Abstract
What is already known about this subject
Calcium antagonists (CA) are listed in textbooks as potential causes of gastro-oesophageal reflux disease (GORD).
There have been two studies which have documented increased use of acid suppressant therapy amongst patients taking CAs.
What this study adds
This study provides the first data on the frequency of exacerbation and precipitation of gastro-oesophageal reflux symptoms amongst users of CAs.
It also provides evidence of the likely potential of the different CAs to cause such symptoms and highlights the need for a prospective study into CA therapy.
The data from the study should heighten prescribers' awareness of the potential of these agents to exacerbate/precipitate GORD, and to consider avoiding CAs in patients with GORD or withdrawing them in patients in whom GORD symptoms develop or worsen.
Aims
A cohort retrospective observational study was undertaken to determine the relationship between calcium antagonist (CA) use and gastro-oesophageal reflux disease (GORD), as well the ability of CAs to precipitate or exacerbate noncardiac chest pain, an atypical symptom of GORD.
Methods
Eligible patients were those prescribed CAs for hypertension without a history of ischaemic heart disease or nitrate use. Patients were recruited through 15 pharmacies (hospital 1, community 14). Patients giving informed consent were administered a standard questionnaire to obtain information including history of reflux symptoms before and during treatment with CAs, and the management of these symptoms.
Results
Three hundred and seventy-one participants were enrolled. Their mean age was 64 years (SD ± 12.7 years), 51.2% were females and 48.8% males. Of the 130 patients with pre-existing gastrointestinal (GI) symptoms, 59 (45.4%) reported a worsening of reflux symptoms during CA therapy. Increases in both frequency and severity of symptoms were most common amongst patients on amlodipine (61.3%; P ≤ 0.0001) and least common amongst those taking diltiazem (12.5%). Reflux-related symptoms developed in 85 (35.3%) of the 241 previously asymptomatic patients during CA therapy, with verapamil having the greatest number of reports (39.1%; P = 0.001) and diltiazem the least (30.7%).
Conclusions
Diltiazem appears the least likely of the CAs to precipitate or exacerbate reflux symptoms. Further research using a prospective design could test whether it may be more appropriate to use diltiazem in patients with ischaemic heart disease and could assess the appropriateness of CA therapy in patients with moderate to severe GORD. Increasing prescriber and pharmacist awareness of these adverse effects may result in better patient outcomes and potentially reduce treatment costs.
Keywords: calcium antagonists, gastro-oesophageal reflux disease, noncardiac chest pain
Introduction
Angina-like chest pain of noncardiac origin is a frequent clinical problem that presents major diagnostic and therapeutic difficulties [1–9]. Between 10 and 50% of patients with ‘anginal’ pain who are referred for arteriography are found to have a normal coronary artery profile. An oesophageal source of noncardiac chest pain is reported in up to 60% of these cases, with the most frequent cause attributable to gastro-oesophageal reflux disease (GORD) [4, 10]. As cardiac and oesophageal pathologies may coexist and interact, the finding of oesophageal symptoms in patients with anginal pain does not allow for the exclusion of a cardiac origin [6].
Since nitrates and calcium antagonists (Cas) are known to decrease the lower oesophageal sphincter (LOS) pressure in a dose-dependent manner, and impair oesophageal clearance, frequent angina or a worsening in its severity could result from reflux induced by antianginal therapy [6]. Thus, a positive feedback mechanism has the potential to develop, whereby worsening angina prompts increasing medical therapy which itself may promote reflux. Therefore, these agents, often used to relieve chest pain of cardiac origin, may in fact produce chest pain due to GORD.
Hallas et al.[11] conducted a study where they screened for drug-related dyspepsia via prescription symmetry. This was found by regression analysis to have a moderately strong association with the use of CAs [relative risk 1.40, 95% confidence interval (CI) 1.18, 1.67] with no apparent specificity within the class and no effect modification by age or dose. The commencement of antiulcer therapy in these patients was in the order of 2.0%.
As a consequence, this study was undertaken to determine the relationship between CA therapy and GORD, to assess the ability of CAs to either precipitate or exacerbate chest pain of noncardiac origin and to identify risk factors that may predispose patients to developing these adverse gastrointestinal (GI) effects. The aims of our study were to determine whether CAs increased the risk of GORD, which in turn may contribute to noncardiac chest pain. This was partitioned according to whether:
CAs could precipitate or exacerbate GORD symptoms.
If there were significant differences in the incidence of reflux symptoms between the dihydropyridines (DHPs: amlodipine, felodipine, nifedipine), phenylalkylamines (diltiazem) and benzothiazepine (verapamil) calcium antagonists.
If a relationship existed between the dose of CA and the frequency and/or severity of reflux symptoms.
If investigation was required for patients with reflux symptoms, as a consequence of CA therapy, and whether the symptoms necessitated the prescription of antiulcer agents.
Methods
Participants
The study adopted a retrospective cohort observation design. Patients eligible for the study were those who were receiving CAs for hypertension, with no documented history of ischaemic heart disease or nitrate use. Due to the fact that GORD may often mimic anginal pain, these exclusion criteria eliminated those patients likely to experience chest pain of cardiac origin. Patients were recruited from 14 Perth metropolitan community pharmacies and through the Pharmacy Department at Fremantle Hospital, Western Australia, over the period January 1999 to April 1999. Ethics approval for the study was obtained from the Curtin University of Technology Human Research Ethics Committee and the Fremantle Hospital Ethics Committee.
Considering the cohort as a whole (pre vs. post exposure, regardless of the type of CA), a sample size of 257 patients was required to show a 20% difference in incidence of oesophageal reflux symptoms, with 90% power, at the 5% significance level [12]. The incidence of developing reflux symptoms from a previous study was approximately 20% in pre vs. post exposure to a CA [13]. Considering the cohort as five separate groups, depending on the type of CA used, a sample size of 133 patients per group, or 665 in total, was required to show a 9% difference (10% nifedipine vs. 1% diltiazem) in incidence of oesophageal reflux symptoms between groups, with 90% power at the 5% significance level. The 9% figure was obtained by assuming a midpoint between the maximum literature incidence value for nifedipine (7.5%) and the value obtained in the preliminary study (12.5%), measured against the minimum literature incidence value obtained for diltiazem (1.2%) [13].
After obtaining informed written consent, a standard questionnaire was administered which determined: patient demographics, indication for the CA, dose and duration of use for each medication, history of reflux symptoms before and during CA treatment, present reflux symptoms and the frequency and management of those symptoms. A medical definition for each symptom was provided to standardize patient recall. In addition to patient data, the Health Insurance Commission (HIC) provided data on the number of prescriptions under the Pharmaceutical Benefit Scheme (PBS) for individual CAs for the 12-month period from April 1998 to April 1999.
All data was then entered into an SPSS (v.11) database for statistical analysis (SPSS Inc., Chicago, IL, USA). A κ test was performed as an indicator of the degree of recall bias in the sample. The primary focus of analysis was to compare the frequency of GI symptoms before and during treatment using the χ2 tests, to determine if CAs caused an overall increase in oesophageal reflux symptoms. From this, it was possible to determine the incidence of GI symptoms associated with each CA. χ2 analysis was also used to compare dose and duration effects for each agent. The impact of confounding variables, such as age, sex, comorbidities, dose, duration and concurrent medications was taken into account by performing logistic regression analysis.
Results
A total of 371 patients were enrolled in the study, which satisfied the power calculations for main time effects. The mean age was 64.9 (SD ± 12.9 years) with approximately equal proportions of females and males (51.2% and 48.8%, respectively). The extent of recall bias was estimated by the κ test. The value of κ was 0.86 (n = 35), which implies a high level of agreement, minimizing the potential for recall bias in the sample. This value was calculated by averaging the κ result across all the drug responses.
Prescribing
Nifedipine was the most frequently prescribed CA and diltiazem the least prescribed (Table 1). The pattern of CA prescribing in this cohort was representative of the Australian distribution at the time of the study, although it should be noted that patients with ischaemic heart disease were excluded from the study and this is an indication for the use of CAs. As the HIC data do not specify indications for use of CAs in these patients, the data presented include use for all relevant pathological conditions.
Table 1.
Relative distribution of calcium antagonist (CA) prescribing
| Sample distribution | Australian distribution (HIC) data | ||||
|---|---|---|---|---|---|
| CA | n | % | n | n per month | % |
| Nifedipine | 91 | 24.5 | 1 369 899 | 114 158 | 17.1 |
| Amlodipine | 84 | 22.6 | 2 109 175 | 175 764 | 26.4 |
| Felodipine | 81 | 21.8 | 1 746 982 | 145 582 | 21.8 |
| Verapamil | 73 | 19.7 | 1 223 491 | 101 958 | 15.3 |
| Diltiazem | 42 | 11.3 | 1 546 254 | 128 854 | 19.3 |
| Total | 371 | 100.0 | 7 995 801 | 666 316 | 100.0 |
HIC, Health Insurance Commission.
Symptom precipitation
Of the sample, 241 had no previous GI symptoms. Of these, 35.3% of asymptomatic patients developed GI symptoms after commencing CA therapy (Table 2). Overall, verapamil was seen to be the most frequent precipitant of GI symptoms, followed by the DHPs and lastly diltiazem (Table 2).
Table 2.
Precipitation of reflux-related symptoms during calcium antagonist (CA) therapy
| Precipitation of reflux-related symptoms | ||||
|---|---|---|---|---|
| CA | n | Total | % | P-value |
| Nifedipine | 23 | 51 | 36.5 | 0.043 |
| Amlodipine | 19 | 50 | 35.8 | 0.005 |
| Felodipine | 17 | 45 | 32.0 | 0.118 |
| Verapamil | 18 | 45 | 39.1 | 0.001 |
| Diltiazem | 8 | 24 | 30.7 | 0.107 |
| Total | 85 | 215 | 35.3 | |
The most common symptoms precipitated were acid reflux by nifedipine, heartburn by verapamil and chest pain by felodipine (Table 3). Diltiazem was shown also to have the lowest proportion of patients with symptom progression, and thus may be a safer alternative.
Table 3.
Development of reflux symptoms in association with calcium antagonist therapy
| Nifedipine | Amlodipine | Felodipine | Verapamil | Diltiazem | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal symptom | n | % | n | % | n | % | n | % | n | % | n | % |
| Heartburn | 13 | 15.3 | 12 | 14.1 | 12 | 14.1 | 14 | 16.5 | 6 | 7.1 | 57 | 67.1 |
| Indigestion | 9 | 10.6 | 2 | 2.4 | 4 | 4.7 | 11 | 12.9 | 1 | 1.2 | 27 | 31.8 |
| Acid reflux | 21 | 24.7 | 13 | 15.3 | 20 | 23.5 | 14 | 16.5 | 7 | 8.2 | 75 | 88.2 |
| Chest pain | 9 | 10.6 | 7 | 8.2 | 10 | 11.8 | 4 | 4.7 | 3 | 3.5 | 33 | 38.8 |
| Acid stomach | 7 | 8.2 | 4 | 4.7 | 2 | 2.4 | 6 | 7.1 | 2 | 2.4 | 21 | 24.7 |
| Bloating | 4 | 4.7 | 5 | 5.9 | 2 | 2.4 | 3 | 3.5 | 0 | 0.0 | 14 | 16.5 |
| Burning throat | 7 | 8.2 | 2 | 2.4 | 4 | 4.7 | 8 | 9.4 | 4 | 4.7 | 25 | 29.4 |
| Bitter taste | 3 | 3.5 | 1 | 1.2 | 3 | 3.5 | 0 | 0.0 | 2 | 2.4 | 9 | 10.6 |
| Other | 2 | 2.4 | 1 | 1.2 | 1 | 1.2 | 0 | 0.0 | 0 | 0.0 | 4 | 4.7 |
n, No. of patients; %, (n÷ 85) × 100 nTotal = Σn; nTotal% = (nTotal÷ 85) × 100.
Symptom exacerbation
Of the entire sample, 130 patients had pre-existing GI symptoms prior to commencing CA therapy. With respect to the exacerbation of reflux symptoms, 45.4% of patients with pre-existing symptoms reported worsening in their symptoms during CA therapy (Table 4).
Table 4.
Exacerbation of reflux-related symptoms after calcium antagonist (CA) exposure
| Patients with pre-existing symptoms prior to CA therapy | Patients with symptom exacerbation after CA therapy | ||||
|---|---|---|---|---|---|
| CA | n | % | n | % | P-value, McNemar's test for paired proportions |
| Nifedipine | 28 | 21.5 | 15 | 53.6 | <0.0001 |
| Amlodipine | 31 | 23.8 | 19 | 61.3 | <0.0001 |
| Felodipine | 28 | 21.5 | 15 | 53.6 | <0.0001 |
| Verapamil | 27 | 20.8 | 8 | 29.6 | <0.0160 |
| Diltiazem | 16 | 12.4 | 2 | 12.5 | <0.5000 |
| Total | 130 | 100.0 | 59 | 45.4 | |
Exacerbation of GI symptoms was most common in patients taking amlodipine and least common during diltiazem therapy. With the exception of diltiazem, a statistically significant increase in symptoms was shown for the remaining CAs. Overall, the DHPs were the most frequent contributors to symptom exacerbation, with acid reflux and heartburn exacerbated by amlodipine and chest pain by nifedipine (Table 5). Patients taking felodipine reported no exacerbations of chest pain. Amongst the other agents, exacerbation of chest pain was more common with nifedipine and amlodipine than the nondihydropyridine (NDHP) CAs (diltiazem and verapamil).
Table 5.
Exacerbation of reflux symptoms in association with calcium antagonst (CA) therapy
| Heartburn | Acid reflux | Chest pain | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Severity | Frequency | Severity | Frequency | Severity | |||||||
| CA | n | % | n | % | n | % | n | % | n | % | n | % |
| Nifedipine | 6 | 31.6 | 6 | 31.6 | 6 | 27.3 | 9 | 40.9 | 4 | 80.0 | 3 | 60.0 |
| Amlodipine | 8 | 40.0 | 5 | 25.0 | 10 | 43.5 | 8 | 34.8 | 3 | 75.0 | 3 | 75.0 |
| Felodipine | 7 | 33.3 | 3 | 14.3 | 6 | 42.9 | 4 | 28.6 | 0 | 0.0 | 0 | 0.0 |
| Verapamil | 5 | 29.4 | 3 | 17.6 | 4 | 22.2 | 3 | 16.7 | 1 | 33.3 | 1 | 33.3 |
| Diltiazem | 0 | 0.0 | 0 | 0.0 | 1 | 7.7 | 0 | 0.0 | 1 | 25.0 | 1 | 25.0 |
| Total | 26 | 44.1 | 17 | 28.8 | 27 | 45.8 | 24 | 40.7 | 9 | 15.2 | 8 | 13.6 |
Chest pain
With respect to the ability of CA therapy to precipitate or exacerbate chest pain of noncardiac origin, 13.7% (n = 33) of patients who were previously asymptomatic developed chest pain during therapy, with the DHPs having the strongest association and diltiazem the least. In patients with pre-existing chest pain, 53% (n = 9) had a worsening in their chest pain, again predominantly in those patients taking the DHPs (seven out of the nine).
Logistic regression
After adjusting for previous reflux symptoms, the use of aspirin and use of drugs to treat reflux symptoms (antacids, H2-antagonists, proton pump inhibitors), the odds of increased reflux symptoms after commencing CA therapy were 2.7 times higher in patients that had taken DHPs compared with those having taken NDHPs (95% CI 1.24, 5.73; P = 0.012). The odds of increased symptom severity and frequency in patients taking certain CAs relative to diltiazem are displayed in Table 6.
Table 6.
Odds ratios of calcium antagonists (CAs) relative to diltiazem
| CA | Odds ratio | 95% CI |
|---|---|---|
| Nifedipine | 4.03 | 1.10, 14.80 |
| Amlodipine | 4.22 | 1.13, 15.70 |
| Felodipine | 3.58 | 0.98, 13.12 |
| Verapamil | 1.80 | 0.49, 6.67 |
Treatment
With respect to treatment, the overall severity of GI symptoms was rated as ‘mild’, and explains why the majority of patients with upper GI symptoms, 46.5% (n = 101), used antacid. However, 12% required further antisecretory medications after the commencement of CA therapy. Sixty-one patients (28.4%) stated they took no treatment for their symptoms, 21.9% (n = 47) used H2-antagonists, and 9.8% (n = 21) used proton pump inhibitors (percentages total to more than 100 as some patients used multiple treatments).
Investigations
In the sample, 44% of patients had diagnostic investigations related to their current reflux symptoms. This was in the form of an endoscopy, barium meal, or both. Patients taking the DHPs accounted for 61% of all investigations. The most frequent result was an ‘unknown’ cause for the symptoms in 45% of patients, followed by oesophagitis in 30% of patients, which may have been precipitated or exacerbated by their CA therapy. In a similar prospective study involving 30 patients, 17% had GI investigations, all were taking the DHPs, and in no cases was a cause for their symptoms found.
Discussion
The results indicate that CAs may contribute to GORD and concomitant noncardiac chest pain. For most patients, these GI symptoms are mild and often only require antacid therapy. The significance of these findings extends to patients with a considerable degree of oesophageal pathology and/or pre-existing symptoms, where a worsening in magnitude could greatly impact on the patient's quality of life. Furthermore, due to the considerable use of these drugs in ischaemic heart disease, any potential for exacerbation of GORD (which in itself has been shown to promote cardiac ischaemic events) or the development of noncardiac chest pain, has consequences both economically and socially for the patient.
With respect to delineation of these effects within the group, the DHPs were more strongly associated with GI symptom development and exacerbation than the NDHPs. These effects were not seen to be dose-related, but a nonsignificant trend was seen for severity of symptoms associated with DHP use. In our study, failure to observe a significant dose relationship was possibly due to the small sample size obtained for each CA.
These findings contrast to those of Chow et al.[14], who conducted a retrospective cohort design study to assess acid-suppressive therapy use associated with antihypertensive agents. They reported that of 15 662 patients receiving hypertensive medications, 20% were receiving acid-suppression therapy. Amongst this cohort, patients more likely to receive acid-suppressant therapy were those receiving nitrates [odds ratio (OR) 1.71, 95% CI 1.49, 1.19; P < 0.005], CAs (OR 1.49, 95% CI 1.32, 168; P < 0.005), α1 antagonists (OR 1.32, 95% CI 1.32, 1.68; P < 0.005), those with asthma (OR 1.77, 95% CI 1.48, 2.12; P < 0.005) and women (OR 1.31, 95% CI 1.20, 1.42; P < 0.005). They noted that with the CA group that there was no significant difference amongst the individual agents. Their findings supported those of Hallas et al.[11], although it should be noted neither specifically examined GORD symptoms, but, rather, assessed the frequency of use of agents to treat them, which might explain the difference in findings.
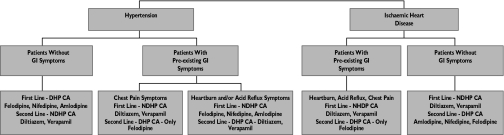
Based on the findings in this study, the following schema (Figure 1) was designed, taking into account the indication for CA therapy and the presence or absence of GI symptoms. In the case of hypertension for patients without GI symptoms, the best alternative would be a DHP CA, due to their enhanced antihypertensive efficacy. This would also be true in patients with pre-existing GI symptoms, except if the patient had chest pain, where a NDHP CA, namely diltiazem, would be the recommended choice of therapy. In the case of patients with ischaemic heart disease, NDHP CAs would be considered the drugs of choice (if the decision was made to use a CA), with diltiazem again favoured in those with pre-existing GI symptoms.
Figure 1.
Illustration of recommended calcium antagonist (CA) prescribing in patients with hypertension or ischaemic heart disease (IHD)
The increased incidence of GI symptoms may be due to a number of factors. First, mild GI symptoms are common in the community and are often precipitated by a number of factors such as lying down, and certain foods. Hence, patients will often attribute their symptoms to an overt cause rather than make the connection between their GI symptoms and medicines. Second, the majority of patients who had mild GI symptoms did not consider them significant enough to mention to their doctor, and treated them with over-the-counter antacids. Finally, the increased incidence of GI symptom reporting may be a result of directly interviewing the patients, who would otherwise not report such a trivial effect.
If the cost of performing diagnostic investigations is taken into consideration, the economic impact of therapy that potentiates GI symptoms is large. Based on the 1998 Medicare scheduled fees, the above GI investigations would cost AU$24 123 (£9659). However, this estimate is conservative and does not include consultant's fees and other charges. In addition, with any presentation of chest pain, a patient may undergo invasive cardiac investigation procedures before a GI origin is considered.
If the findings of this study are extrapolated to the Australian population, of which there were just fewer than eight million prescriptions for CAs on the PBS during April 1998–1999, and if we use the median daily dose, we can estimate that there were approximately 675 000 patients taking these medications. If it is assumed that 25.6% of patients had investigations related to their GI symptoms, this would equate to 173 000 investigations per year. Using the costs from Medicare, this totals just over AU$34.5 million (£13.8 million) per year. When as many as 45% of these patients may have no cause found for their symptoms, a simple trial of drug withdrawal might save as much as AU$16.5 million (£6.6 million) per year.
The study design had several limitations. First, there was no independent control to account for a Hawthorne effect, which may have increased the detection of other known GI confounders such as diet, alcohol and caffeine ingestion, and cigarette use. This may have contributed towards the high incidence of reported GI effects in this study. Second, due to time limitations, the sample sizes were less than the calculated target values required to achieve 90% power, at the 5% level of significance for the individual tests of CAs. This resulted in less power to detect relevant sample differences, which may explain why some P-values were not significant. In particular, due to the smaller patient recruitment for the NDHPs, nifedipine and amlodipine were the only CAs that achieved sufficient power to detect significant individual CA group differences. The majority of comparisons therefore were divided between the DHPs and the NDHPs. Third, as with all retrospective studies, there was a potential for recall bias. However, the result of the κ test of agreement indicated there was minimal recall bias in the sample. Lastly, this study did not allow for the accurate diagnosis of chest pain origin, other than by complying with cardiac exclusion criteria.
Further research in this area using a prospective research design should be undertaken and aim to identify correctly the nature of the chest pain and quantify the number of patients that require cardiac investigations during CA therapy. In addition, an economic assessment would be useful to examine the cost of diagnosing and treating reflux symptoms in these patients. Therefore, this study could serve as a useful framework for patients with ischaemic heart disease to assess both the therapeutic and economic consequences of this adverse effect. There is scope for further investigation into the association between CA dose and GI symptoms, and the use of antireflux treatment during CA therapy.
Conclusions
Whilst CAs have been used in the management of noncardiac chest pain associated with oesophageal motility disorders with varying success, our results suggest that they have the potential to worsen reflux symptoms and contribute to noncardiac chest pain in this way. Diltiazem appears the least likely of the CAs to precipitate or exacerbate reflux symptoms. Therefore, it may be more appropriate to use diltiazem in patients with moderate to severe GORD, especially those with concomitant ischaemic heart disease. Increased awareness of these adverse effects may potentially result in better patient outcomes and reduce treatment costs.
Competing interests: None declared.
References
- 1.Vantrappen G, Janssens J. Gastro-oesophageal reflux disease, an important cause of angina-like chest pain. Scand J Gastroenterol Supplement. 1989;168:73–9. [PubMed] [Google Scholar]
- 2.Bennett JR. Heartburn and gastro-oesophageal reflux. Br J Clin Prac. 1991;45:273–7. [PubMed] [Google Scholar]
- 3.Hamlin TE, Wright DE, Sutherland LR. Gastroesophageal reflux possibly associated with verapamil. Can J Hospital Pharmacy. 1997;44:167–8. [Google Scholar]
- 4.Baldi F, Ferrarini F. Non-cardiac chest pain: a real clinical problem. Eur J Gastroenterol Hepatol. 1995;7:1136–40. doi: 10.1097/00042737-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Vantrappen G, Janssens J, Ghilbert G. The irritable oesophagus – a frequent cause of angina-like pain. Lancet. 1987;1:1232–4. doi: 10.1016/s0140-6736(87)92686-9. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman D. Noncardiac chest pain, there's often an oesophageal cause. Postgrad Med. 1989;86:207–12. doi: 10.1080/00325481.1989.11704398. [DOI] [PubMed] [Google Scholar]
- 7.Bortolotti M, Labriola E, Bacchelli S. Oesophageal angina in patients with angina pectoris. Ital J Gastroenterol. 1992;24:405–8. [PubMed] [Google Scholar]
- 8.Vantrappen G, Janssens J. Angina and oesophageal pain – a gastroenterologist's point of view. Eur Heart J. 1986;7:828–34. doi: 10.1093/oxfordjournals.eurheartj.a061968. [DOI] [PubMed] [Google Scholar]
- 9.Saseen JJ, Carter BL. Dual calcium channel blocker therapy in the treatment of hypertension. Ann Pharmacol. 1996;30:802–10. doi: 10.1177/106002809603000719. [DOI] [PubMed] [Google Scholar]
- 10.Mehta AJ, de Caestecker JS, Camm AJ, Northfield TC. Gastro-oesophageal reflux in patients with coronary artery disease. How common is it? Eur J Gastroenterol Hepatol. 1996;8:973–8. doi: 10.1097/00042737-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hallas J, Byzter P. Screening for drug-related dyspepsia: an analysis of prescription symmetry. Eur J Gastroenterol Hepatol. 1998;10:27–32. doi: 10.1097/00042737-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 12.DuPont WD, Plummer WD. Power and sample size calculations: a review and computer program. J Controlled Clin Trials. 1990;11:116–28. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 13.Hughes J, Keall T, Nguyen T, Paterson T. Calcium channel blockers and oesophageal reflux. Pharmacotherapy. 1999;19:478. (Abstract). [Google Scholar]
- 14.Chow SI, Luzier AB, DiTusa L, Synder BD, Izzo JL., Jr Acid-suppressant therapy use associated with antihypertensive agents. J Clin Pharmacol. 2001;41:750–6. doi: 10.1177/00912700122010654. [DOI] [PubMed] [Google Scholar]