transport. J Nucl Med. 1990;31:441–9. [PubMed] [Google Scholar]
- 18.Casellas F, Aguadé S, Soriano B, Accarino A, Molero J, Guarner L. Intestinal permeability to 99mTc-diethylenetriaminopentaacetic acid in inflammatory bowel disease. Am J Gastroenterol. 1986;81:767–70. [PubMed] [Google Scholar]
- 19.Resnick RH, Royal H, Marshall W, Barron R, Werth T. Intestinal permeability in gastrointestinal disorders. Use of oral [99mTc]DTPA. Dig Dis Sci. 1990;35:205–11. doi: 10.1007/BF01536764. [DOI] [PubMed] [Google Scholar]
- 20.Ersöz G, Aydin A, Erdem S, Yüksel D, Akarca U, Kumanlioglu K. Intestinal permeability in liver cirrhosis. Eur J Gastroenterol Hepatol. 1999;11:409–12. doi: 10.1097/00042737-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH. Standardized lung function testing. Report of the Working Party for Standardisation of Lung Function Tests, European Community for Coal and Steel. Bull Eur Physiopathol Respir. 1983;19(Suppl 5):1–95. [PubMed] [Google Scholar]
- 22.European Pharmacopoeia. 4. pp. 209–19. Section 2.9.; 18.
- 23.Berg E, Asking L. Nebulizer droplet size distribution – refrigerated NGI at 15 L/min. In: Dalby RN, Byron PR, Joanne P, Suman JD, Farr SJ, editors. Respiratory Drug Delivery, IX. River Grove: Davis Healthcare International Publishing; 2004. pp. 361–3. [Google Scholar]
- 24.Berg E, Svensson JO, Asking L. MMAD based on dose to impactor rather than on delivered dose. In: Dalby RN, Byron PR, Joanne P, Farr SJ, editors. Respiratory Drug Delivery, VIII. Raleigh: Davis Horwood International Publishing; 2002. pp. 339–42. [Google Scholar]
- 25.Macey DJ, Marshall R. Absolute quantitation of radiotracer uptake in the lungs using a gamma camera. J Nucl Med. 1982;23:731–5. [PubMed] [Google Scholar]
- 26.Olséni L, Palmer J, Wollmer P. Quantitative evaluation of aerosol deposition pattern in the lung in patients with chronic bronchitis. Physiol Meas. 1994;15:41–8. doi: 10.1088/0967-3334/15/1/003. [DOI] [PubMed] [Google Scholar]
- 27.Bondesson E, Asking L, Borgström L, Nilsson L-E, Trofast E, Wollmer P. In vitro and in vivo aspects of quantifying intrapulmonary deposition of a dry powder radioaerosol. Int J Pharm. 2002;232:149–56. doi: 10.1016/s0378-5173(01)00898-5. [DOI] [PubMed] [Google Scholar]
- 28.Hansson L, Wollmer P, Dahlbäck M, Karlsson J-A. Regional sensitivity of human airways to capsaicin-induced cough. Am Rev Respir Dis. 1992;145:1191–5. doi: 10.1164/ajrccm/145.5.1191. [DOI] [PubMed] [Google Scholar]
- 29.Alexis NE, Hu S-C, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways. Confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164:1964–70. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 30.Bondesson E, Bengtsson T, Borgström L, Nilsson L-E, Norrgren K, Olsson B, Svensson M, Wollmer P. Dose delivery late in the breath can increase dry powder aerosol penetration into the lungs. J Aerosol Med. 2005;18:23–33. doi: 10.1089/jam.2005.18.23. [DOI] [PubMed] [Google Scholar]
- 31.Chinet T, Collignon M-A, Lemarchand P, Barritault L, Huchon G. Effects of smoking on bronchial clearance of technetium-99m-DTPA and indium-113m-DTPA. J Nucl Med. 1995;36:1569–72. [PubMed] [Google Scholar]
- 32.Royston D, Braude S, Nolop KB. Failure of aerosolised 99mTc DTPA clearance to predict outcome in patients with adult respiratory distress syndrome. Thorax. 1987;42:494–9. doi: 10.1136/thx.42.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monaghan P, Provan I, Murray C, Mackey DWJ, van der Wall H, Walker BM, Jones PD. An improved radionuclide technique for the detection of altered pulmonary permeability. J Nucl Med. 1991;32:1945–9. [PubMed] [Google Scholar]
- 34.Nilsson K, Evander E, Wollmer P. Pulmonary clearance of [99mTc]DTPA and [99mTc]albumin in smokers. Clin Physiol. 1997;17:183–92. doi: 10.1046/j.1365-2281.1997.02424.x. [DOI] [PubMed] [Google Scholar]
- 35.Klopper JF, Hauser W, Atkins HL, Eckelman WC, Richards P. Evaluation of 99mTc-DTPA for the measurement of glomerular filtration rate. J Nucl Med. 1972;13:107–10. [PubMed] [Google Scholar]
- 36.Dahlström K, Thorsson L, Larsson P, Nikander K. Systemic availability and lung deposition of budesonide via three different nebulizers in adults. Am Allergy Asthma Immunol. 2003;90:226–32. doi: 10.1016/S1081-1206(10)62146-1. [DOI] [PubMed] [Google Scholar]