Abstract
Aims
To develop a population pharmacokinetic model for stavudine in children and to investigate the consistency of the currently recommended dose based on adult target concentrations.
Methods
The pharmacokinetics of stavudine were investigated using a population approach. Individual estimates of CL/F were used to calculate the stavudine dose required to achieve the area under the concentration-time curve reported in adults given recommended doses.
Results
Stavudine pharmacokinetics were well described by a one-compartment model with zero-order absorption. Typical population estimates (% interindividual variability) of the apparent distribution volume (V/F) and plasma clearance (CL/F) were 40.9 l (32%) and 16.5 l h−1 (38%), respectively. Stavudine V/F and CL/F were similarly related to age. Mean calculated doses (0.61 mg kg−1 for children less than 2 weeks, 1.23 mg kg−1 for children more than 2 weeks with bodyweight less than 30 kg, and 31.5 mg for children with a bodyweight between 30 and 60 kg) were in agreement with the current paediatric doses (0.5 mg kg−1, 1 mg kg−1, and 30 mg, respectively).
Conclusions
Our findings support the current recommended paediatric dosage regimens for stavudine, as they result in the same exposure to the drug as in adults.
Keywords: children, HIV, pharmacokinetics, stavudine
Introduction
Stavudine is a nucleoside analogue used for the treatment of HIV infection. It can be administered to children as a liquid as well as a solid oral formulation, at recommended doses of 0.5 mg kg−1 twice a day from birth to 13 days, 1 mg kg−1 twice daily for children older than 13 days and weighing less than 30 kg, and 30 mg twice daily for children with a bodyweight between 30 and 60 kg. For children with a bodyweight greater than 60 kg, the adult 40 mg twice daily dose is recommended, and has been approved by the FDA.
As no pharmacokinetic-pharmacodynamic relationship has been identified for stavudine, the current paediatric dosage regimen was established in order to achieve the same mean area under the concentration time curve from time 0–12 h (AUC(0,12 h) as that obtained in adults at the recommended dose [1]. Stavudine pharmacokinetics in children have only been investigated in a small number of low powered studies, which may have precluded the identification of covariates such as age or bodyweight [2–5]. Accordingly, we have developed a population pharmacokinetic model for stavudine in a large group of children in order to determine the relationship between stavudine pharmacokinetics and age or bodyweight, and to verify the consistency of the current recommended paediatric dosage regimen.
Methods
Patients and treatment
The population comprised 272 paediatric patients (from which were obtained 671 plasma samples), ranging in age from 3 days to 16 years (median 8.15 years), and in bodyweight from 2.1 to 76 kg (median 24 kg). Children received stavudine for the treatment of HIV infection or for the prevention of mother-to-child transmission, and the median stavudine dose was 0.9 mg kg−1 (range 0.30–4.65 mg kg−1). Plasma concentrations of antiretroviral drugs were obtained in the context of routine drug monitoring. In France ethics committee approval is not required for studies using routine drug monitoring data. The population comprised 25 neonates younger than 13 days, 170 children older than 13 days and weighing less than 30 kg, 71 children weighing between 30 and 60 kg, and six children weighing more than 60 kg. In 95%, 52%, and 31% of the samples, patients had been taking stavudine with at least one nucleoside reverse transcriptase inhibitor (NRTI), one protease inhibitor (PI), or a non nucleoside reverse transcriptase inhibitor (NNRTI), respectively. Sixty-four percent of the samples corresponded to the tablet formulation. Serum creatinine (SCr) was recorded for 158 children (301 samples), and viral load at sampling time was available from 105 children (296 samples). Median SCr and viral load were 54.5 µmol l−1 (range 19.3–209 µmol l−1) and 3450 copies ml−1 (range <50–540000 copies ml−1), respectively.
Drug analysis
Plasma concentrations of stavudine were determined using a validated HPLC-UV method. A solid-phase extraction procedure was performed on 0.1 ml of plasma to which had been added the internal standard (2-acetamidophenol). The drug was separated on a satisfaction C8 Plus column (5 µm; 250 × 3mm) using an elution gradient. The initial conditions were: eluant A (0.01% trifluoroacetic acid containing 2% of methanol): 98% and eluant B (acetonitrile 95% in water): 2% which were changed at 11 min to: A: 90% and B: 10% for 20 min. The mobile flow rate was 0.5 ml min−1 and the column effluent was monitored at 278 nm. The assay was linear over the concentration range 0.02–4 mg l−1, and the limit of determination was 0.02 mg l−1. Mean precision and accuracy were less than 13 and 15%, respectively, over the calibration range.
Population pharmacokinetic modelling
Concentration-time data were analyzed using the first-order conditional estimation method and by non linear mixed effects modelling NONMEM (version V, level 1.1, double precision [6]). Several structural pharmacokinetic and error models were investigated. Covariate selection and bootstrap validation were performed as previously described [7]. Because SCr values were not available for all samples, the first analysis was performed on the database containing the 301 samples from patients in whom SCr had been measured. The addition of a database for SCr to the model did not result in a decrease in the objective function. Thus, the analysis was performed again using the whole dataset, including the samples for which SCr was unknown.
Calculation of the theoretical stavudine dose
Individual Bayesian estimates of CL/F were used to calculate the stavudine dose required to achieve a value of 1.60 mg l−1 h for AUC(0,12 h), which is the mean value reported in adults for the 40 mg twice daily dose [8, 9].
Results
The best fit to the data was obtained with a one-compartment model with zero-order absorption, the duration of the absorption phase being the estimated parameter. This model provided a further 89 points decrease in the objective function (OF) compared with the one-compartment model with first-order absorption. The two-compartment model did not improve the fit. Inter-patient variability was described by an exponential error model, and residual variability by a combined exponential and additive error model. A significant covariance term was found between the interindividual variability in CL/F and V/F. Inter-patient variability in the duration of absorption could not be estimated. Although the large standard deviation suggested that the latter parameter was not significant, the additive residual error had to be maintained in the model to allow a successful convergence. The addition of bodyweight to the CL/F and V/F models resulted in significant decreases of −158 and −22 points, respectively, in the OF compared with the basic model, and the addition of age resulted in decreases of −196 and −31 points. The effect of gender and the possibility of an influence of drug interactions on CL/F and V/F were systematically investigated, but they did not produce any decrease in the OF. Dosage form showed no influence on bioavailability. When age and bodyweight were included, a significant increase in the OF was obtained with the deletion of age on CL/F (+44 points) and on V/F (+7 points) but not with the deletion of bodyweight.
The final covariate models were
The coefficient of variation of CL/F and V/F decreased from 68% (CL/F) and 83% (V/F) in the basic model to 38% and 32%, respectively, in the final model. Table 1 summarizes the population parameter estimates. A previously reported allometric weight-scaling approach was also tested [10], but it did not improve the fit compared with our final model.
Table 1.
Population pharmacokinetic parameters for stavudine in 272 children and the results of the boostrap validation
| Final model | ||||
|---|---|---|---|---|
| Original data set | Boostrap* | |||
| Parameter | Mean | SE | Median | CI |
| TV (CL/F) (l h−1) | 16.5 | 0.74 | 16.1 | 12.8–17.9 |
| CL/F, θage | 0.40 | 0.023 | 0.40 | 0.34–0.45 |
| TV (V/F) (l) | 40.9 | 2.9 | 39.9 | 33.7–46.7 |
| V/F, θage | 0.40 | 0.034 | 0.39 | 0.29–0.46 |
| D (h) | 1.72 | 0.13 | 1.65 | 0.50–2.00 |
| ω2CL/F | 0.143 | 0.024 | 0.137 | 0.09–0.18 |
| ω2V/F | 0.102 | 0.043 | 0.087 | 0.01–0.19 |
| COVCL,V | 0.090 | 0.030 | 0.081 | 0.02–0.14 |
| Proportional residual variability, σ12 | 0.23 | 0.022 | 0.23 | 0.19–0.27 |
| Additive residual variability, σ22 | 0.00041 | 0.00022 | 0.00037 | 3.7 × 10–11–0.0012 |
Results of 1000 boostrap analyses; SE standard error of the estimate; TV typical value of the corresponding PK parameter;
θ covariateinfluential factor for covariate; D the duration of the absorption phase; ω2interindividual variability; COVCL,Vcovariance between ηs of CL/F and V/F; CI nonparametric 95% confidence interval.
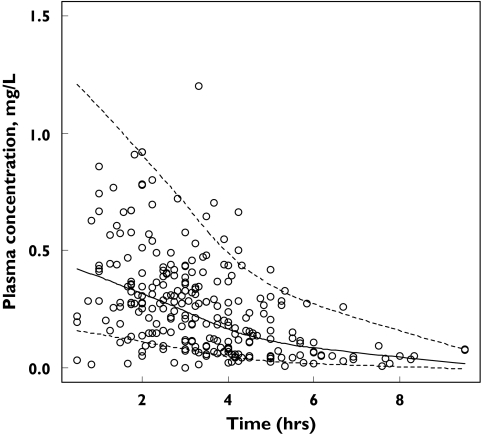
The accuracy of the final model was evaluated by a posterior visual predictive check obtained from 1000 simulations of the database [Figure 1].
Figure 1.
Accuracy of the final model evaluated by posterior visual predictive assessments obtained from 1000 simulations of the database. Curves corresponded to the 5th, 50th, and 95th percentiles of simulated concentrations, and 5.2% of the observed data were outside the 5th-95th percentiles
As shown in Table 1, the estimates previously obtained with the original dataset were validated by the bootstrap results.
For the age and weight categories defined by the dosing recommendations made by regulatory bodies, the calculated doses were 0.61 ± 0.16 mg kg−1 for neonates younger than 2 weeks, 1.23 ± 0.47 mg kg−1 for children older than 2 weeks and weighing up to 30 kg, and 31.5 ± 8.52 mg kg−1 for children weighing between 30 and 60 kg.
Discussion
The plasma pharmacokinetics of stavudine in children were well described by a one-compartment model with zero-order absorption, confirming the findings of others [2].
The mean pharmacokinetic parameter estimates were also close to the values previously reported in children [1–5].
Approximately 40% of a stavudine dose is excreted unchanged in the urine as a result of both glomerular filtration and net renal tubular secretion [11]. To our knowledge, the metabolic pathways involved in the elimination of the remaining 60% of the dose have not been clearly established. However, in children, both renal and hepatic function progressively mature with age [12]. Therefore, the presence of a relationship between age and stavudine pharmacokinetic parameters in a paediatric population was expected. Our results also indicated that stavudine CL/F reaches the reported adult value of 0.44 l h−1 kg−1 [13] at approximately 15 years of age.
As no pharmacokinetic/pharmacodynamic relationship has been reported for stavudine, the current dosing guidelines in children were made in order to achieve the same AUC(0,12 h) as in adults. For the current recommended 40 mg twice daily adult dosage regimen mean values for the AUC(0,12 h) of 1.25 [8] and 1.95 mg l−1 h [9] have been reported. Therefore, we used the mean of these two values (i.e. 1.60 mg l−1 h) as the target AUC(0,12 h) for our population. The calculated doses obtained from our analysis were consistent with the current recommended dosage regimen (i.e. 0.5 mg kg−1 twice daily for children younger than 2 weeks, 1 mg kg−1 twice daily for children older than 2 weeks and with bodyweight up to 30 kg, and 30 mg twice daily for children with a bodyweight between 30 and 60 kg). A small discrepancy was observed for children with a bodyweight less than 30 kg, as our model suggested about a 20% increase in the recommended dose. Furthermore, the high interindividual variability in calculated doses, especially for children weighing less than 30 kg, suggests the need for individual dose adjustments. However; there are no pharmacokinetic/pharmacodynamic data supporting such a dose adjustment. Since, a correlation between plasma stavudine and intracellular stavudine triphosphate concentrations has been reported [14], further studies are needed to determine whether plasma concentration data can be used as a guide to dose.
In conclusion, the present study, in a large paediatric population, found that CL/F and V/F of stavudine were similarly related to age, and supported the current dosing guidelines in children.
Acknowledgments
This work was supported by l'Institut National de la Santé et de la Recherche Médicale (INSERM, Contrat de Recherche Stratégique 2002).
References
- 1.Kaul S, Kline MW, Church JA, Dunkle LM. Determination of dosing guidelines for stavudine (2′,3′-didehydro-3′-deoxythymidine) in children with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2001;45:758–63. doi: 10.1128/AAC.45.3.758-763.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher CV, Brundage RC, Remmel RP, Page LM, Weller D, Calles NR, Simon C, Kline MW. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob Agents Chemother. 2000;44:1029–34. doi: 10.1128/aac.44.4.1029-1034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wade NA, Unadkat JD, Huang S, Shapiro DE, Mathias A, Yasin S, Ciupak G, Watts DH, Delke I, Rathore M, Hitti J, Frenkel L, Samelson R, Smith ME, Mofenson L, Burchett SK. Pharmacokinetics and safety of stavudine in HIV-infected pregnant women and their infants: Pediatric AIDS Clinical Trials Group protocol 332. J Infect Dis. 2004;190:2167–74. doi: 10.1086/425903. [DOI] [PubMed] [Google Scholar]
- 4.Kline MW, Dunkle LM, Church JA, Goldsmith JC, Harris AT, Federici ME, Schultze ME, Woods L, Loewen DF, Kaul S, et al. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96(2)(Part 1):247–52. [PubMed] [Google Scholar]
- 5.Rongkavilit C, Thaithumyanon P, Chuenyam T, Damle BD, Limpongsanurak S, Boonrod C, Srigitsanapol A, Hassink EA, Hoetelmans RM, Cooper DA, Lange JM, Ruxrungtham K, Phanuphak P. Pharmacokinetics of stavudine and didanosine coadministered with nelfinavir in human immunodeficiency virus-exposed neonates. Antimicrob Agents Chemother. 2001;45:3585–90. doi: 10.1128/AAC.45.12.3585-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beal SL, Sheiner LB. NONMEM User's GuideNONMEM Project Group. San Francisco: University of California at San Francisco; 1991. [Google Scholar]
- 7.Jullien V, Treluyer JM, Chappuy H, Dimet J, Rey E, Dupin N, Salmon D, Pons G, Urien S. Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients. Br J Clin Pharmacol. 2005;59:183–8. doi: 10.1111/j.1365-2125.2004.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seifert RD, Stewart MB, Sramek JJ, Conrad J, Kaul S, Cutler NR. Pharmacokinetics of co-administered didanosine and stavudine in HIV-seropositive male patients. Br J Clin Pharmacol. 1994;38:405–10. doi: 10.1111/j.1365-2125.1994.tb04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul S, Mummaneni V, Barbhaiya RH. Dose proportionality of stavudine in HIV seropositive asymptomatic subjects: application to bioequivalence assessment of various capsule formulations. Biopharm Drug Dispos. 1995;16:125–36. doi: 10.1002/bdd.2510160207. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BJ, Woollard GA, Holford NH. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol. 2000;50:125–34. doi: 10.1046/j.1365-2125.2000.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley MN, Graham KK, Kaul S, Geletko S, Dunkle L, Browne M, Mayer K. Pharmacokinetics of stavudine in patients with AIDS or AIDS-related complex. J Infect Dis. 1992;166:480–5. doi: 10.1093/infdis/166.3.480. [DOI] [PubMed] [Google Scholar]
- 12.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 13.Horton CM, Dudley MN, Kaul S, Mayer KH, Squires K, Dunkle L, Anderson R. Population pharmacokinetics of stavudine (d4T) in patients with AIDS or advanced AIDS-related complex. Antimicrob Agents Chemother. 1995;39:2309–15. doi: 10.1128/aac.39.10.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becher F, Landman R, Mboup S, Kane CN, Canestri A, Liegeois F, Vray M, Prevot MH, Leleu G, Benech H. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. AIDS. 2004;18:181–7. doi: 10.1097/00002030-200401230-00006. [DOI] [PubMed] [Google Scholar]