Abstract
Type III collagen is a fibrillar forming collagen comprising three α1(III) chains and is expressed in early embryos and throughout embryogenesis. In the adult, type III collagen is a major component of the extracellular matrix in a variety of internal organs and skin. Mutations in the COL3A1 gene have been implicated as a cause of type IV Ehlers–Danlos syndrome, a disease leading to aortic rupture in early adult life. To directly study the role of Col3a1 in development and disease, we have inactivated the Col3a1 gene in embryonic stem cells by homologous recombination. The mutated allele was transmitted through the mouse germ line and homozygous mutant animals were derived from heterozygous intercrosses. About 10% of the homozygous mutant animals survived to adulthood but have a much shorter life span compared with wild-type mice. The major cause of death of mutant mice was rupture of the major blood vessels, similar to patients with type IV Ehlers–Danlos syndrome. Ultrastructural analysis of tissues from mutant mice revealed that type III collagen is essential for normal collagen I fibrillogenesis in the cardiovascular system and other organs.
Keywords: gene targeting, Ehlers–Danlos syndrome type IV, aortic rupture
Patients with type IV Ehlers–Danlos syndrome, a genetic disorder associated with fragile blood vessels and skin, often carry mutations in the COL3A1 gene coding for type III procollagen (1–3). This suggests that type III collagen is important for the development of skin and the cardiovascular system and for maintaining the normal physiological functions of these organs in adult life (4, 5). Type III collagen is a member of the fibrillar collagen family and is colocalized with the most abundant member of the family, type I collagen, in such tissues as blood vessels and skin (6–10). Previous studies have shown that fibrillogenesis may involve coassembly of different types of collagens. For example, type III collagen is found to be colocalized with type I collagen within the same fibril (11–14). It was thought that type III collagen may modulate the size of type I collagen fibrils because the diameter of these fibrils formed by self-assembly in vitro appeared uniform (15–17), but variable in different tissues or in the same tissue at different developmental stages which have different ratios of type III to type I fibrils (11, 12, 18, 19). To define the role of type III collagen in fibrillogenesis, we derived Col3a1−/− mutant mice by gene targeting. Most homozygous Col3a1 mutant mice died perinatally. The phenotype of surviving mutant mice resembled the clinical manifestations of patients with type IV Ehlers–Danlos syndrome (20) including sudden death due to rupture of the large vessels. Electron microscopic analysis revealed that, in Col3a1−/− mice, collagen fibrils in the media of aorta were missing and collagen fibrils in the adventitia of the aorta and skin were irregular in size.
MATERIALS AND METHODS
Targeting Vector.
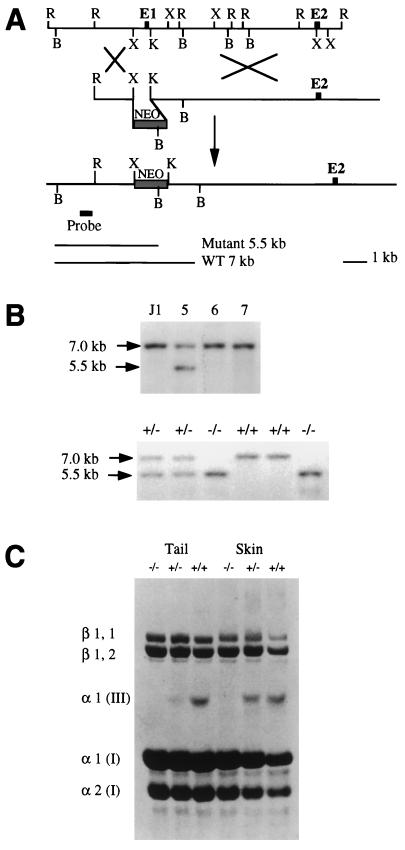
Genomic DNA of the Col3a1 gene was cloned from a J1 library (21), and a 14-kb fragment that covers the promoter and the first two exons of the gene was subcloned into a pGEM7 vector. At the 5′ portion of this 14-kb genomic DNA fragment, a 0.7-kb XbaI–KpnI fragment containing the promoter and the first exon of the col3a1 gene was replaced with a 1.8-kb PGKneo cassette (see Fig. 1).
Figure 1.
Targeted deletion of the Col3a1 gene in mouse ES cells and generation of mutant mice. (A) Schematic diagram of the strategy used to target the Col3a1 gene. (Top) Restriction map of the Col3a1 gene genomic DNA fragment which covers the promoter, the first two exons, and the first intron of the Col3a1 gene. (Middle) The 18-kb replacement-type targeting vector carrying a neo gene driven by a phosphoglycerate kinase promoter, which replaced the XbaI and KpnI DNA fragment of the Col3a1 gene. (Bottom) The targeted allele in which the promoter region and the first exon that codes for the signal peptide of type III procollagen were deleted by homologous recombination; the genomic probe external to the 5′ homologous arm is indicated, which hybridizes to a 5.5-kb and a 7-kb DNA fragment from mutant and wild-type alleles, respectively. B, BamHI; K, KpnI; R, EcoRI; X, XbaI. (B Upper) Southern blot of ES cell clones. DNA from parental J1 ES cells and independently cloned G418-resistant ES cell clones number 5, 6, and 7 was digested with BamHI and hybridized to the external probe shown in A. Clones containing the expected 5.5-kb BamHI fragment diagnostic for homologous recombinant were obtained at a frequency of 1 in 30. The blot was rehybridized to a neo probe to verify a single integration. Three independent recombinant ES cell clones contributed to the germ line of recipient embryos after blastocyst injection. (Lower) Southern blot of offspring from a Col3a1+/− × Col3a1+/− cross. Tail DNA was extracted and digested with BamHI and analyzed as described in ref. 22. (C) Collagens from tails and skin of wild-type mice and type III collagen mutants. Collagens were resolved by SDS/PAGE and stained with Coomassie blue. The type III collagen molecule consists of three α1 (III) chains. The β1,1 and β1,2 dimers and the α1(I) and α2(I) chains are all from type I collagen and serve as internal controls for the loading. Skin collagens from wild-type mice (far right lane) were underloaded.
Production of Col3a1 Mutant Mice.
About 50 μg of the targeting vector DNA linearized at the 3′ end of the 14-kb fragment was electroporated into 2 × 107 J1 embryonic stem (ES) cells. The cells were subsequently cultured in the presence of G418, and 384 clones were picked after 9 days of culture. Among the 384 clones, 192 were analyzed by BamHI digestion and hybridization to probe (see Fig. 1), and 6 of them, including clones 5 and 67, were identified as correctly targeted clones. Clones 5 and 67 were injected into C57-BL/6 and BALB/c embryos as described (22). Chimeras were identified on the basis of agouti pigmentation in the coat and backcrossed. Their agouti offspring were genotyped by Southern blot analysis.
Collagen Analysis of Col3a1 Mutant Mice.
Collagens were extracted from mouse tail or skin by digestion with pepsin, 50 μg/mg, in 0.5 M acetic acid at 0–4°C for 2–4 days. The collagen extracts were resolved with SDS/PAGE. When the dye front had migrated into the running gel for about 1.5 cm, the sample buffer was changed and 2% of 2-mercaptoethanol was added. This method permits the separation of disulfide-bonded type III collagen from type I collagen. The collagens were visualized by staining with Coomassie blue.
Histological Analysis.
Samples were fixed in 10% buffered formalin. They were then placed in successive ethanol and xylene baths and finally embedded in Paraplast Plus (Oxford Labware, Oxford, U.K.) using an Autotechnicon embedder (Technicon). The embedded tissues were sectioned to 5 μm using a Reichert–Jung (Vienna) microtome and stained with Masson’s trichrome (23) where collagens are blue in color.
Transmission Electron Microscopy.
Samples were fixed with 2.5% glutaraldehyde in 0.1 M Na cacodylate buffer (pH 7.2) for 1 hr on ice. Samples were then rinsed in 10% sucrose in 0.1 M Na cacodylate buffer, postfixed with 1% OsO4 in cacodylate buffer, and en bloc stained with 1% aqueous uranyl acetate. Samples were dehydrated in graded ethanol and embedded in polyBed 812 (Polysciences). Thin sections were cut on a Ultracut E (Reichert–Jung), stained with uranyl acetate and lead citrate, and examined under the Philips 410 transmission election microscope.
RESULTS AND DISCUSSION
ES (J-1) cells (22) were transfected with a targeting vector containing a Col3a1 gene genomic DNA fragment in which the promoter region of the gene and the first exon encoding the signal peptide were deleted (Fig. 1A). Targeted ES cell clones were identified by Southern blot analysis and used for blastocyst injection (Fig. 1B Upper). Mutant mice were derived from two independently targeted ES cell clones and identified by Southern blot analysis (Fig. 1B Lower). Protein analysis of tail and skin collagen showed that heterozygous mutant mice have about a 50% reduction in type III collagen whereas no type III collagen was detected in homozygous mutant animals (Fig. 1C).
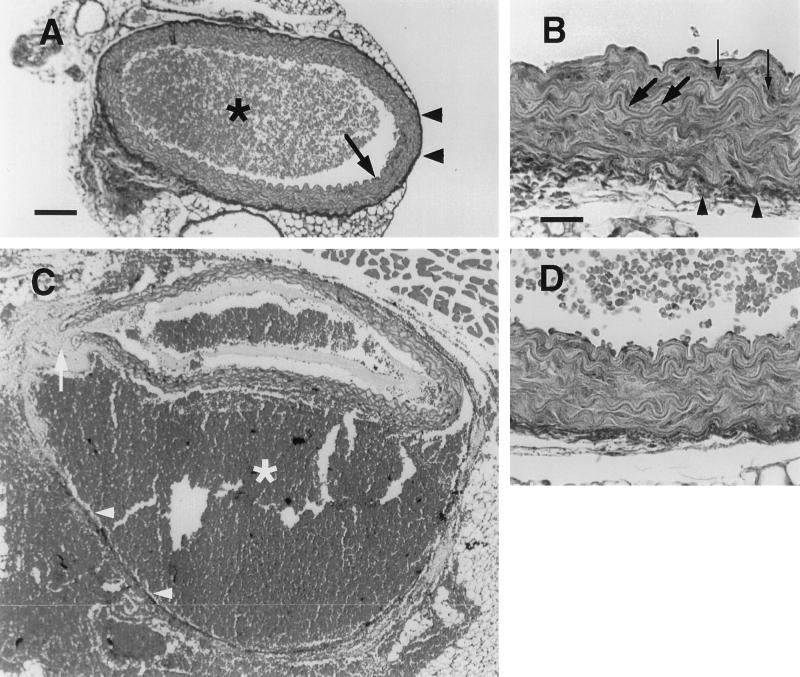
Mutant animals were fertile but only heterozygous mice were phenotypically normal. Homozygous mutant mice displayed an average survival rate of 5% at weaning age, with most deaths occurring within the first 48 hr after birth (Table 1). The precise cause of the neonatal lethality is not clear because the dead pups were cannibalized before they could be examined, and light microscopic histological analysis of live newborn homozygous mutants did not detect any gross abnormality. Adult homozygous mutant mice appeared normal except that they were about 15% smaller than their wild-type littermates of the same sex (data not shown). The average life span of the homozygous mutant mice was, however, about 6 months or one-fifth of the normal life span. Autopsy showed that blood vessel rupture was the major cause of the shortened life of these mice (Table 2). The sites of rupture were sporadic and mostly associated with large blood vessels. Histochemical analysis was carried out to find the defect that caused the fragility of the blood vessel wall. Fig. 2 A and B show a cross section of normal abdominal aorta. The wall of the aorta consists of intima and media (Fig. 2A, arrow) and adventitia (Fig. 2 A and B, arrowheads). The medium consists of elastic fibers (Fig. 2B, large arrows) and smooth muscle cells (Fig. 2B, small arrows) and provides the elasticity of the aorta while the adventitia comprises mostly type I collagen fibrils and limits the dilatation of the aorta. Fig. 2C shows a cross section of a dissecting aneurysm of the abdominal aorta from a homozygous mutant mouse. The rupture crossed the media (arrow), which led to a blood-filled channel (star) between media and adventitia (arrowheads) and partially collapsed the lumen of the aorta. The adventitia eventually ruptured elsewhere, and blood leaked into the peritoneal cavity as is the case in humans with lethal aortic aneurysm (20). The overall arrangement of the elastic fibers and the smooth muscle cells (Fig. 2D) was similar to that of the controls (Fig. 2B). The intensity and distribution of extracellular matrix material and the darker staining in the adventitia and between elastic fibers and smooth muscle cells in the intima of mutant mice (Fig. 2D) were comparable to that of wild-type controls (Fig. 2B). No obvious defects of heart and midsize and small arteries in mutant animals were observed under the light microscope (data not shown).
Table 1.
Survival of homozygous Col3a1 mutant mice
| Age | Total no. of animals | Wild type (+/+) | Heterozygous (+/−) | Homozygous (−/−) |
|---|---|---|---|---|
| E18 | 64 | 14 | 33 | 17 |
| Newborn | 35 | 7 | 20 | 8 |
| 3 weeks | 143 | 42 | 96 | 5 |
Table 2.
Autopsy results from the dead homozygous mutant adult mice
| Aneurism
|
Intestinal rupture | Infection | Cause not determined | |
|---|---|---|---|---|
| Chest | Abdominal | |||
| 6 | 9 | 2 | 4 | 4 |
Figure 2.
Masson’s trichrome staining of cross sections of aorta of wild-type and Col3a1 mutant mice. (A) Wild-type aorta composed of intima and media (arrow) and adventitia (arrowheads). There are blood cells (star) in the lumen. (B) High magnification of wild-type aorta. Elastic fiber (large arrows) and smooth muscle cells (small arrows) of the intima can be seen. (C) A dissecting aneurysm of mutant aorta. The intima and media (arrow) were ruptured and blood (star) filled in between media and adventitia (arrowheads). (D) High magnification of mutant aorta. [Bar = 100 μm (A and C) and 25 μm (B and D).]
In addition to aneurysm, mutant mice showed frequent intestinal enlargement and occasional intestinal rupture resulting in death (Table 2). About 60% of homozygous mutant mice displayed skin lesions, the most severe of which presented as an open wound of ≈1 cm length in the shoulder area, which completely penetrated the skin and exposed subdermal tissue (Fig. 3). The wounds were not due to fighting between animals, since they were observed in animals caged separately. Light microscopic analysis of skin, intestine, and other internal organs including liver and lung did not detect any overt abnormality in mutant animals (data not shown).
Figure 3.
A type III collagen-deficient mouse with a skin wound on its left shoulder.
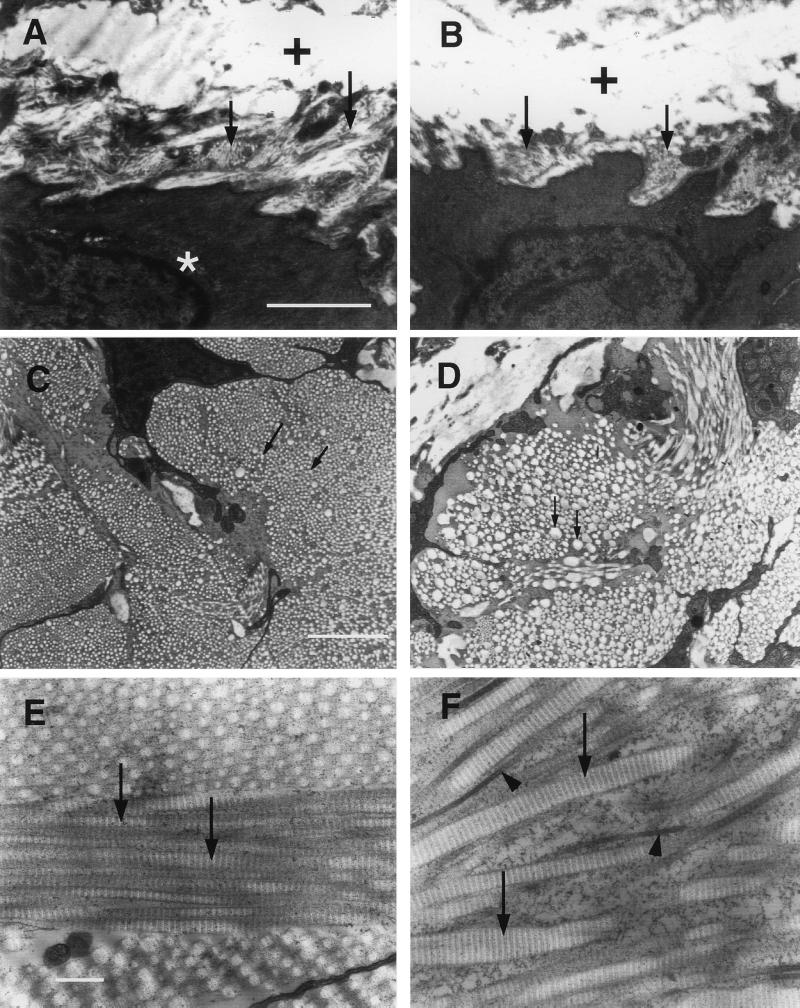
To define the structural defects in mutant animals, electron microscopic analyses of aorta and heart were carried out. Fig. 4 shows that the collagen fibrils located between smooth muscle cells or between smooth muscle cell and elastic fibers (Fig. 4A, arrows) were absent or severely reduced in the media of mutant aorta (Fig. 4B, arrows). Most strikingly, in the adventitia where the majority of collagen is type I, the diameter of collagen fibrils of the mutant aorta was highly variable (compare Fig. 4 D to C). When the fibrils were counted and the diameter of these fibrils measured in a given area of the adventitia, we found that the number of fibrils in mutants was reduced to approximately one-third of that of wild-type while the mean diameter of the fibrils in mutants was approximately twice that of wild-type mice (Fig. 5). Similar to the aorta, the collagen fibrils between epicardium and myocardium were reduced or missing and the microvilli of the epicardium were underdeveloped in the heart of mutant mice (data not shown).
Figure 4.
Transmission electron microscopic analyses of aorta and skin from wild-type and mutant mice. (A) Collagen fibrils (arrows) are around the smooth muscle cell (star) in the media of wild-type aorta. White areas marked with crosses are elastic fibers. (B) Collagen fibrils are missing around the smooth muscle cell (arrows) in the media of mutant aorta. (C) Cross section of the collagen fibrils in the adventitia of wild-type aorta. Arrows point to individual fibrils. The diameter of the collagen fibrils is smaller and relatively uniform compared with the fibrils (arrows) of mutant aorta in D. (E) Skin section of wild-type mouse. The collagen fibrils (arrows) are uniform in diameter. (F) Skin section of mutant mouse. The collagen fibrils are often thicker (arrows) or thinner (arrowheads) than control fibrils and are not uniform in diameter. [Bar = 1 μm (A–D) and 0.2 μm (E and F).]
Figure 5.
Comparison of the diameters of collagen fibrils in the adventitia of aorta of wild-type and mutant mice. A 2 μm × 2 μm area in the adventitia of either wild-type or mutant aorta was randomly chosen, and all the fibrils in this area were measured for their diameters and counted.
In addition to the cardiovascular system, the skin, intestine, liver, and lung of mutant mice were examined by electron microscopy. As with the adventitia of the aorta, the collagen I fibrils in mutant skin (Fig. 4F, arrows and arrowheads) were disorganized and were highly variable in diameter as compared with those of wild-type mice (Fig. 4E, arrows). This alteration was also seen in liver and lung of mutant mice (data not shown). Moreover, collagen fibrils were missing or highly reduced in the submucosa and serosa of mutant intestines (data not shown) suggesting that collagen fibrils in these areas are mostly type III collagen fibrils.
Previous studies introducing targeted mutations into Col1a1, Col2a1, Col5a2, and Col9a1 genes have brought important insights into the function of these collagens (24–27). Here we show that type III collagen has a critical role in fibrillogenesis, which is an important part of the development of such organs as the cardiovascular system, intestine, and skin. Lack of type III collagen disturbed fibrillogenesis and resulted in defective development and functional failure of these organs. Under physiological conditions, type III collagen is not only an essential component of fibrils in tissues such as the media of aorta but also an important regulatory element in type I collagen fibrillogenesis. Our results suggest that type III collagen regulates the diameter of type I collagen fibrils, which serves as a mechanism to meet the physiological requirements of different tissues or of a tissue at different developmental stages. The phenotype of type III collagen deficient mice closely resembles the clinical manifestations of Ehlers–Danlos syndrome type IV patients in whom death results from blood vessel or intestinal rupture (20). These mutant mice should, therefore, prove to be good animal models for understanding this disease and possibly testing therapeutic approaches.
Acknowledgments
We thank Jessie Dausman and Ruth Curry for technical assistance. This research was supported by National Institutes of Health Grants R35-CA44339 to R.J. and AR-3564 to S.K. X.L. was supported by a fellowship from the Leukemia Society.
ABBREVIATION
- ES cell
embryonic stem cell
References
- 1.Prockop D J, Kivirikko K I. N Engl J Med. 1984;311:376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- 2.Kontusaari S, Tromp G, Kuivaniemi H, Romanic A M, Prockop D J. J Clin Invest. 1990;86:1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuivaniemi H, Tromp G, Bergfeld W F, Kay M, Helm T N. J Invest Dermatol. 1995;105:352–356. doi: 10.1111/1523-1747.ep12320704. [DOI] [PubMed] [Google Scholar]
- 4.Olsen B R. Experientia. 1995;51:194–195. doi: 10.1007/BF01931087. [DOI] [PubMed] [Google Scholar]
- 5.Wong R S, Follis F M, Shively B K, Wernly J A. Ann Thorac Surg. 1995;60:1439–1443. doi: 10.1016/0003-4975(95)00706-Q. [DOI] [PubMed] [Google Scholar]
- 6.Vuorio E, de Crombrugghe B. Annu Rev Biochem. 1990;59:837–872. doi: 10.1146/annurev.bi.59.070190.004201. [DOI] [PubMed] [Google Scholar]
- 7.Piez K. In: Extracellular Matrix Biochemistry. Piez K, Reddi A, editors. New York: Elservier; 1984. pp. 1–40. [Google Scholar]
- 8.Kuhn K. In: Structure and Function of Collagen Types. Mayne R, Burgeson R, editors. New York: Academic; 1987. pp. 1–37. [Google Scholar]
- 9.Epstein E H, Jr, Munderloh N H. J Biol Chem. 1975;250:9304–9012. [PubMed] [Google Scholar]
- 10.Bornstein P, Sage H. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- 11.Fleischmajer R, Olsen B R, Timpl R, Perlish J S, Lovelace O. Proc Natl Acad Sci USA. 1983;80:3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischmajer R, Perlish J S, Timpl R, Olsen B R. J Histochem Cytochem. 1988;36:1425–1432. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- 13.Timpl R, Wick G, Gay S. J Immunol Methods. 1977;18:165–182. doi: 10.1016/0022-1759(77)90168-5. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmajer R, Timpl R, Tuderman L, Raisher L, Wiestner M, Perlish J S, Graves P N. Proc Natl Acad Sci USA. 1981;78:7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams B R, Gelman R A, Poppke D C, Piez K A. J Biol Chem. 1978;253:6578–6585. [PubMed] [Google Scholar]
- 16.Gelman R A, Williams B R, Piez K A. J Biol Chem. 1979;254:180–186. [PubMed] [Google Scholar]
- 17.Kadler K E, Hojima Y, Prockop D J. Biochem J. 1990;268:339–343. doi: 10.1042/bj2680339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parry D A D, Craig A S. In: Ultrastructure of the Connective Tissue Matrix. Rugerri A, Motta P M, editors. Boston: Nijhoff; 1984. pp. 34–64. [Google Scholar]
- 19.Keene D R, Sakai L Y, Bachinger H P, Burgeson R E. J Cell Biol. 1987;105:2393–2402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uitto J, Bauer E A. Diseases Associated with Collagen Abnormalities. New York: Churchill Livingstone; 1982. [Google Scholar]
- 21.Wu H, Liu X, Jaenisch R. Proc Natl Acad Sci USA. 1994;91:2819–2823. doi: 10.1073/pnas.91.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 23.Bancroft J D, Cook H C. Manual of Histological Techniques. New York: Churchill Livingston; 1984. pp. 49–51. [Google Scholar]
- 24.Fassler R, Schnegelsberg P N, Dausman J, Shinya T, Muragaki Y, McCarthy M T, Olsen B R, Jaenisch R. Proc Natl Acad Sci USA. 1994;91:5070–5074. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrikopoulos K, Liu X, Keene D R, Jaenisch R, Ramirez F. Nat Genet. 1995;9:31–36. doi: 10.1038/ng0195-31. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Wu H, Byrne M, Jeffrey J, Khane S, Jaenisch R. J Cell Biol. 1995;130:227–237. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S W, Prockop D J, Helminen H, Fassler R, Lapvetelainen T, et al. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]