Abstract
What is already known about this subject
Gliclazide has been considered metabolized by CYP2C9.
Its modified release formulation, gliclazide MR, shows low pharmacokinetic variability in Whites but high variability in Chinese.
What this study adds
The results of this study show that the pharmacokinetics of gliclazide MR are affected mainly by CYP2C19 genetic polymorphism instead of CYP2C9 genetic polymorphism.
CYP2C19 genetic polymorphism might be responsible for the high pharmacokinetic variability of gliclazide MR in Chinese.
Aims
To investigate the influence of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics of gliclazide modified release (MR) in healthy Chinese subjects.
Methods
In a single-dose pharmacokinetic study, 24 healthy male subjects with various CYP2C9 and CYP2C19 genotypes received an oral dose of 30 mg gliclazide MR and plasma was sampled for 72 h postdose. In a multiple-dose pharmacokinetic study, 17 other CYP2C9*1 homozygotes with various CYP2C19 genotypes received 30 mg gliclazide MR once daily for 6 days and plasma was sampled after the last dose. The plasma concentrations of gliclazide were measured using a validated LC/MS/MS method. CYP2C9 and CYP2C19 genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism analysis.
Results
In the single-dose study, no significant difference in any pharmacokinetic parameters was found in CYP2C9*1/*1, *1/*3 and *1/*13 subjects. In contrast, the AUC0–∞ of gliclazide was significantly increased by 3.4-fold [95% confidence interval (CI) 2.5, 4.7; P< 0.01] in CYP2C19 poor metabolizer (PM) subjects compared with CYP2C19*1 homozygotes. The half-life (t1/2) was prolonged from 15.1 to 44.5 h (P< 0.01). Similar differences were found in the multiple-dose study. The parameters of gliclazide AUCss, AUC0–∞ and Cmax were 3.4-fold (95% CI 2.9, 4.0), 4.5-fold (95% CI 3.8, 5.4) and 2.9-fold (95% CI 2.4, 3.4) increased (P< 0.01) in CYP2C19 PM subjects, respectively, compared with CYP2C19*1 homozygotes, and t1/2 was increased from 13.5 to 24.6 h (P< 0.01).
Conclusions
The pharmacokinetics of gliclazide MR are affected mainly by CYP2C19 genetic polymorphism instead of CYP2C9 genetic polymorphism.
Keywords: clinical pharmacokinetics, CYP2C19, CYP2C9, gliclazide, polymorphism
Introduction
Both cytochrome P450 (CYP) 2C9 and CYP2C19, which are responsible for metabolizing over 20% of clinical therapeutic drugs, are known as genetically polymorphic enzymes [1, 2]. CYP2C9*2 (Arg144Cys) and *3 (Ile359Leu), with allele frequencies of 0.08–0.125 and 0.03–0.085, respectively, in Whites, have been recognized in humans as the main CYP2C9 variants and have reduced catalytic activity compared with the wild type (CYP2C9*1) [1]. In Asian populations, CYP2C9*2 is rare, and CYP2C9*3 and *13 (Leu90Pro), with allele frequencies of 0.043–0.077 and 0.00–0.012, respectively, are the main CYP2C9 variants [3]. CYP2C9*13 has been identified in a poor metabolizer (PM) of lornoxicam [4, 5]. In vitro and in vivo studies have indicated that this mutation is associated with impaired activity of CYP2C9 [6, 7].
In contrast to CYP2C9, Asian populations have a greater incidence of PMs of CYP2C19 phenotype than Whites and Africans, and 97% of PMs were identified to carry two mutant alleles of the CYP2C19 gene, CYP2C19*2/*2, *2/*3 or *3/*3[8, 9]. Therefore, it is important to know whether the genetic polymorphism of CYP2C19 influences the disposition of a drug whose metabolism is mediated by CYP2C19 in Asian populations.
Gliclazide is a second-generation of sulphonylurea oral hypoglycaemic agent used in the treatment of Type 2 diabetes mellitus. Compared with other hypoglycaemic agents, it is well tolerated, with a relatively low incidence of hypoglycaemia, and may have beneficial effects beyond the reduction of blood glucose [10]. Gliclazide undergoes extensive metabolism to several inactive metabolites in humans, mainly methylhydroxygliclazide and carboxygliclazide [11]. Rieutord et al. have reported that tolbutamide hydroxylase, which is CYP2C9 in man, is involved in the formation of hydroxygliclazde in rats in vitro[12] and Park et al. have found that rifampin increases gliclazide metabolism in humans in vivo[13]. However, it remains uncertain which enzyme mediates the metabolism of gliclazide in humans. Consequently, the influence of enzyme polymorphisms on its disposition has not been reported.
Gliclazide modified release (MR) is a new formulation of gliclazide and is given once daily. Clinical studies have shown that gliclazide MR 30–120 mg once daily has similar efficacy to immediate release (IR) 80–320 mg day−1 in patients with Type 2 diabetes mellitus [14]. The major advantage of this formulation over the IR preparation is a lower incidence of hypoglycaemia [15]. Its once-daily dosing regimen is also helpful to improve patient compliance. It has been reported that gliclazide MR shows high bioavailability and low variability in pharmacokinetics, and the absorption of gliclazide is unaffected by coadministration with food [16]. However, our previous data have shown that this formulation exhibits large variability in pharmacokinetics in Chinese subjects.
In the present study, we compared the relative effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics of gliclazide MR in Chinese subjects with a single-dose treatment. The influence of CYP2C19 polymorphism on the pharmacokinetics of gliclazide MR after multiple-dose exposure was also assessed.
Methods
Subjects
Twenty-four healthy male Chinese subjects, with a mean age of 22 years (range 20–30 years) and a mean weight of 64 kg (range 56–80 kg), were enrolled in the single-dose study. CYP2C9 and CYP2C19 genotypes of these subjects are listed in Table 1.
Table 1.
Demographic characteristics and genotypes of 24 subjects in the single-dose study of gliclazide MR
| Genotype | Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Weight (kg) | CYP2C9 | CYP2C19 | Subject | Age (years) | Weight (kg) | CYP2C9 | CYP2C19 |
| 1 | 22 | 70 | *1/*1 | *1/*2 | 13 | 20 | 66 | *1/*1 | *1/*3 |
| 2 | 22 | 57 | *1/*1 | *1/*1 | 14 | 23 | 70 | *1/*3 | *1/*1 |
| 3 | 21 | 68 | *1/*1 | *1/*2 | 15 | 21 | 65 | *1/*3 | *1/*2 |
| 4 | 22 | 52 | *1/*1 | *1/*1 | 16 | 21 | 70 | *1/*3 | *1/*1 |
| 5 | 21 | 67 | *1/*1 | *1/*3 | 17 | 22 | 60 | *1/*3 | *1/*1 |
| 6 | 20 | 56 | *1/*1 | *1/*3 | 18 | 22 | 60 | *1/*3 | *1/*1 |
| 7 | 22 | 60 | *1/*1 | *1/*2 | 19 | 22 | 56 | *1/*3 | *1/*1 |
| 8 | 23 | 58 | *1/*1 | *1/*3 | 20 | 22 | 75 | *1/*3 | *1/*2 |
| 9 | 26 | 70 | *1/*1 | *2/*3 | 21 | 23 | 68 | *1/*3 | *1/*1 |
| 10 | 21 | 57 | *1/*1 | *2/*2 | 22 | 22 | 65 | *1/*13 | *1/*2 |
| 11 | 21 | 57 | *1/*1 | *1/*1 | 23 | 23 | 66 | *1/*13 | *1/*2 |
| 12 | 22 | 80 | *1/*1 | *1/*2 | 24 | 30 | 60 | *1/*13 | *2/*2 |
Another 17 healthy male Chinese subjects, with a mean age of 21 years (range 19–26 years) and a mean weight of 65 kg (range 57–74 kg), were enrolled in the multiple-dose study. They were all homozygous for CYP2C9*1, but with different CYP2C19 genotypes. As shown in Table 2, five of these subjects were CYP2C19*1 homozygotes, nine were CYP2C19*1 heterozygotes and three were CYP2C19 PM genotype subjects (subjects with CYP2C19*2/*2 or *2/*3 genotypes).
Table 2.
Demographic characteristics and genotypes of 17 subjects in the multiple-dose study of gliclazide MR
| Genotype | Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) | Weight (kg) | CYP2C9 | CYP2C19 | Subject | Age (years) | Weight (kg) | CYP2C9 | CYP2C19 |
| 1 | 20 | 60 | *1/*1 | *1/*1 | 10 | 21 | 68 | *1/*1 | *1/*2 |
| 2 | 21 | 63 | *1/*1 | *1/*1 | 11 | 22 | 57 | *1/*1 | *1/*2 |
| 3 | 23 | 68 | *1/*1 | *1/*1 | 12 | 20 | 66 | *1/*1 | *1/*2 |
| 4 | 21 | 65 | *1/*1 | *1/*1 | 13 | 21 | 57 | *1/*1 | *1/*3 |
| 5 | 22 | 62 | *1/*1 | *1/*1 | 14 | 21 | 57 | *1/*1 | *1/*3 |
| 6 | 20 | 74 | *1/*1 | *1/*2 | 15 | 21 | 65 | *1/*1 | *2/*2 |
| 7 | 26 | 70 | *1/*1 | *1/*2 | 16 | 23 | 70 | *1/*1 | *2/*2 |
| 8 | 22 | 65 | *1/*1 | *1/*2 | 17 | 19 | 67 | *1/*1 | *2/*3 |
| 9 | 22 | 70 | *1/*1 | *1/*2 | |||||
Before enrolment for the pharmacokinetic studies, each subject was shown to be in good health through medical history, physical examination, electrocardiograms (ECGs) and routine laboratory tests. No medication was used for at least 2 weeks before the study and alcohol was forbidden within 72 h prior to drug administration.
Ethics
The study was approved by the Independent Ethics Committee of the People's Hospital of Liaoning Province and was in full compliance with the principles of the Declaration of Helsinki (current revision) and Good Clinical Practice guideline. Written informed consent was obtained from each subject before the study.
Determination of CYP2C9 and CYP2C19 genotypes
All subjects were genotyped for the main variants of both CYP2C9 and CYP2C19. CYP2C9 and CYP2C19 genotypes were determined by polymerase chain reaction (PCR)-restriction fragment length polymorphism, using genomic DNA isolated from leucocytes of peripheral venous blood with an extraction kit (Sino-American, Luoyang, China). CYP2C9*3 allele was analysed using the modified method of Wang et al.[17]. Briefly, the PCR reaction was performed with forward and reverse primers 5′-TGC ACG AGG TCC AGA GGT AC-3′ and 5′-CTA TGA ATT TGG GGA CTT CG-3′, and the resulting 152-bp product was digested with restriction enzyme KpnI and electrophoresed on agarose gels. CYP2C9*13 was identified by the method previously described [4]. For CYP2C19, PCR was carried out using the primer pair 5′-AGA GCT TGG CAT ATT GTA TC-3′ and 5′-GCA TTA CTC CTT GAC CTG TT-3′ for CYP2C19*2 mutation, and the primer pair 5′-GCT TTT AAG GGA ATT CAT AG-3′ and 5′-AAA ATA AAG AAC TTT GCC AT-3′ for CYP2C19*3. Amplified fragments were digested with restriction enzymes SmaI and BamHI for the CYP2C19*2 and CYP2C19*3, respectively.
Study design
Both single-dose and multiple-dose pharmacokinetic studies were carried out in bioequivalence studies. In these studies, both the test and the reference were MR formulations containing 30 mg of gliclazide, and the reference was Diamicron MR (Servier, Neuilly sur Seine, France). On the day of the single-dose study, each subject received one tablet of gliclazdie MR with 250 ml of 20% glucose solution in a fasted state. Venous blood (4 ml) was collected and placed in heparinized tubes just before dosing and at 1, 2, 3, 4, 6, 8, 10, 12, 14, 24, 36, 48 and 72 h postdose. In the multiple-dose study, each subject received one tablet of gliclazide MR once daily for 6 days. Venous blood (4 ml) was collected at the same time points as those in the single-dose study after the last dose (on day 6). Separated plasma was stored frozen (−20°C) until assay.
On study days, subjects continued in the fasting condition for 2 h after drug administration, during which period 60 ml of 20% glucose solution were given orally every 30 min to reduce the risk of hypoglycaemic episodes. Standard meals were provided at 2, 5 and 10 h postdose.
Safety assessment
The blood glucose level was determined using a glucometer before drug administration. Symptoms suggestive of hypoglycaemia were monitored closely during treatment. Glucose solution was supplied when any symptoms suggestive of hypoglycaemia such as nervousness, sweating, intense hunger, trembling, weakness and palpitations were observed during treatment.
Gliclazide assay
Plasma concentrations of gliclazide were determined using a validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) method [18]. Gliclazide and glyburide, the internal standard, were kindly provided by Dongrui Pharmaceutical Co. Ltd. (Suzhou, China). For the sample preparation, the plasma was acidified with 0.25 m phosphoric acid (100 µl) and extracted with 3 ml of hexane-dichloromethane (1 : 1, v/v). A Shimadzu LC-10AD pump (Kyoto, Japan) was used for delivering the mobile phase. Chromatography was performed on a Zorbax XDB C8 column (particle size 5 µm, 150 × 4.6 mm; Agilent, Wilmington, DE, USA) using a mobile phase of acetonitrile–water–formic acid (90 : 10 : 0.5, v/v/v). The flow rate was 0.7 ml min−1. A Thermo Finnigan TSQ™ triple quadrupole mass spectrometer equipped with an atmospheric pressure chemical ionization (APCI) source (San Jose, CA, USA) was used for mass analysis and detection. Quantification was performed using selected reaction monitoring (SRM) of the transitions m/z 324 → m/z 127 for gliclazide and m/z 494 → m/z 369 for the internal standard.
The lower limit of quantification of gliclazide was 1.0 ng ml−1. The intra- and interrun precision values for the concentrations of 2.5, 80, 1800 and 3600 ng ml−1 were all <3.8% and the accuracy ranged from 99.4% to 101% of the nominal value.
Data analysis
Only the data of the reference formulation (Diamicron MR) are presented in this paper. Pharmacokinetic parameters were calculated using standard noncompartmental methods. Maximum concentration (Cmax) and the time to reach Cmax (tmax) were determined by inspection of the plasma concentration–time curves. The elimination rate constant (k) was determined by liner regression of the terminal linear portion of the ln-concentration–time curve, and the apparent elimination half-life (t1/2) was calculated as 0.693/k. Area under the plasma concentration–time curve from zero to the last point (AUC0–t) and the AUC within a dosing interval (AUCss) at steady-state were calculated by the linear trapezoidal method. The AUC from zero to infinity (AUC0–∞) was calculated as AUC0–t + Ct/k, where Ct is the last measurable concentration. The software utilized for pharmacokinetic analysis was WinNonlin 5.0.1 (Pharsight, Mountain View, CA, USA).
Statistical analysis
The calculated parameters were compared across the three genotyped groups by one-way anova. A post hoc Dunnett's test was used to assess the presence of statistical differences between the genotyped groups when a statistically significant association was described by anova. For all analyses, P < 0.05 was considered to be statistically significant.
Results
Single-dose study
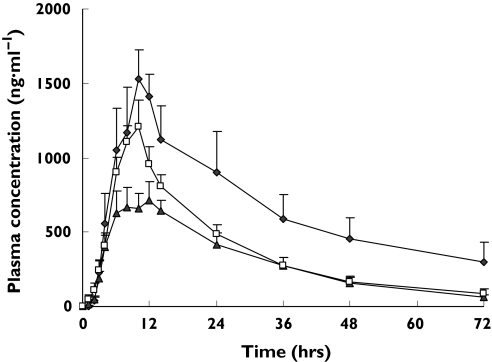
Plasma concentration–time curves of gliclazide in CYP2C9*1/*1,*1/*3 and *1/*13 subjects after an oral dose of 30 mg gliclazide MR are shown in Figure 1. The calculated parameters are listed in Table 3. Although CYP2C9*1/*13 subjects had higher AUC and Cmax values than *1/*1 subjects, no statistically significant differences were observed in subjects with these CYP2C9 genotypes.
Figure 1.
Plasma concentration–time curves of gliclazide in CYP2C9*1/*1 subjects (n = 13) (□), CYP2C9*1/*3 subjects (n = 8) ( ) and CYP2C9*1/*13 subjects (n = 3) (
) and CYP2C9*1/*13 subjects (n = 3) ( ) after a single oral dose of 30 mg of gliclazide MR. All data indicate mean ± SE
) after a single oral dose of 30 mg of gliclazide MR. All data indicate mean ± SE
Table 3.
Pharmacokinetic parameters of gliclazide in subjects with various CYP2C9 genotypes in the single-dose study (mean and 95% CI)
| Parameters | CYP2C9*1/*1 (n = 13) | CYP2C9*1/*3 (n = 8) | CYP2C9*1/*13 (n = 3) |
|---|---|---|---|
| AUC0−72 (µg ml−1 h−1) | 26.9 (18.5, 35.2) | 21.5 (15.5, 27.6) | 46.8 (−0.88, 94.6) |
| AUC0–∞ (µg ml−1 h−1) | 30.4 (18.5, 42.3) | 23.3 (16.5, 30.0) | 65.6 (−33.4, 164.7) |
| Cmax (µg ml−1) | 1.32 (0.83, 1.81) | 0.906 (0.636, 1.18) | 1.63 (1.18, 2.08) |
| tmax (h) | 9.08 (7.81, 10.4) | 9.75 (8.28, 11.2) | 10.7 (3.07, 18.3) |
| t1/2 (h) | 18.7 (11.6, 25.8) | 16.7 (13.9, 19.5) | 33.5 (−18.9, 86.0) |
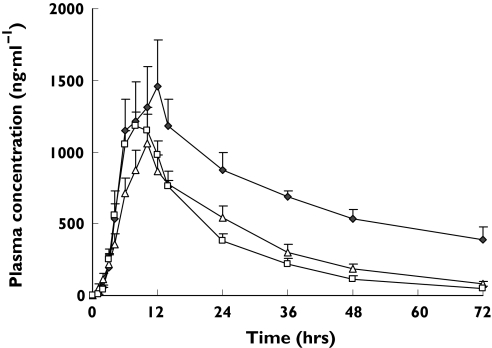
In contrast, the AUC0–∞ of gliclazide was significantly greater in CYP2C19 PM genotype subjects than that in subjects with the CYP2C19*1/*1 genotype, with CYP2C19 PM subjects demonstrating a 3.4-fold increase [95% confidence interval (CI) 2.5, 4.7; P< 0.01] and t1/2 was significantly longer (15.1 h vs. 44.5 h, 2.9-fold, 95% CI 2.3, 3.8) in CYP2C19 PM subjects than in CYP2C19*1/*1 subjects (P< 0.01). Plasma concentration–time curves of gliclazide in various CYP2C19 genotype groups are shown in Figure 2 and the calculated parameters are listed in Table 4.
Figure 2.
Plasma concentration–time curves of gliclazide in CYP2C19*1 homozygotes (n = 9) (□), CYP2C19*1 heterozygotes (n = 12) (▵) and CYP2C19 poor metabolizers (n = 3) ( ) after a single oral dose of 30 mg of gliclazide MR. All data indicate mean ± SE
) after a single oral dose of 30 mg of gliclazide MR. All data indicate mean ± SE
Table 4.
Pharmacokinetic parameters of gliclazide in subjects with various CYP2C19 genotypes in the single-dose study (mean and 95% CI)
| Parameters | CYP2C19*1 homozygotes (n = 9) | CYP2C19*1 heterozygotes (n = 12) | CYP2C19 poor metabolizers (n = 3) |
|---|---|---|---|
| AUC0−72 (µg ml−1 h−1) | 21.5 (15.4, 27.6) | 26.4 (18.9, 33.8) | 50.7 (21.5, 79.8)* |
| AUC0–∞ (µg ml−1 h−1) | 22.7 (15.9, 29.5) | 28.4 (20.0, 36.9) | 77.6 (14.2, 141.1)** |
| Cmax (µg ml−1) | 1.16 (0.75, 1.56) | 1.20 (0.79, 1.61) | 1.48 (0.09, 2.87) |
| tmax (h) | 8.67 (7.30, 10.0) | 9.83 (8.25, 11.4) | 10.7 (4.93, 16.4) |
| t1/2 (h) | 15.1 (11.2, 18.9) | 17.4 (15.5, 19.2) | 44.5 (6.12, 82.8)** |
P < 0.05
P < 0.01, compared with CYP2C19*1 homozygotes.
Multiple-dose study
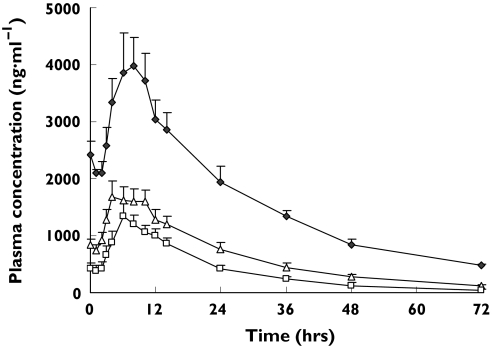
After multiple doses of gliclazide MR for 6 days, concentrations of gliclazide reached steady state. Plasma concentration–time curves of gliclazide in various CYP2C19 genotype groups at the steady state are shown in Figure 3 and the calculated parameters are listed in Table 5. Parameters AUCss, AUC0–∞ and Cmax of gliclazide were 3.4-fold (95% CI 2.9, 4.0; P < 0.01), 4.5-fold (95% CI 3.8, 5.4; P < 0.01) and 2.9-fold (95% CI 2.4, 3.4; P < 0.01) increased in CYP2C19 PM subjects, respectively, compared with CYP2C19*1 homozygotes. The half-life (t1/2) was significantly longer (13.5 h vs. 24.6 h, 1.8-fold, 95% CI 1.6, 2.1) in CYP2C19 PM subjects than that in CYP2C19*1 homozygotes (P< 0.01). Although these parameters were also increased in CYP2C19*1 heterozygotes, no statistically significant differences were found between CYP2C19*1 heterozygotes and CYP2C19*1 homozygotes.
Figure 3.
Plasma concentration–time curves of gliclazide at steady state in CYP2C19*1 homozygotes (n = 5) (□), CYP2C19* heterozygotes (n = 9) (▵) and CYP2C19 poor metabolizers (n = 3) ( ) after multiple oral doses of 30 mg of gliclazide MR. All data indicate mean ± SE
) after multiple oral doses of 30 mg of gliclazide MR. All data indicate mean ± SE
Table 5.
Pharmacokinetic parameters of gliclazide in subjects with various CYP2C19 genotypes in the multiple-dose study (mean and 95% CI)
| Parameters | CYP2C19*1 homozygotes (n = 5) | CYP2C19*1 heterozygotes (n = 9) | CYP2C19 poor metabolizers (n = 3) |
|---|---|---|---|
| AUC0−24 (µg ml−1 h−1) | 20.4 (13.4, 27.4) | 28.9 (20.4, 37.3) | 69.1 (37.8, 100.4)** |
| AUC0−72 (µg ml−1 h−1) | 28.9 (16.3, 41.5) | 44.5 (30.7, 58.3) | 118.0 (76.5, 159.5)** |
| AUC0–∞ (µg ml−1 h−1) | 29.9 (16.1, 43.7) | 47.5 (33.0, 61.9) | 135.1 (100.0, 170.1)** |
| Cmax (µg ml−1) | 1.51 (0.93, 2.08) | 1.89 (1.26, 2.52) | 4.32 (2.15, 6.49)** |
| tmax (h) | 8.00 (3.35, 12.6) | 6.67 (4.24, 9.10) | 8.00 (3.03, 13.0) |
| t1/2 (h) | 13.5 (9.23, 17.7) | 18.3 (16.8,19.8) | 24.6 (12.6, 36.7)** |
P < 0.01, compared with CYP2C19 homozygotes.
Safety assessment
Gliclazide MR was well tolerated in both the single-dose and the multiple-dose study. No signs or symptoms of hypoglycaemia were found, even in CYP2C19 PM subjects, and no other adverse effects were observed except for mild diarrhoea, which is a normal reaction to glucose administration.
Discussion
Type 2 diabetes mellitus is a growing global epidemic, especially in Asian populations. Treatment of this disease with oral antidiabetic drugs is characterized by considerable interindividual variability in pharmacokinetics, clinical efficacy and adverse effects, making the therapy complicated. Individual differences can result from many factors such as age, sex, disease, drug and food interactions and comorbidity, and genetic factors play an important role. The polymorphic enzyme CYP2C9 is known to be responsible for the metabolism of warfarin, phenytoin, many nonsteroidal anti-inflammatory drugs, and apparently a number of oral sulphonylurea antidiabetic drugs, including tolbutamide, glyburide and glimepiride. Kirchheiner et al. reported that total oral clearance of these sulphonylureas was only 20% in persons with the CYP2C9*3/*3 genotype compared with carriers of the wild-type genotype CYP2C9*1/*1, and clearance in the heterozygous carriers was between 50% and 80% of that of the wild-type genotypes [19]. Another polymorphic enzyme, CYP2C19, is mainly responsible for the metabolism of mephenytoin and some proton pump inhibitors such as omeprazole. In vitro investigations have suggested that CYP2C19 might also catalyse the oxidation of tolbutamide and glyburide, but no in vivo genotype-based clinical studies have revealed any relevant contribution of CYP2C19 to the metabolism of these drugs [20, 21].
In the present study, we had assumed that gliclazide, like some other sulphonylureas mentioned above, was also metabolized by CYP2C9 in humans, and the initial objective of the study was to investigate the effect of CYP2C9 polymorphism on the pharmacokinetics of gliclazide MR in a single-dose study, but no statistically significant differences were observed in subjects with various CYP2C9 genotypes. As CYP2C19 is closely associated with CYP2C9, and its genetic polymorphisms are more important in Asian populations, CYP2C19 genotyping was then performed. We incidentally found that the pharmacokinetic profile of gliclazide MR was in good relation to CYP2C19 polymorphism. To exclude the effect of CYP2C9 polymorphisms, a multiple-dose study was conducted in CYP2C9*1/*1 subjects with various CYP2C19 genotypes, and we found that gliclazide was highly accumulated in CYP2C19 PM subjects after 6 days' administration of gliclazide MR, which was in line with our findings in the single-dose study. Both studies indicated that CYP2C19 might play an important role in the disposition of gliclazide in humans. This observation is contrary to previous suggestions that CYP2C9 is the major metabolizing enzyme for gliclazide [13]. In prior studies, Park et al. found that rifampin decreased the mean AUC for gliclazide by 70% and the mean t1/2 from 9.5 to 3.3 h. They attributed this interaction to the induction of CYP2C9 by rifampin [13]. However, more investigations have demonstrated that rifampin is a general inducer of several drug-metabolizing enzymes. It can induce not only CYP2C9, but also other CYP enzymes such as CYP2B6, CYP2C8, CYP2C19 and CYP3A4. CYP2C19 has been reported more inducible by rifampin than CYP2C9 [22, 23]. Therefore, it is more reasonable to conclude that CYP2C19 is mainly involved in the metabolism of gliclazide, and this can better explain the pharmacokinetic interaction between rifampin and gliclazide.
In the single-dose study, we found that subjects with the CYP2C9*1/*13 genotype had higher AUC values and longer elimination half-life than subjects from other groups. No statistically significant difference was observed, probably due to the small sample size of the CYP2C9*1/*13 group. It has been reported that CYP2C9*13 is strongly linked to CYP2C19*2[24]. As shown in Table 1, among the three CYP2C9*1/*13 subjects, two were CYP2C19*1/*2 carriers and one was a CYP2C19*2/*2 carrier. Furthermore, all CYP2C9*1/*13 subjects we have found up to now are either heterozygous or homozygous for CYP2C19*2. Therefore, it is difficult to assess the impact of CYP2C9*13 polymorphism on gliclazide pharmacokinetics in in vivo studies. In our studies, no CYP2C9 PM subjects (subjects with CYP2C9*3/*3, CYP2C9*3/*13 or CYP2C9*13/*13 genotypes) were included due to the low frequencies of CYP2C9*3 and CYP2C9*13 alleles in Chinese, and the pharmacokinetic profile of gliclazide MR is unclear and requires further investigations in these subjects.
In the present study, no pharmacodynamic assessments were made and no significant hypoglycaemia-related adverse events were found throughout the treatments due to the adequate supply of glucose. The relationship between CYP2C19 genotype and the pharmacodynamic reponse to gliclazide MR remains unknown. With regard to many antidiabetic agents, elevated drug concentrations are likely to increase the risk of hypoglycaemia. Because of the magnitude of the AUC ratio between CYP2C19 PM subjects and CYP2C19*1 homozygotes (4.5-fold in multiple-dose regimens) and the high frequency of CYP2C19 PMs in Asian populations, the presence of CYP2C19 polymorphisms could be clinically significant.
In conclusion, our in vivo study has shown that the pharmacokinetics of gliclazide MR are mainly affected by CYP2C19 genetic polymorphism. No statistically significant differences were found between subjects with CYP2C9*1/*1, CYP2C9*1/*3 and CYP2C9*1/*13 genotypes. These findings are likely to be clinically meaningful in Chinese and other Asian populations.
Acknowledgments
Competing interests: None to declare.
We acknowledge Professor R. Q. Li and the nursing staff of the People's Hospital of Liaoning Province for their help in performing the clinical studies. We also thank Dr J. Zheng for his help in preparation of the manuscript. The study was supported by the National Natural Science Foundation of China, nos. 39930180 and 30472062.
References
- 1.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–38. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome CYP2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–58. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY. Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol. 2005;60:418–22. doi: 10.1111/j.1365-2125.2005.02448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Si DY, Guo YJ, Zhang YF, Zhou H, Zhong DF. Identification of a novel variant of CYP2C9 allele in Chinese. Pharmacogenetics. 2004;14:465–9. doi: 10.1097/01.fpc.0000114749.08559.e4. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YF, Zhong DF, Si DY, Guo YJ, Chen XY, Zhou H. Lornoxiam pharmacokinetics in relation to cytochrome P450 2C9 genotype. Br J Clin Pharmacol. 2005;59:14–7. doi: 10.1111/j.1365-2125.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo YJ, Zhang YF, Wang Y, Si DY, Chen XY, Zhong DF, Fawcett JP, Zhou H. Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in human. Drug Metab Dispos. 2005;33:749–53. doi: 10.1124/dmd.105.003616. [DOI] [PubMed] [Google Scholar]
- 7.Guo YJ, Wang Y, Si DY, Fawcett PJ, Zhong DF, Zhou H. Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica. 2005;35:853–61. doi: 10.1080/00498250500256367. [DOI] [PubMed] [Google Scholar]
- 8.Persson I, Aklillu E, Rodrigues F, Bertilsson L, Ingelman-Sundberg M. S-mephenytoin hydroxylation phenotype and CYP2C19 genotype among Ethiopians. Pharmacogenetics. 1996;6:521–6. doi: 10.1097/00008571-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the effective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Palmer KJ, Brogden RN. Gliclazide, an update of its pharmacological properties and therapeutic efficacy in non-insulin-dependent diabetes mellitus. Drugs. 1993;46:92–125. doi: 10.2165/00003495-199346010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AR, Brownsill RD, Grandon H, Lefoulon F, Petit A, Luijten W, Kopelman PG, Walther B. Synthesis of putative metabolism and investigation of the metabolic fate of gliclazide, [1-(3-azabicyclo (3,3,0) oct-3-yl)-3-(4--methylphenylsulfonyl) urea], in diabetic patients. Drug Metab Dispos. 1996;24:55–64. [PubMed] [Google Scholar]
- 12.Rieutord A, Stupans I, Shenfield GM, Gross AS. Gliclazide hydroxylation by rat liver microsomes. Xenobiotica. 1995;25:1345–54. doi: 10.3109/00498259509061922. [DOI] [PubMed] [Google Scholar]
- 13.Park JY, Kim KA, Park PW, Park CW, Shin JG. Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide. Clin Pharmacol Ther. 2003;74:334–40. doi: 10.1016/S0009-9236(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 14.McGavin JK, Perry CM, Gua KL. Gliclazide modified release. Drugs. 2002;62:1357–66. doi: 10.2165/00003495-200262090-00010. [DOI] [PubMed] [Google Scholar]
- 15.Schernthaner G. Gliclazide modified release: a critical review of pharmacodynamic, metabolic, and vasoprotective effects. Metabolism. 2003;52:29–34. doi: 10.1016/s0026-0495(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 16.Delrat P, Paraire M, Jochemsen R. Complete bioavailability and lack of food-effect on pharmacokinetics of gliclazide 30 mg modified release in healthy volunteers. Biophar Drug Dispos. 2002;23:151–7. doi: 10.1002/bdd.303. [DOI] [PubMed] [Google Scholar]
- 17.Wang SL, Huang J, Lai MD, Tsai JJ. Detection of CYP2C9 polymorphism based on the polymerase chain reaction in Chinese. Pharmacogenetics. 1995;5:37–42. doi: 10.1097/00008571-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Su J, Chen XY, Duan XT, Zhang YF, Zhong DF. Rapid determination of gliclazide in human plasma by LC/MS/MS. Chinese Pharmaceut J. 2006;41:864–7. [Google Scholar]
- 19.Kirchheiner J, Roots I, Goldammer M, Rosenkranz B, Brockmoller J. Effect of genetic polymorphisms in cytochrome P450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet. 2005;44:1209–25. doi: 10.2165/00003088-200544120-00002. [DOI] [PubMed] [Google Scholar]
- 20.Yin OQ, Tomlinson B, Chow MS. CYP2C9, but not CYP2C19, polymorphism affects the pharmacokinetics and pharmacodynamics of glyburide in Chinese subjects. Clin Pharmacol Ther. 2005;78:370–7. doi: 10.1016/j.clpt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Kirchheiner J, Bauer S, Meineke I, Rohde W, Prang V, Meisel C, Roots I, Brockmoller J. Impacts of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and on the insulin and glucose response in healthy volunteers. Pharmacogenetics. 2002;12:101–9. doi: 10.1097/00008571-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Madan A, Graham RA, Carroll KM, Mudra DR, Burton LA, Krueger LA, Downey AD, Czerwinski M, Forster J, Ribadeneira MD, Gan LS, LeCluyse EL, Zech K, Robertson P, Jr, Koch P, Antonian L, Wagner GYuL, Parkinson A. Effects of prototypical microsomal enzyme inducers in cytochrome P450 expression in cultured human hepatocytes. Drug Metab Dispos. 2003;31:421–31. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 23.Branch RA, Adedoyin A, Frye RF, Wilson JW, Romkes M. In vivo modulation of CYP enzymes by quinidine and rifampin. Clin Pharmacol Ther. 2000;68:401–11. doi: 10.1067/mcp.2000.110561. [DOI] [PubMed] [Google Scholar]
- 24.Maekawa K, Fukushima-Uesaka H, Tohkin M, Hasegawa R, Kajio H, Kuzuya N, Yasuda K, Kawamoto M, Kamatani N, Suzuki K, Yanagawa T, Saito Y, Sawada J. Four novel defective alleles and comprehensive haplotype analysis of CYP2C9 in Japanese. Pharmacogenet Genomics. 2006;16:497–514. doi: 10.1097/01.fpc.0000215069.14095.c6. [DOI] [PubMed] [Google Scholar]