Abstract
What is already known about this subject
There is a major gap between the use of statins in clinical trial settings and in actual practice.
Unfortunately, little is known about the impact of suboptimal use of statins on clinical outcomes.
What this study adds
Patients who filled more than 90% of the prescribed doses began to achieve significant reductions in nonfatal coronary artery disease events.
Statin effectiveness is apparent after one full year of treatment.
Aims
To evaluate the impact of adherence to statins on nonfatal coronary artery disease (CAD). Statins reduce cardiovascular morbidity and mortality after 1–2 years of continuous treatment. Studies have shown that < 40% of patients take ≥80% of prescribed doses 1 year after starting therapy and that approximately half discontinue medication within 6 months of starting therapy.
Methods
A cohort of 20 543 patients was reconstructed using the Régie de l'assurance maladie du Québec databases. Patients aged 50–64 years, without cardiovascular disease, and newly treated with statins between 1998 and 2000 were eligible. A nested case–control design was used to study nonfatal CAD. Every case was matched with 20 randomly selected controls. The adherence level was defined as the percentage of the prescribed medication doses used over a specified period and classified as ≥90% or < 90%. Rate ratios (RR) of nonfatal CAD were determined through conditional logistic regression adjusted for age, sex, socioeconomic status, diabetes and hypertension.
Results
The mean patient age was 58 years, 45% had hypertension and 19% had diabetes. Men represented 37% of the cohort. Among patients followed for >1 year, adherence of ≥90% was associated with fewer nonfatal CAD events (RR 0.81; 0.67, 0.97) compared with adherence < 90%. In the multivariate model, male gender (RR 1.37; 1.16, 1.63), welfare recipients (RR 1.24; 1.04, 1.48), newly diagnosed hypertension (RR 3.54; 2.62, 4.77) and newly diagnosed diabetes mellitus (RR 1.97; 1.20, 3.24) were risk factors for CAD.
Conclusion
The incidence of nonfatal CAD events decreases when >90% of the prescribed medications is used over at least 1 year.
Keywords: adherence to medical regimen, coronary artery disease, statins
Introduction
Cardiovascular diseases are responsible for the highest healthcare utilization costs in most industrialized countries [1]. In Canada, one person in five dies from coronary artery disease (CAD) [1]. Many risk factors are involved in the development of the disease but, according to observational studies, one of the strongest predictors is dyslipidaemia [2–4]. The lifetime risk of CAD is 1.5–2 times higher for people with elevated total cholesterol concentrations than for those with normal lipid concentrations [5]. In Canada, 45% of the population has higher than the recommended cholesterol concentration, making dyslipidaemia a major public-health concern [1].
Clinical trials have shown that decreased low-density lipoprotein cholesterol (LDL-C) levels resulting from statin therapy can reduce cardiovascular morbidity and mortality among patients with dyslipidaemia [6–15]. Clinical trials of primary prevention have shown that statins reduce the incidence of CAD by approximately 30% [9, 11]. Statins could reach their full therapeutic potential after 1 or 2 years of continuous treatment [16]. Despite this evidence, statins are not used optimally and the efficacy shown in randomized clinical trials may therefore be irrelevant in real life [17, 18].
We recognize that there is a major gap between the use of statins in clinical trial settings and in actual practice. Unfortunately, little is known about the impact of suboptimal use of statins on clinical outcomes. The aim of this study was to evaluate the impact of statin adherence on nonfatal CAD events among middle-aged patients free of cardiovascular disease.
Methods
Data sources
This population-based study used the databases of the Régie de l'assurance maladie du Québec (RAMQ), which administers public healthcare insurance programmes in Quebec, Canada. The RAMQ databases contain three types of files. The demographic file lists age, gender, post code and year of death for all registered individuals. The medical-services file comprises claims for all inpatient or ambulatory services and includes data such as the nature of the medical procedure, date and site (office, emergency room, hospital) of the procedure and the diagnostic code [19]. Diagnosis is coded by International Classification of Disease (ICD-9). Procedure codes follow the Canadian classification of diagnostic, therapeutic and surgical procedures [19, 20]. These codes are linked to the doctor's payment and are carefully audited [21, 22]. The pharmaceutical file contains data on all prescriptions for covered drugs prescribed to patients living in the community whose medications are insured by RAMQ. The file includes the name, dose and quantity of the drug; the date; and the duration of therapy as indicated by the pharmacist.
The first two databases include all residents covered by provincial health insurance, i.e. the entire population. The pharmaceutical file covers all residents insured under the public drug plan, comprising about 40% of the population aged from 50 to 64 years [23]. Each of the computerized files contains the individual's health insurance number, which serves as a link between them. The pharmaceutical file has been validated for research and used in pharmacoepidemiological research studies [24]. The prescription claims database in Quebec is also one of the most accurate validity means of determining drugs dispensed to individuals [24]. Validity studies have been performed specifically for the medical services claims of Quebec administrative databases. The validity of the diagnostic codes identifying episodes of hospitalizations for acute myocardial infarction was assessed among patients >65 years old [25]. Compared with the information in the patient hospital discharge summary, the sensitivity and specificity of the diagnostic code identifying the first hospital stay for acute myocardial infarction were 81.1% and 98.8%, respectively [25]. We enhanced the sensitivity of the RAMQ database for nonfatal CAD by adding procedural codes and medication to the diagnostic code. Procedural codes and medication reclamation are linked to the physician's and the pharmacist's payments and are carefully audited [21, 22].
Cohort study
From the RAMQ database, we selected a cohort of patients who started treatment with atorvastatin, fluvastatin, lovastatin, pravastatin or simvastatin treatment between 1 January 1998 and 31 December 2000, but had not taken lipid-lowering drugs in the year preceding entry into the cohort. The index date was defined as the date of the first prescription of a statin agent and was the date of entry into the cohort. Patients had to be between 50 and 64 years old at the index date and to have been insured for their drugs by RAMQ for at least 1 year before the index date.
To be eligible, subjects could not have any indication of cardiovascular disease as evidenced by the absence of a diagnosis and a medical procedure in the 3 years before the index date and the absence of a drug marker 1 year before the index date. Patients had to be free of any marker of cardiovascular disease such as (i) CAD: diagnosis of myocardial infarction or angina (ICD-9 codes 410–414), a medical procedure, i.e. coronary artery bypass grafting, angiography or angioplasty, use of nitrate, including nitroglycerin; (ii) stroke: diagnosis (430–438) and medical procedures; (iii) peripheral vascular disease: diagnosis (440–447), medical procedure of noncoronary angioplasty and use of pentoxifylline; (iv) congestive heart failure: diagnosis (428) or the use of furosemide alone or with digoxin, angiotensin converting enzyme (ACE) inhibitors, spironolactone or β-blockers; and (v) arrhythmia: diagnosis (427), a medical procedure using a pacemaker and the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone or sotalol). The RAMQ drug database was also used to exclude patients who received other drugs such as antiplatelets, low-dose aspirin (acetylsalicylic acid) or anticoagulants during the year preceding the index date.
The final study cohort included 20 543 subjects who were followed from the date of issuance of their first prescription of statin until the first nonfatal CAD event or the end of the study (30 June 2001). During the follow-up period, subjects were censured if they met an exclusion criterion, switched or had a prescription for another class of lipid-lowering drug, were no longer covered by the RAMQ drug-insurance plan, or died. Subjects were followed for a minimum of 6 months and a maximum of 3.5 years.
Nested case–control study
The nested case–control approach was used to estimate the rate ratio (RR) of the first nonfatal CAD event associated with adherence to statin agents. A nonfatal CAD event was defined by a composite end-point of nonfatal myocardial infarction or angina; a revascularization procedure, angioplasty, coronary artery bypass graft; or initiation of treatment with a nitrate drug. All cases of nonfatal CAD were identified and 20 controls were randomly selected from the risk set for each case using density sampling (i.e. among noncases who had at least the same follow-up time as the case) [26]. Accordingly, a subject might be selected as a control before being a case and might be selected as a control for more than one case.
Assessment of exposure
For each case and control, we reported the adherence defined as the percentage of the prescribed doses of medication actually taken by the patient over a specified period. Calculations were based on the quantity dispensed and the number of days supplied for each filled prescription. The patient's adherence was calculated from the start of follow-up to the time of a nonfatal CAD event; the control's cumulative adherence was calculated from the start of follow-up to the time of selection. The time of selection was determined by the addition of the case's follow-up time to the control's index date. The exposure was dichotomized into two levels: adherence to >90% of the prescribed doses and non-adherence to < 90%.
Confounding variables
The potential confounders for which it is possible to control were age, sex, socioeconomic status based on social-assistance status and cardiovascular risk factors (hypertension and diabetes). Age, sex and social-assistance status were identified at the entry into the cohort from data in the beneficiary's file of the RAMQ database. Hypertension and diabetes were time dependent and identified before the index date and during follow-up. Those comorbidities were defined as follows: diabetes by ICD-9 code 250 or by the use of insulin or an antidiabetic agent; and hypertension by essential hypertension ICD-9 code 401 or by the use of thiazides, ACE inhibitors without furosemide, calcium channel blockers or β-blockers without other markers of CAD. Patients with hypertension or diabetes diagnosed in the year preceding the date of a nonfatal CAD (for the patients) or the date of selection (for the controls) were considered newly diagnosed with hypertension or diabetes mellitus. For the other patients, the use of antihypertensive or antidiabetic agents in the year before the date of nonfatal CAD (for the cases) or the date of selection (for the controls) was dichotomized into two levels: adherence to >80% of the prescribed doses and non-adherence to < 80%.
Statistical analysis
The crude and adjusted RRs for nonfatal CAD events were determined through a conditional logistic regression. With the nested case–control approach, the exposure and covariate information for controls reflects values corresponding to the time of selection of their respective case [27]. The timescale used in the model was the time since the issuing of the first prescription of a statin.
To account for the possible effect of modification of time on the incidence of CAD, we stratified the analysis by the time of case presentation (in the first year of follow-up and after 1 year of follow-up). We carried out backward selection of variables to identify confounding variables to be retained in the final model [28].
To assess the robustness of our findings, we performed two sensitivity analyses. The first was done to assess the effect of different levels of adherence over the risk of nonfatal CAD. There were two categories of adherence and we used different cut-offs to compare adherence vs. non-adherence (≥70% of the prescribed doses vs. < 70% and ≥80% vs. < 80%).
The second sensitivity analysis assessed the robustness of our findings about potential biases introduced by unmeasured confounders. We used the approach proposed by Greenland which considered an unmeasured risk factor less frequent among those adhering to therapy than among those not adhering to therapy [29]. We created several scenarios with different risk factors between the confounder and nonfatal CAD. For each scenario, we changed the prevalence of the unmeasured confounder across adherence categories. Using this analysis, we determined how the RR changes after adjusting for the unmeasured confounder.
All analyses were performed using Statistical Analysis System Software (version 9; SAS Institute, Cary, NC, USA). All analyses with 95% confidence intervals (CI) are presented.
Ethical considerations
No patient or physician identifiers were provided to the researchers; only scrambled identifiers were used throughout the study. The Research and Ethics Committee of the University of Montreal approved the study.
Results
Patient characteristics
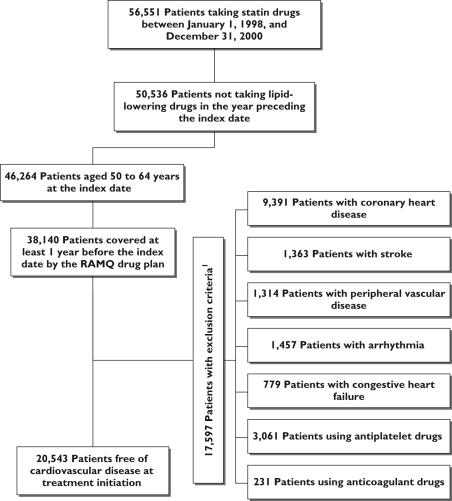
The distribution of exclusion criteria is shown in Figure 1. Of the 20 543 identified patients, 10 208 (50%) had filled a prescription for atorvastatin, 1254 (6%) for fluvastatin, 442 (2%) for lovastatin, 4850 (24%) for pravastatin and 3789 (18%) for simvastatin (Table 1). The mean age of the subjects was 58 years [standard deviation (SD) ±5). The mean follow-up was 1.6 years.
Figure 1.
Flow chart of inclusion and exclusion criteria. Exclusion criteria were assessed in the year preceding the index date for the medication and in the 3 years preceding the index date for diagnosis and medical procedures
Table 1.
Characteristics of patients starting a new statin treatment in the Régie de l'assurance maladie du Québec database in 1998–2000
| Entire cohort | Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Simvastatin | |
|---|---|---|---|---|---|---|
| No. patients | 20 543 | 10 208 | 1254 | 442 | 4850 | 3789 |
| Nonfatal CAD events (%) | 1 535 (7.5) | 647 (6.0) | 95 (7.6) | 44 (10) | 421 (8.7) | 328 (8.7) |
| Mean age (continuous)* | 58 (±5) | 58 (±5) | 58 (±5) | 58 (±5) | 58 (±5) | 58 (±5) |
| Mean dose | 18 (±11)† | 12 (±5) | 27 (±9) | 21 (±4) | 19 (±7) | 15 (±7) |
| Follow-up time (days) (continuous) | 586 (±322) | 510 (±278) | 688 (±349) | 700 (±367) | 660 (±345) | 649 (±340) |
| Sex (male vs. female) (%) | 37 | 39 | 33 | 37 | 35 | 36 |
| Social assistance (yes vs. no) (%)* | 29 | 31 | 33 | 33 | 26 | 26 |
| Diabetes mellitus (yes vs. no) (%)‡ | 19 | 20 | 16 | 14 | 17 | 20 |
| Hypertension (yes vs. no) (%)‡ | 45 | 45 | 48 | 41 | 45 | 46 |
| Dose distribution (mg) (%)* | ||||||
| 5 | 1 | 0 | 0 | 0 | 6 | |
| 10 | 79 | 0 | 6 | 19 | 52 | |
| 20 | 19 | 66 | 89 | 75 | 39 | |
| 40 | 1 | 34 | 5 | 6 | 3 | |
| 80 | 0 | 0 | 0 | 0 | 0 | |
At treatment initiation.
Statins equivalent to simvastatin dose during follow-up [46].Simvastatin 10 mg = lovastatin 20 mg = pravastatin 20 mg = fluvastatin 40 mg = atorvastatin 5 mg.
ICD-9 or pharmacological treatment before the index date.
A comparison of baseline demographic and clinical characteristics revealed no major differences across the statin groups (Table 1). Notable exceptions were that lovastatin users tended to have fewer comorbidities. Nevertheless, no pattern emerged of preferential prescribing of a particular statin to sicker or healthier patients. Regarding the distribution of daily doses, we found that in most cases lower doses of the statins were prescribed (10–20 mg) (Table 1), which are approximately equivalent in lowering LDL-C levels [30]. Very few subjects were prescribed the highest dose of each statin.
In the full cohort, 37% were men, 29% welfare recipients, 45% had hypertension and 19% had diabetes. The 37% proportion of men in the study was related to the exclusion criteria (e.g. men 56% and women 44%). Of the 20 543 individuals who fulfilled the inclusion and exclusion criteria, 1538 (7.5%) had a nonfatal CAD event. The percent of total death and death from coronary heart disease during follow-up was 0.09% and 1%, respectively.
A total of 5953 subjects were followed for < 1 year and 958 (4.7%) of them developed the main outcome measure. Of the 14 590 subjects followed for >1 year, 580 (4.0%) developed the outcome, yielding an incidence rate of two per 100 person-years. The prevalence of risk factors (male sex, diabetes and hypertension) was statistically higher among patients followed for < 1 year compared with those followed for >1 year (data not shown).
Characteristics of cases and controls
There were 32 298 cases and controls in the nested case–control which were divided into two groups: cases occurring in the first year of follow-up and their controls (n = 20 118) and cases occurring after 1 year of follow-up and their controls (n = 12 180) (Table 2). This time division was chosen based on data in the literature that suggest that statins are effective after a minimum of 1 year of use, particularly for older drugs. Table 2 shows the social and demographic characteristics of the cases and controls. The proportions of men, welfare recipients and patients with diabetes or hypertension were statistically higher among the cases. To assess if there was a difference between the statin doses received by patients during follow-up, we converted the doses of different statins into equivalent doses (Table 2). No matter what type of statin, every prescription was considered to be equivalent to simvastatin after the transformation. Based on literature data, the rule of conversion was that 10 mg of simvastatin is equivalent to 5 mg of atorvastatin, 40 mg of fluvastatin, 20 mg of lovastatin and 20 mg of pravastatin [31, 32]. Finally, the mean dose equivalent to simvastatin was 16 mg and was the same across the cases and controls (Table 2).
Table 2.
Characteristics of patients with nonfatal coronary artery disease event and matched controls
| Cases occurring in the first year of follow-up and their controls | Cases occurring after 1 year of follow-up and their controls | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | P-value* | Cases | Controls | P-value* | |
| Number | 958 | 19 160 | 580 | 11 600 | ||
| Age (years) (continuous)† | 58 (±5) | 58 (±5) | 0.11 | 58 (±5) | 58 (±5) | 0.21 |
| Mean equivalent dose‡ | 19 (±13) | 18 (±11) | 0.01 | 16 (±11) | 16 (±10) | 0.78 |
| Statin adherence (%)§ (yes vs. no) | ||||||
| < 90% | 35 | 36 | 0.41 | 67 | 63 | 0.06 |
| ≥90% | 65 | 64 | 33 | 37 | ||
| Sex (male vs. female) (%) | 43 | 37 | 0.001 | 41 | 35 | 0.004 |
| Social assistance (yes vs. no) (%)† | 34 | 29 | 0.003 | 38 | 33 | 0.01 |
| Diabetes (%) | 21 | 19 | 0.039 | 22 | 19 | 0.031 |
| Newly diagnosed diabetes mellitus¶ (yes vs. no) | 6 | 4 | 3 | 2 | ||
| Antidiabetic drug adherence < 80%** (yes vs. no) | 7 | 7 | 11 | 9 | ||
| Antidiabetic drug adherence ≥80%** (yes vs. no) | 8 | 8 | 9 | 9 | ||
| Hypertension (%) | 62 | 46 | < 0.0001 | 65 | 50 | < 0.0001 |
| Newly diagnosed hypertension¶ (yes vs. no) | 20 | 12 | 11 | 5 | ||
| Antihypertensive drug adherence < 80%** (yes vs. no) | 17 | 15 | 25 | 20 | ||
| Antihypertensive drug adherence ≥80%** (yes vs. no) | 25 | 20 | 29 | 26 | ||
The P-values are related to analyses made to compare cases with controls.
At treatment initiation.
Statins equivalence in simvastatin dose during the follow-up time [46]. Simvastatin 10 mg = lovastatin 20 mg = pravastatin 20 mg = fluvastatin 40 mg = atorvastatin 5 mg.
Proportion of days covered (%).
ICD-9 or pharmacological treatment; new diabetes or new hypertension were detected in the year before the index date.
Proportion of days covered (%) in the year before the index date.
Risk factors for CAD
Univariate analysis (Table 3) shows that sex, socioeconomic status and newly diagnosed diabetes or hypertension are risk factors for a nonfatal CAD event. In the multivariate model, male sex (RR 1.37; 1.16, 1.63), social-assistance status (RR 1.31; 1.10, 1.57), newly diagnosed diabetes (RR 1.97; 1.20, 3.24) and hypertension (RR 3.54; 2.62, 4.77) increased the risk of a nonfatal CAD event after 1 year of follow-up. The risk associated with these variables was the same in the full cohort and in the two subgroups defined by follow-up time. Among patients already diagnosed with diabetes, being adherent with more or less than 80% of their prescriptions did not significantly reduce the risk of nonfatal CAD compared with patients without diabetes. However, among patients already diagnosed with hypertension, adherence by more or less than 80% still presented an increasing risk of CAD compared with patients without hypertension; but these risks decreased significantly compared with newly diagnosed patients.
Table 3.
Rate ratio (RR) of nonfatal coronary artery disease event
| RR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Entire case–control | Cases occurring in the first year of follow-p and their controls | Cases occurring after 1 year of follow-up and their controls | ||||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| Atorvastatin | Reference | Reference | Reference | Reference | Reference | Reference |
| Fluvastatin | 0.92 | 0.91 (0.73, 1.14) | 0.76 | 0.75 (0.55, 1.02) | 1.16 | 1.13 (0.82, 1.57) |
| Lovastatin | 1.09 | 1.13 (0.81, 1.57) | 1.34 | 1.40 (0.93, 2.11) | 0.78 | 0.81 (0.46, 1.43) |
| Pravastatin | 1.04 | 1.08 (0.95, 1.23) | 1.08 | 1.12 (0.95, 1.31) | 0.98 | 1.03 (0.84, 1.26) |
| Simvastatin | 1.04 | 1.07 (0.93, 1.23) | 1.15 | 1.17 (0.98, 1.39) | 0.87 | 0.93 (0.74, 1.18) |
| Statin adherence* | ||||||
| < 90% | Reference | Reference | Reference | Reference | Reference | Reference |
| ≥90% | 0.97 | 0.92 (0.82, 1.03) | 1.07 | 1.02 (0.87, 1.18) | 0.84 | 0.81 (0.67, 0.97) |
| Age (continuous)† | 1.01 | 1.01 (1.00, 1.03) | 1.01 | 1.01 (0.99, 1.03) | 1.01 | 1.02 (0.99, 1.04) |
| Sex (male vs. female) | 1.26 | 1.34 (1.21, 1.49) | 1.24 | 1.32 (1.16, 1.51) | 1.28 | 1.37 (1.16, 1.63) |
| Social assistance† (yes vs. no) | 1.24 | 1.29 (1.16, 1.44) | 1.23 | 1.28 (1.11, 1.48) | 1.25 | 1.31 (1.10, 1.57) |
| New diagnosed diabetes mellitus‡ (yes vs. no) | 1.64 | 1.41 (1.11, 1.81) | 1.52 | 1.30 (0.98, 1.72) | 2.16 | 1.97 (1.20, 3.24) |
| Antidiabetic drug adherence < 80%§ (yes vs. no) | 1.16 | 1.06 (0.88, 1.28) | 1.04 | 0.94 (0.73, 1.22) | 1.33 | 1.21 (0.92, 1.59) |
| Antidiabetic drug adherence ≥80%§ (yes vs. no) | 1.08 | 0.92 (0.76, 1.11) | 1.13 | 0.95 (0.75, 1.21) | 1.01 | 0.87 (0.64, 1.18) |
| New diagnosed hypertension‡ (yes vs. no) | 2.67 | 2.69 (2.30, 3.14) | 2.47 | 2.48 (2.07, 2.98) | 3.41 | 3.54 (2.62, 4.77) |
| Antihypertensive agent adherence < 80%§ (yes vs. no) | 1.70 | 1.71 (1.48, 1.98) | 1.64 | 1.67 (1.38, 2.03) | 1.80 | 1.77 (1.42, 2.21) |
| Antihypertensive agent adherence = 80%§ (yes vs. no) | 1.68 | 1.74 (1.52, 1.99) | 1.72 | 1.76 (1.48, 2.09) | 1.64 | 1.74 (1.40, 2.15) |
Proportion of days covered (%).
At treatment initiation.
ICD-9 or pharmacological treatment; new diabetes or new hypertension was detected in the year before the index date.
Proportion of days covered (%) in the year before the index date.
Impact of adherence level on CAD
In the current study, we focused on the results from the patients followed for >1 year because the efficacy of statins is apparent only after that period of time. The mean adherence level was 62% (SD = 35) for the cases and 65% (SD = 34) for the controls. The proportion of patients taking ≥90% of the prescribed doses did not differ significantly between controls (37%) and cases (33%) (Table 2). Moreover, the level of adherence in the total cohort was similar among the statin drugs (except for fluvastatin and lovastatin), given that 38%, 29%, 25%, 37% and 39% of patients used ≥90% of atorvastatin, fluvastatin, lovastatin, pravastatin and simvastatin dosage, respectively.
In multivariate analysis, the nonfatal CAD event rate did not decrease in the adherent group compared with the non-adherent group (RR 0.92; 0.82, 1.03) (Table 3) in the entire cohort. When the analysis was stratified for follow-up time, we found that good adherence had an impact on nonfatal CAD events after 1 year of treatment (RR 0.81; 0.67, 0.97), but not during the first year of treatment (RR 1.02; 0.87, 1.18). In the multivariate model, adherence ≥90% reduced the RR of nonfatal CAD events by 19% (RR 0.81; 0.67, 0.97) compared with adherence < 90%. With patients taking atorvastatin as the reference group, we observed no significant differences among the statin drugs.
Sensitivity analyses revealed that the greater the adherence, the greater the reduction in the risk of nonfatal CAD events (Table 4). The effect of adherence became statistically significant at the 90% level (RR 0.81; 0.67, 0.97) compared with adherence < 90%. Irrespective of the cut-off, coefficients associated with confounding covariables remained constant.
Table 4.
Rate ratio (RR) of nonfatal coronary artery disease event for cases occurring after 1 year of follow-up and their controls
| RR (95% CI) | |||
|---|---|---|---|
| Drug at initiation | Adherence 70% | Adherence 80% | Adherence 90% |
| Atorvastatin | Reference | Reference | Reference |
| Fluvastatin | 1.14 (0.82, 1.57) | 1.14 (0.82, 1.57) | 1.13 (0.82, 1.57) |
| Lovastatin | 0.81 (0.46, 1.44) | 0.81 (0.46, 1.44) | 0.81 (0.46, 1.43) |
| Pravastatin | 1.03 (0.84, 1.27) | 1.03 (0.83, 1.26) | 1.03 (0.84, 1.26) |
| Simvastatin | 0.94 (0.74, 1.18) | 0.94 (0.74, 1.18) | 0.93 (0.74, 1.18) |
| Statin adherence* | |||
| < 70%, 80%, 90%, respectively, | Reference | Reference | Reference |
| ≥70%, 80%, 90%, respectively, | 0.86 (0.72, 1.02) | 0.86 (0.72, 1.03) | 0.81 (0.67, 0.97) |
| Age (continuous)† | 1.02 (0.99, 1.04) | 1.02 (0.99, 1.04) | 1.02 (0.99, 1.04) |
| Sex (male vs. female) | 1.38 (1.16, 1.63) | 1.38 (1.16, 1.63) | 1.37 (1.16, 1.63) |
| Social assistance (yes vs. no)† | 1.30 (1.09, 1.55) | 1.30 (1.09, 1.55) | 1.31 (1.10, 1.57) |
| Newly diagnosed diabetes mellitus (yes vs. no)‡ | 1.97 (1.20, 3.24) | 1.97 (1.20, 3.23) | 1.97 (1.20, 3.24) |
| Antidiabetic drug adherence < 80%§ (yes vs. no) | 1.21 (0.92, 1.60) | 1.21 (0.92, 1.60) | 1.21 (0.92, 1.59) |
| Antidiabetic drug adherence ≥80%§ (yes vs. no) | 0.86 (0.63, 1.17) | 0.86 (0.63, 1.17) | 0.87 (0.64, 1.18) |
| Newly diagnosed hypertension (yes vs. no)‡ | 3.50 (2.59, 4.72) | 3.50 (2.60, 4.74) | 3.54 (2.62, 4.77) |
| Antihypertensive drug adherence < 80%§ (yes vs. no) | 1.77 (1.42, 2.22) | 1.78 (1.42, 2.22) | 1.77 (1.42, 2.21) |
| Antihypertensive drug adherence ≥80%§ (yes vs. no) | 1.72 (1.38, 2.13) | 1.72 (1.38, 2.13) | 1.74 (1.40, 2.15) |
Proportion of days covered (%).
At treatment initiation.
ICD-9 or pharmacological treatment; new diabetes or new hypertension was detected in the year before index date.
Proportion of days covered (%) in the year before the index date.
Finally, sensitivity analyses to assess the robustness of our results by accounting for unmeasured confounders revealed that our conclusions might be overturned when a confounder had a RR for a nonfatal CAD event of 3 and when there was an important difference in the confounder prevalence of risk factors between the two groups (e.g. present in 5% of adherent patients and >15% of non-adherent patients or present in 10% of adherent patients compared with 20% of non-adherent patients or present in 20% of adherent patients compared with 30% of non-adherent patients).
Discussion
As expected, our results indicated that adherence to statins that exceeds 90% is associated with a significant reduction in nonfatal CAD events. The coefficients associated with CAD risk factors such as diabetes, hypertension and socioeconomic status agreed with findings from other studies [3, 4]. Previous observational studies have revealed that patients with hypertension have 1.5–2times the risk of CAD compared with individuals without hypertension and that individuals with low or middle incomes have 1.2 times the risk of those with higher incomes [3, 4]. A coefficient associated with diabetes trends in the same direction, as reported in previous studies that there is a risk for CAD of 2–4 times higher among patients with diabetes [3, 4, 33].
The reduction in nonfatal CAD events found in the current study is comparable to that reported in the literature [16]. For an absolute reduction in LDL-C of 1 mmol l−1 (average of the clinical trials), the reduction in ischaemic heart disease was 11% (range 4–18%) after 1 year, 24% (range 17–30%) after 2 years and 33% (range 28–37%) after 3–5 years [16]. We observed similar results among subjects who used at least 90% of the prescribed medications compared with those who used < 90% (RR 0.81; 0.67, 0.97) after a mean follow-up of 1.6 years. The discrepancy in the reduction of CAD after 1 year may be related to the fact that a large proportion of patients who received treatment failed to achieve lipid goals [34]. Many patients who begin statin treatment remain at the initial dose and dose titration is uncommon in actual practice [35].
Statin drugs reduce the incidence of recurrent acute myocardial and death among patients who have had an acute myocardial infarction [6–8]. The benefit also has been evident in recent trials that enrolled subjects with and without previous cardiovascular diseases but who were at high risk of future cardiovascular events compared with placebo [10, 36]. It is unclear if the effect size observed across trials varied because of the characteristics of the trials or because the statin drugs had different effects. The results of the PROVE IT-TIMI 22 study [37] suggested that high-dose atorvastatin could provide additional benefits, but atorvastatin at 80 mg was not frequently prescribed in our study. In our population-based setting representative of daily practice, we observed similar relative effectiveness of the five statins. Similar results were also observed for secondary prevention in elderly patients after an acute myocardial infarction [38].
Our study has several limitations. First, databases do not allow adjustment for clinical severity. We thus do not have – and so cannot adjust for – cholesterol values before and after treatment. This shortcoming may be of minimal importance given a recent study of patients with diabetes and dyslipidaemia which reported that adherence to statin therapy is related closely to the attainment of the LDL goal and appears to increase substantially when adherence is >80% [39]. To investigate possible bias, we evaluated the rate of switching to other doses and found that most patients (72%) were taking the same dose (equivalent to simvastatin during the follow-up period).
Second, we could not adjust for blood pressure or glycaemia values, two well-known CAD risk factors. If patients were using drugs to treat hypertension or diabetes, we defined the categories of adherence levels for these therapies to take into account the adherence level to them more precisely to estimate the CAD risk reduction.
Third, the RAMQ databases did not allow for adjustments for lifestyle. Smoking, lack of exercise, obesity and poor diet are important CAD risk factors [4]. Furthermore, since they are more likely to be present among patients who do not adhere to a medication regimen, they may introduce a bias [40]. The level of adherence to medications may be a marker for a better prognosis [41, 42]. We cannot exclude the fact that adherent patients may have more healthy lifestyles and may be healthier than non-adherent patients [34]. In sensitivity analyses, our conclusions could be invalidated with an unmeasured confounder having a RR of 3 and a difference in the prevalence of the confounder between adherent groups of 5% compared with >15% of non-adherents. A RR of 3 could be attributed to the risk associated with smoking, as reported in the Interheart Study [4]. However, bias of that magnitude is unlikely because patients may have adopted a better lifestyle early after treatment initiation, but these changes could be substantially reduced over time.
Fourth, the databases included data for insured drugs only. Our concern here is aspirin, which has been beneficial in preventing CAD events among patients with some risk factors who are free of cardiovascular disease [43]. To ensure that our cohort was free of cardiovascular disease, subjects with an aspirin prescription were excluded, but some may have been taking aspirin obtained over the counter. However, there is no reason to believe that one adherence category should include more such patients than another.
Fifth, some subjects may have had a previous CAD event that did not appear in the databases for which we have information 3 years before the index date. Such patients are at higher risk than primary-prevention patients – the reinfarction rate is approximately 5% annually [44, 45] – but they also have better compliance with statins. Still, the likelihood of such a misclassification is minimal because we have pharmaceutical data for 1 year and medical data for 3 years before the index date. It is thus reasonable to believe that our subjects did not have cardiovascular disease.
Finally, another possible misclassification error is related to the determination of statin exposure. It is assessed on the basis of the pharmaceutical files completed by community pharmacists; but we cannot know with absolute certainly if patients took their drugs. However, patients pay a proportion of the cost of the drugs, so they may be more likely to take their medication and the chances of bias are lowered.
Despite those limitations, we believe that the study results are reliable and could provide a first estimation of the impact of adherence to medical regimens on clinical outcomes. Moreover, it is an innovative and promising way to analyse that kind of data because it leads to a precise estimation of adherence and accurate comparisons among subjects.
In summary, our results showed that at currently used dosages patients who filled >90% of the prescribed doses began to achieve significant reductions in nonfatal CAD events. Our study corroborates the findings that statin effectiveness is apparent after one full year of treatment. These results are also supported by meta-analyses reports [16]. It is important to raise the awareness of health professionals of the need to improve adherence to therapy. Clinicians must emphasize this factor to patients during long-term treatment.
Acknowledgments
The Canadian Institutes Health Research (CIHR) supported this work. S.P. and D.P. are research scholars who receive financial support from the Fonds de recherche en santé du Québec. L.B. and A.B. are research scholars who receive financial support from the CIHR.
References
- 1.Fondation des maladies du Coeur du Canada. Le Fardeau Croissant des Maladies Cardiovasculaires et des Accidents Vasculaires Cérébraux au Canada, 2003. Ottawa: Heart and Stroke Foundation of Canada; 2003. Report no. 1-89624-32-4. [Google Scholar]
- 2.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. Eur Heart J. 2002;23:620–6. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lana F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Wilson PW, Larson MG, Leip R, Beiser A, D'Agostino RB, Cleeman JI, Levy D. Lifetime risk of coronary heart disease by cholesterol levels at selected ages. Arch Intern Med. 2003;163:1966–72. doi: 10.1001/archinte.163.16.1966. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 7.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 8.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 10.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 11.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–22. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Sever PS, Poulter NR, Dahlöf B, Wedel H. Different time course for prevention of coronary and stroke events by atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOTT-LLA) Am J Cardiol. 2005;96:39F–44F. doi: 10.1016/j.amjcard.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Colhoum HM, Betteridge DJ, Durrington PN, Hitman GA, Neil AW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 14.Larosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJP, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 15.Ray KK, Cannon CP, McCabe CH, Cairns R, Tonkin AM, Sacks FM, Jackson G, Braunwald E. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes. Results from the PROVE IT-TIMI 22 Trial. J Am Coll Cardiol. 2005;46:1405–10. doi: 10.1016/j.jacc.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 16.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ. 2003;326:1423. doi: 10.1136/bmj.326.7404.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 18.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 19.WHO. International Classification of DiseasesManual of the International Statistical Classification of Diseases, Injuries, and Cause of Death. 9. Geneva: World Health Organization; 1977. Publication no PHS 80-1260. [Google Scholar]
- 20.Canada: Supply and ServicesCanadian Classification of Diagnostic, Therapeutic, and Surgical Procedures. 2. Ottawa: Statistics Canada Health Division; 1986. [Google Scholar]
- 21.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 22.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–94. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 23.Régie de l'Assurance MaladieListe Des Médicaments Québec Canada. 15. Québec: Ministère de la Santé; 2003. [Google Scholar]
- 24.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 25.Monfared A, Rahme E, LeLorier J. First Canadian Therapeutics Congress. Canada: Halifax; 2004. Accuracy of ICD-9 Diagnosis Code ‘410’ to Identify Episodes of Hospitalizations for Acute Myocardial Infarction in RAMQ. [Google Scholar]
- 26.Lubin J, Gail M. Biased selection of controls for case control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 27.Essebag V, Platt RW, Abrahamowicz M, Pilote L. Comparison of nested case–control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5:5. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–9. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25:1107–16. [PubMed] [Google Scholar]
- 30.Jones P, Kafonet S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia. Am J Cardiol. 1998;81:582–7. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 31.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 32.Robets WC. The role of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol. 1997;80:106–7. [PubMed] [Google Scholar]
- 33.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 34.Middleton A, Fuat A. Achieving lipid goals in real life: the DISCOVERY-UK Study. Br J Cardiol. 2006;13:72–6. [Google Scholar]
- 35.Foley KA, Simpson RJ, Jr, Crouse JR, 3rd, Weiss TW, Markson LE, Alexander CM. Effectiveness of statin titration on low density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol. 2003;92:79–81. doi: 10.1016/s0002-9149(03)00474-0. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 37.Ray KK, Cannon CP, McCabe CH, Cairns R, Tonkin AM, Sacks FM, Jackson G, Braunwald E. PROVE IT-TIMI 22 Investigators. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2005;46:1405–10. doi: 10.1016/j.jacc.2005.03.077. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Z, Rahme E, Abrahamowicz M, Tu JV, Eisenberg MJ, Humphries K, Austin PC, Pilote L. Effectiveness of statins for secondary prevention in elderly after acute myocardial infarction: an evaluation of class effect. CMAJ. 2005;172:1187–94. doi: 10.1503/cmaj.1041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28:595–9. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 40.Kim YS, Sunwoo S, Lee HR, Lee KM, Park YW, Shin HC, Kim CH, Kim DH, Kim BS, Cha HS, Hub BY. Determinants of non-compliance with lipid-lowering therapy in hyperlipidemic patients. Pharmacoepidemiol Drug Saf. 2002;11:593–600. doi: 10.1002/pds.730. [DOI] [PubMed] [Google Scholar]
- 41.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Arch Intern Med. 1997;157:1921–9. [PubMed] [Google Scholar]
- 42.Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303:1038–41. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 43.Lam J. Lignes Directrices Pratiques Quant au Traitement Antiplaquettaire. Vol. 2004. Montréal: Le Groupe de Travail Sur la Thrombose Du Canada; 2000. [Google Scholar]
- 44.Kannel WB. Risk factors for atherosclerotic cardiovascular outcomes in different arterial territories. J Cardiovasc Risk. 1994;1:333–9. [PubMed] [Google Scholar]
- 45.Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, Gardner M, Stewart D, Plante S, Dupuis R, Lauzon C, Ferguson J, Mikes E, Balnozan V, Savard P. Myocardial infarction patients in the 1990s – their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27:1119–27. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 46.LaRosa JC, He J, Vupputuri S. Effect of statins risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]