Abstract
What is already known about this subject
Despite encouraging effects of N-methyl-D-aspartate (NMDA) receptor antagonists in reducing neuropathic pain of different aetiologies, the clinical use of these agents has been limited by their mainly psychotropic side-effects.
In a recent study in healthy volunteers, CNS 5161, a novel noncompetetive NMDA receptor antagonist, was well tolerated up to a dosage of 2000 µg without psychotropic side-effects.
This is the first study to evaluate the maximal tolerated dosage of CNS 5161 and to gain experience about the analgesic effect of CNS 5161 in patients with different pain syndromes.
What this study adds
In patients with neuropathic pain CNS 5161 is well tolerated up to a dosage of 500 µg with the most common side-effect of increasing blood pressure, mild visual disturbances and headaches.
While no therapeutic effect can be observed in a dosage up to 250 µg, treatment with 500 µg CNS 5161 provides some indications of analgesic activity.
It appears that this effect occurs predominantly in patients with diabetic neuropathy.
Aims
The purpose of the current study was to establish the safety and maximal tolerated dose of CNS 5161 HCl.
Methods
Forty patients with chronic neuropathic pain (23 male, 17 female) were treated with escalating dosages of CNS 5161. All adverse events to study drug, blood pressure, heart rate, ECG, drug level and clinical laboratory values were monitored. Actual pain was measured on a 100-mm visual analogue scale (VAS) and ordinal verbal pain scores.
Results
The most commonly occurring nervous system disorder was headache, which was found more often during placebo than during CNS 5161 HCl treatment. Visual disturbances were experienced by 16.7% of patients receiving 250 µg and by 33.3% receiving 500 µg CNS 5161 HCl, but not during placebo treatment. An increase in blood pressure was observed in 8.3% of patients receiving 250 µg and in 50% of patients receiving 500 µg CNS 5161 HCl, compared with 15.4% during placebo treatment. The study was abandoned after two patients entered the 750 µg cohort due to a sustained systolic blood pressure response. Although this study was underpowered for the confirmation of efficacy, some indications of greater pain relief after 500 µg CNS 5161 compared with placebo could be observed (change in VAS between baseline and 12 h 10 ± 22 mm vs. 2 ± 19 mm; P = 0.11).
Conclusions
CNS 5161 HCl was reasonably well tolerated up to 500 µg. The most common adverse events were hypertension, headache and mild visual disorders.
Keywords: analgesic therapy, NMDA receptor antagonist, painful neuropathy
Introduction
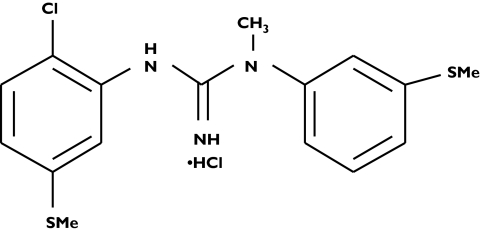
Pain is a symptom of a variety of acute and chronic neurological syndromes, including truncal, proximal motor, acute sensory and chronic distal sensorimotor neuropathies. Development and persistence of neuropathic pain is a multifaceted process in which peripheral nerve injury induces a cascade of degeneration, biochemical and regulatory changes at the levels of the peripheral axons, spinal root, spinal cord and brain [1]. Neurotransmission of pain is regulated by complex excitatory and inhibitory interactions from several spinal cord cells, acting on neurokinin, N-methyl-D-aspartate (NMDA) and opiate receptors embedded in the postsynaptic membrane [2]. Neuropathic pain appears to be less responsive to opioid drugs than nociceptive pain. Recent evidence suggests that NMDA receptor antagonists may be effective in the treatment of neuropathic pain of several aetiologies [2–7]. However, treatment has been significantly limited by the psychotropic side-effects seen with such substances. CNS 5161 HCl (N-(2-chloro-5-(methylmercapto)phenyl)-N′-(3-(methylmercapto)phenyl)-N′-methylguanidine) (HCl) is a novel and selective noncompetitive NMDA antagonist with a Ki of 1.8 nm for the ion-channel binding site of the NMDA receptor complex [8]. The chemical structure of CNS 5161 is shown in Figure 1.
Figure 1.
Chemical structure of CNS 5161 HCl (N-(2-chloro-5-(methylmercapto)phenyl)-N′-(3-(methylmercapto)phenyl)-N′-methylguanidine) (HCl)
CNS 5161 appears to be different from traditional high-affinity NMDA antagonists since in preliminary preclinical and clinical studies no psychometric effects have been observed. In a previous study, CNS 5161 was well tolerated in human healthy volunteers, the major observation at high doses being a rise in blood pressure [9]. The purpose of the current study was to establish a maximum tolerated dose of CNS 5161 HCl by evaluating its safety at escalating dose levels in patients with neuropathic pain. In addition, the effects of CNS 5161 HCl on a visual pain analogue scale (VAS), a verbal pain intensity (VPI) and a verbal pain relief (VPR) score were recorded.
Methods
Study procedure
This was a multicentre, double-blind, placebo-controlled, crossover study of CNS 5161 HCl in patients with chronic neuropathic pain. Each patient received a single dose of CNS 5161 HCl or placebo in a randomized sequence with a wash-out period of at least 7 days in between both treatments. Patients were randomly assigned to their treatment sequence according to a computer-generated randomization list. Cohorts of 12 patients were intended to receive escalating dosages of 125, 250, 500 and 750 µg CNS 5161, after safety data from the preceding cohort had been assessed by a safety review board. The study participants received the study treatment intravenously as a controlled infusion in 50 ml saline. Fifteen millilitres of the total volume was given as a bolus within the first 10 min and the remaining 35 ml were continuously infused over 5 h 50 min. The patients remained in hospital overnight and were released the next morning after blood and urine samples had been provided for laboratory safety evaluations and vital signs, ECG, concomitant medications and adverse events (AEs) had been recorded and the pain questionnaires had been completed.
Patients were instructed to continue on their regular medication if the medications had been taken in a stable dosage for the previous 3 months. No change in analgesic medication was allowed during the course of the study.
Local ethics approval was granted and all study participants provided written informed consent.
Study population
The trial inclusion criteria comprised: males or females aged 21–75 years, neuropathic pain of at least 6 months' duration, current medication stable for 3 months before the start of the study, ability and willingness to participate, and a stable pain perception [with a mean of ≥35 mm on VAS of 100 mm during the 6-day (±2 days) screening period]. The exclusion criteria were: uncontrolled hypertension defined as systolic blood pressure >150 mmHg or diastolic >90 mmHg, concurrent administration of ketamine, history of spontaneous nontraumatic intracranial haemorrhage and concurrent severe mental deficit.
Study medication
Study drug was supplied as 5-ml vials containing CNS 5161 HCl 1 mg ml−1 (as base) sterile solution for injection, and 5-ml vials of matching placebo. At the investigator sites, the study drug was diluted in a 50-ml syringe using sterile physiological saline. Initially a slow bolus dose of 30% of the total dose of study drug was administered over 10 min. The remaining dose (containing 70% of the total dose) was administered as a continuous infusion from 10 min to 6 h.
Pharmacokinetic analysis
Blood samples for pharmacokinetic analysis were taken at baseline, at 10 min, 2, 6, 9, 12 and 24 h after the commencement of infusion on each treatment day. A liquid chromatography–tandem mass spectrometry bioanalytical assay was used for determination of plasma CNS 5161 levels. Pharmacokinetic analysis was conducted using WinNonlinProb, version 4.1.
Safety measurements
Physical examinations were performed at screening, before drug administration at each treatment visit and at the 7-day follow-up visit. Standard laboratory tests were performed at screening, at baseline for each treatment period, at 24 h after the end of each drug administration and at the 7-day follow-up visit. Safety laboratory analyses comprised: haemoglobin, haematocrit, red blood cells, platelet count, white blood cells, neutrophils, lymphocytes, monocytes, eosinophils and basophils, sodium, potassium, creatinine, urea, alanine aminotransferase, aspartate aminotransferase, total bilirubin and γ-glutamyl transferase. Changes in physical examination and laboratory test values were evaluated for safety considerations. Vital signs, blood pressure and ECG were monitored throughout drug administration every 30 min, thereafter hourly up to 12 h and once more after 24 h. All AEs over the entire duration of study participation were documented.
Efficacy measurements
Pain was evaluated at baseline before the medication was applied, at 2 h into drug administration, at the end of drug administration and at 12 and 24 h. A VAS (0 mm = no pain and 100 mm = worst possible pain) was used to record the pain level. The distance from the start of the line (0 mm) to the point where the patient's mark crossed the line was recorded [10, 11]. In addition, a VPI and a VPR were used. The VPI is an ordinal scale, rating pain intensity between 0 (no pain) and 5 (worst pain imaginable), while the VPR scale is used for the rating of pain change over time providing scores from 0 (pain worse) to 5 (complete relief of pain).
Statistical analysis
Summary statistics for quantitative data were recorded as n and mean ± SD (or range). For categorical data, a frequency table (showing n and %) replaced this summary. All safety analyses were performed on the safety analysis set, which included all patients randomized in the study. The primary efficacy variable was the VAS for pain intensity. Secondary efficacy variables were the VPI and VPR scales. Efficacy variables were analysed on the per protocol (PP) analysis set. Within each cohort, the study drug was compared with placebo using Koch's nonparametric test of treatment difference (i.e. a Mann–Whitney test between treatment sequences of the differences between treatment period 1 and treatment period 2) for each scheduled time post dose.
Results
A total of 40 patients were recruited and received one dose of study drug on one occasion and placebo on another occasion in randomized order. The clinical characteristics, the origin of neuropathic pain and the number of hypertensive patients according to the different dose cohorts are given in Table 1.
Table 1.
Clinical characteristics and origin of neuropathic pain in the different cohorts [n; mean ± SD; (range); safety analysis set]
| Cohort 1 125 µg | Cohort 2 250 µg | Cohort 3 500 µg | Cohort 4 750 µg | |
|---|---|---|---|---|
| n | 12 | 12 | 14 | 2 |
| History of hypertension | 3 | 4 | 8 | 0 |
| Age (years) | 54.8 ± 9.7 | 53.0 ± 14.5 | 56.4 ± 10.2 | 62.5 ± 16.3 |
| (range) | (40–74) | (32–73) | (40–72) | (51–74) |
| Sex (male/female) | 8/4 | 5/7 | 8/6 | 2/0 |
| Height (cm) | 173 ± 11 | 170 ± 9 | 171 ± 8 | 185 ± 0 |
| Weight (kg) | 83 ± 18 | 79 ± 19 | 81 ± 18 | 101 ± 8 |
| Postherpetic pain (n) | 3 | 2 | 0 | 1 |
| Diabetic neuropathy (n) | 0 | 3 | 8 | 0 |
| Post-traumatic injury (n) | 6 | 6 | 4 | 1 |
| CRPS I (n) | 2 | 0 | 1 | 0 |
| CRPS 2 (n) | 1 | 1 | 1 | 0 |
CRPS, chronic regional pain syndrome.
Twelve patients joined each of cohorts 1 (125 µg) and 2 (250 µg) and all of them completed the study. A further patient was randomized to cohort 2, but by mistake received a partial dose of treatment assigned for cohort 3. This patient was included in the cohort 3 safety analysis and withdrawn from efficacy analysis population. Thirteen patients were initially randomized to cohort 3 (500 µg), which made a total of 14 included in this cohort. One withdrew consent before treatment, the other was withdrawn following the above discribed dosing error. Twelve patients completed cohort 3. Two patients were randomized to cohort 4, which was terminated after one patient completed both treatment periods and one patient completed the first period due to a sustained increase in systolic blood pressure for >6 h.
The pharmacokinetic characteristics of CNS 5161 at escalating dose steps are summarized in Table 2. Thirty percent of the total dose was applied within the first 10 min and the remaining 70% were infused over 5 h 50 min. The terminal half-life of the drug was comparable in between the 125 µg and 500 µg. A linear relationship of plasma concentration was found between the 250-µg and 500-µg doses. The mean plasma concentration in the 125 µg dose group was approximately one-third that of the 250-µg group.
Table 2.
Pharmcokinetic data of CNS 5161 (mean ± SD) in the different dosage cohorts
| Cohort 1 125 µg 12 | Cohort 2 250 µg 12 | Cohort 3 500 µg 12 | |
|---|---|---|---|
| t1/2 (h) | 8.2 ± 3.4 | 8.7 ± 3.7 | 8.3 ± 1.6 |
| Cmax (pg ml−1) | 331.6 ± 204.9 | 876.0 ± 582.0 | 1056.7 ± 484.0 |
| AUC0−24 h (h pg−1 ml−1 µg−1) | 1772.3 ± 565.6 | 4348.2 ± 915.9 | 7017.3 ± 2093.3 |
One serious AE was reported: a case of cerebral ischaemia in a 51-year-old patient 8 days after administration of 750 µg of CNS 5161 HCl, which required hospitalization but resolved in 14 days. This event was unexpected and considered unlikely to be related to study treatment or procedure; it was possibly related to the patient's history of diabetes and smoking.
Nervous system disorders, classified as treatment related (probable or possible association with study drug) occurred with increasing frequency during dose escalation of CNS 5161 HCl. The most commonly occurring was headache, which was reported approximately once per affected patient at 125 µg (two events in two of 12 patients), 250 µg (five events in four of 12 patients) and 500 µg (four events in three of 12 patients), but also at a rate of approximately two events per affected patient in those receiving placebo (14 events in seven of 39 patients). Blurred vision was experienced by 16.7% of patients receiving CNS 5161 HCl at 250 µg, and 33.3% receiving 500 µg. No patients experienced visual disturbances during placebo treatment. There were cases of flatulence (16.7% in those receiving CNS 5161 HCl at 500 µg), dyspepsia and abdominal discomfort (8.3% at 250 µg) and nausea (8.3% at 500 µg) during study drug application.
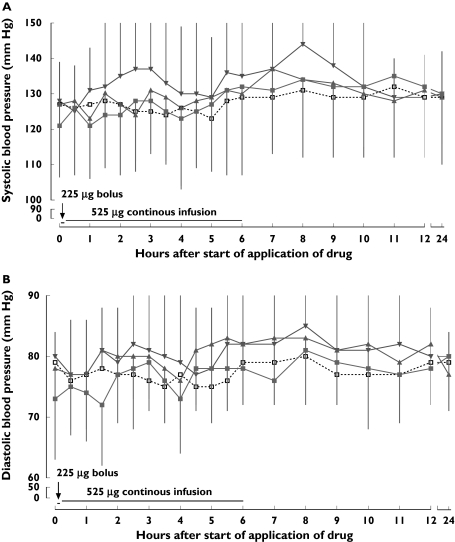
Infusion of CNS 5161 at all dosages was associated with an increase in mean systolic, and to a lesser extent also in diastolic blood pressure. The changes in blood pressure were found to be dose related. The time course of mean systolic and diastolic blood pressure values for each dose cohort is given in Figure 2a,b.
Figure 2.
(a, b) Time course of systolic (a) and diastolic (b) blood pressure during the different treatment periods (mean ± SD;  , 125 µg;
, 125 µg;  , 250 µg;
, 250 µg;  , 500 µg CNS 5161 HCl; □, placebo)
, 500 µg CNS 5161 HCl; □, placebo)
At 125 µg and 250 µg of CNS 5161, a slight systolic blood pressure elevation was found within the first 6 h of study drug appliacation, which remained clinically negligible.
In the 500-µg cohort, onset of the increase in mean systolic pressure was at about 1–1.5 h into the infusion, initially peaking at 2.5–4 h (mean difference from baseline at 2.5 h = 9.1 ± 24.3 mmHg and declining towards baseline levels at 5 h). There was a second peak in systolic pressure, reaching a maximum at 8 h (mean difference from baseline 16.3 ± 19.9 mmHg), followed by a decline towards baseline by 11 h after the start of infusion. Mean diastolic pressure appeared largely undisturbed, except for a peak at 8 h when the mean change was 5.8 ± 12.4 mmHg.
At 750 µg, mean systolic blood pressure increased 30 min after the start of the infusion (increase over baseline of 8.5 ± 9.2 mmHg), from which it continued to rise, reaching a peak increase over baseline of 28.0 ± 45.3 mmHg after 6 h, and thereafter rapidly declined towards baseline levels. In this small patient group, diastolic blood pressure was essentially unaffected.
Elevation of blood pressure was experienced by one (8.3%) patient receiving CNS 5161 HCl at 250 µg, by six (50.0%) receiving 500 µg and by one of the two patients who received 750 µg, compared with 15.4% during placebo treatment. In most cases there was a history of controlled hypertension. Heart rate fell slightly with all treatments, including placebo. There were no consistent ECG abnormalities associated with CNS 5161 HCl treatment, and no clinically significant alterations in safety laboratory parameters.
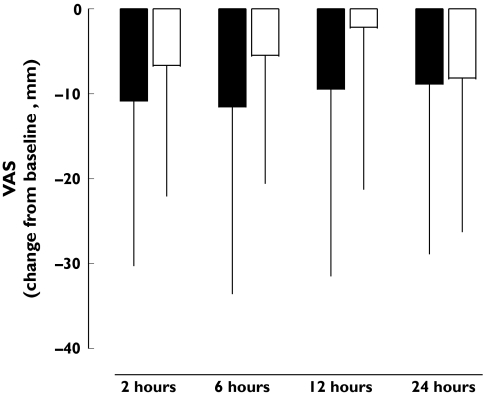
This early safety study was insufficiently powered to demonstrate analgesic efficacy of the drug, but the opportunity was taken to study some measures of pain relief. Since only one patient finished treatment cohort 4, the 750-µg cohort was excluded from efficacy evaluation. The time course of the VAS score for the different dosage cohorts is presented in Table 3. At the dose level of 125 µg and 250 µg CNS 5161 HCl, no difference in the VAS score could be found between CNS 5161 and placebo. In patients receiving 500 µg CNS 5161, the VAS score was found to be slightly lower in the CNS 5161 group within the first 12 h after application compared with the placebo group. As shown in Figure 3, there is some indication of a stronger reduction in pain after CNS 5161 HCl over the first 12 h measured by VAS compared with placebo.
Table 3.
Visual analog scale for the 125–500-µg cohorts at baseline and during the observational period (mean ± SD)
| Baseline | 2 h | 6 h | 12 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNS5161 | Placebo | CNS5161 | Placebo | CNS5161 | Placebo | CNS5161 | Placebo | CNS5161 | Placebo | |
| 125 µg | 64 ± 16 | 64 ± 17 | 50 ± 25 | 51 ± 22 | 50 ± 28 | 47 ± 27 | 51 ± 30 | 46 ± 29 | 53 ± 26 | 52 ± 30 |
| 250 µg | 62 ± 13 | 55 ± 10 | 48 ± 20 | 43 ± 21 | 50 ± 18 | 41 ± 22 | 50 ± 19 | 44 ± 21 | 50 ± 20 | 50 ± 15 |
| 500 µg | 65 ± 20 | 65 ± 18 | 54 ± 23 | 58 ± 20 | 53 ± 25 | 59 ± 23 | 55 ± 27 | 63 ± 23 | 56 ± 31 | 57 ± 24 |
Figure 3.
Change in pain visual analogue scale (VAS) from baseline (mm; mean ± SD; ▪, 500 µg CNS 5161 HCl; □, placebo)
No differences in the VPI or VPR scores or the change in VPI or VPR scores could be observed betweenplacebo and CNS 5161 HCl for any dose of CNS 5161 HCl.
Discussion
Pharmacological treatment of neuropathic pain is still a challenge, and conventional analgesics such as morphine and nonsteroidal anti-inflammatory drugs have shown limited therapeutic efficacy. Tricyclic antidepressants, serotonin-noradrenalin reuptake inhibitors and pregabalin, often used for the treatment of neuropathic pain, do not give sufficient pain reduction in up to 50% of patients [2, 12–15]. Therefore, there is still a need for the development of drugs providing adequate pain relief in patients with chronic neuropathic pain syndromes. Excessive activation of glutamate receptors, especially of the NMDA subtype, has been associated with neuropathic pain. Noncompetitive NMDA antagonists such as ketamine have been reported to ameliorate neuropathic pain, but their clinical use for the treatment of neuropathic pain is limited by their side-effects, which include auditory and visual disturbances, hallucinations, feelings of unreality, feelings of detachment, dizziness, sedation, ataxia, motor incoordination, etc. [16–18]. Therefore, there has been a strenuous effort to develop new NMDA antagonists with pharmacological properties allowing pain reduction with a reduced side-effect profile. Noncompetitive NMDA antagonists with low affinity to the ion channel appeared to evoke lower neurotoxicity [19, 20]. CNS 5161 HCl is a novel agent that interacts with the NMDA receptor/ion channel site to produce a noncompetitive blockade of the actions of glutamate [8]. In a previous study in healthy volunteers it was well tolerated at a dose range between 30 and 2000 µg, with a dose-dependent rise in systolic and diastolic blood pressure reaching a maximum between 60 and 120 min following drug application, but no psychomimetic effects [9].
The most common AEs reported by the patients in our study were mild headaches and visual disturbances. While the frequency of headaches was not different between CNS 5161 HCl and placebo treatment, visual disturbances occurred more often after CNS 5161 HCl compared with placebo. No other psychomimetic effects of CNS 5161 HCl were observed at any dosage in any patient. As previously shown with other noncompetitive NMDA antagonists [6, 16], CNS 5161, at dosages up to 500 µg, revealed less psychomimetic effects compared with ketamin and similar NMDA antagonists.
NMDA receptors in the central nervous system are involved in blood pressure and heart rate regulation, and treatment with NMDA antagonists is well known to increase systolic and diastolic blood pressure in animals and humans [9, 21–23]. In agreement with these studies, the most common side-effect of CNS 5161 in our study was an elevation of systolic blood pressure. It occurred in 50% of the patients receiving 500 µg CNS 5161 HCl and resulted in the termination of the study after two patients entered the 750-µg cohort. Therefore, the maximum tolerated dose of CNS 5161 seems to be <750 µg, due to increasing haemodynamic effects.
This study was not sufficiently powered to investigate the analgesic efficacy of CNS 5161. Nevertheless, the observed efficacy results further indicate an analgesic effect of CNS 5161 HCl at a dosage of 500 µg. Analysis of the diagnostic subgroups of neuropathic pain within the cohorts showed no clear effect of CNS 5161 HCl at 125 µg and 250 µg on the VAS. Following the administration of 500 µg, CNS 5161 HCl seemed to exert differential efficacy in pain of different aetiologies. CNS 5161 HCl seemed most effective in patients suffering from diabetic neuropathy, who showed reductions in VAS of 16.7 ± 20.5, 21.5 ± 25.9 and 20.3 ± 24.8 mm compared with 11.7 ± 20.42, 13.8 ± 17.1 and 6.2 ± 2.7 mm during placebo treatment at 2, 6 and 12 h, respectively. This finding should be interpreted with care due to the small sample size and it remained unresolved whether the apparent analgesic effect might be related to the higher dose level or the increased prevalence of diabetic patients in that dose cohort. The results of the present study support further work with CNS 5161 HCl in homogeneous neuropathic pain populations with sufficient patient numbers to validate a clinically relevant analgesic effect of the drug.
In conclusion, in patients with neuropathic pain of various aetiologies, CNS 5161 HCl was reasonably well tolerated at single infusions of up to 500 µg, and some indications of analgesic efficacy could be observed. The most common adverse events were hypertension, headache and mild visual disorders. CNS 5161's psychotropic profile appears promising, which might lead to improved tolerability in the treatment of neuropathic pain syndromes compared with other NMDA receptor antagonists. Further studies will attempt to clarify the clinical efficacy of CNS 5161 HCl in distinct patient groups, especially in diabetic neuropathy.
Acknowledgments
This study was financially supported by CeNeS Ltd, Cambridge, UK. T.F. and A.P. have received research funds from CeNeS Ltd. P.M. is an independent pharmaceutical medicine consultant retained by CeNeS Ltd, the study sponsor. T.S. is an employee of CeNeS Ltd.
References
- 1.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–10. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Singleton JR. Evaluation and treatment of painful peripheral polyneuropathy. Semin Neurol. 2005;25:185–95. doi: 10.1055/s-2005-871327. [DOI] [PubMed] [Google Scholar]
- 3.Pud D, Eisenberg E, Spitzer A, Adler R, Fried G, Yarnitsky D. The NMDA receptor antagonist amantadine reduces surgical neuropathic pain in cancer patients: a double blind, randomized, placebo controlled trial. Pain. 1998;75:349–54. doi: 10.1016/s0304-3959(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 4.Stannard CF, Porter GE. Ketamine hydrochloride in the treatment of phantom limb pain. Pain. 1993;54:227–30. doi: 10.1016/0304-3959(93)90214-A. [DOI] [PubMed] [Google Scholar]
- 5.Backonja M, Arndt G, Gombar KA, Check B, Zimmermann M. Response of chronic neuropathic pain syndromes to ketamine: a preliminary study. Pain. 1994;56:51–7. doi: 10.1016/0304-3959(94)90149-X. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson KC, Hoem NO, Moberg ER, Mathisen LC. Analgesic effect of dextromethorphan in neuropathic pain. Acta Anaesthesiol Scand. 2004;48:328–36. doi: 10.1111/j.0001-5172.2004.0325.x. [DOI] [PubMed] [Google Scholar]
- 7.Amin P, Sturrock ND. A pilot study of the beneficial effects of amantadine in the treatment of painful diabetic peripheral neuropathy. Diabet Med. 2003;20:114–8. doi: 10.1046/j.1464-5491.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- 8.Hu LY, Guo J, Magar SS, Fischer JB, Burke-Howie KJ, Durant GJ. Synthesis and pharmacological evaluation of N-(2,5-disubstituted phenyl)-N-(3-substituted phenyl)-N-methylguanidines as N-methyl-D-aspartate receptor ion-channel blockers. J Med Chem. 1997;40:4281–9. doi: 10.1021/jm970459c. [DOI] [PubMed] [Google Scholar]
- 9.Walters MR, Bradford AP, Fischer J, Lees KR. Early clinical experience with the novel NMDA receptor antagonist CNS 5161. Br J Clin Pharmacol. 2002;53:305–11. doi: 10.1046/j.0306-5251.2001.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–84. [PubMed] [Google Scholar]
- 11.Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–9. doi: 10.1038/clpt.1983.159. [DOI] [PubMed] [Google Scholar]
- 12.Barbano R, Hart-Gouleau S, Pennella-Vaughan J, Dworkin RH. Pharmacotherapy of painful diabetic neuropathy. Curr Pain Headache Report. 2003;7:169–77. doi: 10.1007/s11916-003-0070-9. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116:109–18. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–38. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254–63. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Chizh BA, Headley PM. NMDA antagonists and neuropathic pain – multiple drug targets and multiple uses. Curr Pharm Des. 2005;11:2977–94. doi: 10.2174/1381612054865082. [DOI] [PubMed] [Google Scholar]
- 17.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 18.Hocking G, Cousins MJ. Ketamine in chronic pain management: an evidence-based review. Anesth Analg. 2003;97:1730–9. doi: 10.1213/01.ANE.0000086618.28845.9B. [DOI] [PubMed] [Google Scholar]
- 19.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–69. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 20.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist – a review of preclinical data. Neuropharmacology. 1999;38:735–67. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang WZ, Yuan WJ, Su DF. Blockade of N-methyl-D-aspartate receptors within the rostral ventrolateral medulla antagonizes clonidine-induced cardiovascular effects. Auton Neurosci. 2003;109:21–8. doi: 10.1016/j.autneu.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Sitniewska EM, Wisniewska RJ, Wisniewski K. The role of ionotropic receptors of glutaminic acid in cardiovascular system. A. The influence of ionotropic receptor NMDA agonist – 1R,3R-ACPD and antagonist – DL-AP7 on the systemic pressure in rats. Amino Acids. 2003;24:397–403. doi: 10.1007/s00726-002-0342-4. [DOI] [PubMed] [Google Scholar]
- 23.Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001;286:2673–82. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]