Abstract
What is already known about this subject
The functionality of the HPA axis through cortisol production has been the subject for several investigations.
Modelling of cortisol production has been described by endogenous substance models utilizing a combination of indirect response functions and sum of cosine functions.
The effect of glucocorticoid drugs on cortisol production has been investigated using these models.
What this study adds
Previous investigations (models) have not primarily considered the combined action of ACTH and cortisol.
Since ACTH drives cortisol production we adopted, for our modelling, the same approach as previous investigations but we distinguished between two types of models for the production of cortisol (driven by ACTH), one being the sum of cosine functions, the other being described by surges.
The presented surge-based model can serve as a tool for further understanding of the HPA axis and may also prove useful in the development of drugs interacting with the axis.
Aims
Budesonide, a glucocorticosteroid, is used as a first-line treatment for asthma. The aim of the study was to develop a PK/PD model for the effect of budesonide on ACTH and cortisol.
Methods
The modelling data were generated by conducting a single-blind, randomized, placebo-controlled cross-over study. Ten healthy volunteers inhaled placebo (Placebo Turbohaler) and 1600 µg budesonide (Pulmicort Turbohaler), with a wash-out period of 7 days between treatments. Baseline concentrations of cortisol and ACTH were measured after placebo treatment and concentrations of cortisol, ACTH and budesonide were assessed after budesonide treatment. A one-compartment disposition model was used for budesonide disposition. Based on indirect response models, two types of models, distinguishing between production driven by a sum of cosine functions and production driven by surges, were used in parallel to describe the data.
Results
The surge-based approach was the most appropriate, based on goodness-of-fit, objective function values and number of parameters. The surge-based model that integrated both ACTH and cortisol data was chosen as the final model. The estimated half-lives of endogenous ACTH and cortisol were 9 and 113 min, respectively. The budesonide and ACTH concentrations producing 50% of the maximal response (IC50 and A50) were 0.325 µg l−1 and 4.96 pmol l−1.
Conclusions
The present PK/PD model of the effect of budesonide on ACTH and cortisol can serve as a tool for further understanding of the hypothalamic-pituitary-adrenal (HPA) axis and be useful in the development of drugs interacting with the axis.
Keywords: ACTH, circadian variation, cortisol, HPA axis, modelling, population pharmacokinetics-pharmacodynamics
Introduction
Inhaled corticosteroids are recognized as the first-line anti-inflammatory treatment for asthma. Clinically, the glucocorticosteroid budesonide is used in the treatment of respiratory diseases such as asthma and rhinitis. Budesonide is a moderately lipophilic compound that shows rapid uptake into the airway mucosa [1], a high systemic clearance and an extensive first-pass metabolism after absorption in the gut. Although it is well established that both endogenous and exogenous glucocorticosteroids suppress cortisol secretion by the adrenal gland through feed-back mechanisms acting simultaneously on suprahypothalamic centres, the hypothalamus, the pituitary and possibly the adrenal cortex itself·[4], there is a need for a physiological model to describe the system since the mechanism involves many components including feed-back and feed-forward (i.e. a sequential cascade of effects) systems and circadian variation.
One of the most sensitive markers for detecting the systemic bioactivity of inhaled corticosteroids is the suppression of the hypothalamic-pituitary-adrenal (HPA) axis, measured as a reduction in serum cortisol concentrations over 24 h [2]. One of the main functions of the HPA axis is to control the production of endogenous steroids, including cortisol, both in basal and stress-related states. Cortisol (hydrocortisone, 11,17,21-trihydroxypreg-4-ene-3,20-dione) is the principal glucocorticoid of the human body and it is synthesized in the adrenal cortex from cholesterol via several enzyme-catalyzed steps [3]. Under normal circumstances the rate-limiting step for adrenal steroidogenesis is the conversion of cholesterol to pregnenolone, an intermediate for major biologically active corticosteroids [4]. The synthesis and release of glucocorticoids are regulated by the pituitary adrenocorticotrophic hormone (ACTH). ACTH secretion, on the other hand, is regulated by the hypothalamic production of corticotrophin releasing factor (CRF). Finally, circulating cortisol molecules provide negative feed-back regulation along the pituitary-hypothalamus axis thus maintaining homeostasis. The secretion and serum concentration of ACTH, and consequently cortisol, fluctuate according to a circadian rhythm that follows sleeping and waking periods [3]. Under normal circumstances, plasma cortisol concentrations reach peak concentrations in the morning and after awakening, cortisol concentrations decrease irregularly throughout the day with the lowest hormone concentrations being found after the onset of sleep [3]. The circadian rhythm of cortisol production is also affected by other factors including trauma, intense heat, intense cold and stress [3].
The most sensitive tests of the function of the HPA axis include measurement of cortisol concentrations in multiple blood samples with frequent sampling, particularly overnight or for 12–24 h, measurements in urine collected overnight or for 24 h, and stimulation tests with ACTH [1, 3].
In this study, healthy volunteers inhaled a single dose of budesonide and placebo, followed by frequent blood sampling. The data from this study have been used to create integrated PK/PD models for the action of budesonide on ACTH and cortisol.
Methods
Subjects
Ten healthy male and female volunteers entered the study. Their mean age was 23 years (range 20–28 years), mean height 176 cm (167–190 cm) and mean weight 65 kg (54–86 kg). All subjects gave written informed consent to participate in the study which was approved by the Ethics Committee of Uppsala University and the Swedish Medical Products Agency. The study was performed according to the Declaration of Helsinki and its amendments and to ICH-GCP guidelines.
Study design
This single-blind, randomized, placebo-controlled cross-over study consisted of two treatment periods, each lasting 24 h. At approximately 08.00 h, subjects inhaled a single dose of placebo on one occasion and 1600 µg budesonide on the other. The treatment periods were separated by a 7-day wash-out period. Budesonide was administered through a Pulmicort Turbohaler® and placebo through a similar Turbohaler®.
On the study days the subjects arrived at the study site in the morning after fasting for at least 10 h. A venous catheter was inserted into a forearm vein and baseline blood samples were collected. Subjects inhaled the treatment at approximately 08.00 h under the supervision of two staff members. After the treatment was inhaled, the subjects rinsed their mouths with water. Blood samples for ACTH (4 ml) and cortisol (5 ml) were collected at the following time points: predose and 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22 and 24 h after administration. Blood samples for budesonide (2 × 7 ml) were collected at the following time points: predose and 10, 20, 30, 45 min, 1, 2, 4, 6, 8 and 10 h after drug administration. The ACTH samples were collected in ethylene-diaminetetraacetate (EDTA) tubes. The cortisol and budesonide samples were collected in heparinized tubes. The plasma was transferred to polypropylene tubes for ACTH and cortisol and to cryotubes for budesonide, and all samples were stored at −18°C until analyzed.
Bioanalytical methods
The assay of budesonide in plasma was performed at Analytical Services Quintiles AB, Uppsala, Sweden. Budesonide in plasma was determined by liquid chromatography with mass-spectrophotometry detection (LC-MS/MS), using a reversed phase LC column (Kromasil, C18), isocratic elution of the substance and MS/MS detection. Desonide was used as the internal standard. The mobile phase consisted of 70% aqueous methanol with 7.5 mm ammonia and 10 mm formic acid. The assay had a lower limit of quantification (LOQ) of 0.043 µg l−1. Blank samples, calibration samples and quality control samples of three different concentrations were analyzed with each batch to ascertain selectivity, precision, and accuracy. The coefficient of variation (%CV) at 0.086, 0.86 and 3.23 µg l−1 was 7.0, 4.7 and 2.1, respectively.
The assay of cortisol was performed at the Department of Clinical Chemistry, University Hospital, Uppsala, Sweden. The plasma concentrations of cortisol were determined by a fluoroimmuno-assay (AutoDELFIA Cortisol, Wallac OY). The assay was based on a competitive reaction between europium-labelled cortisol and sample cortisol for a limited amount of binding sites on cortisol-specific, monoclonal antibodies. The measured fluorescence was inversely proportional to the quantity of cortisol in the sample. The cross-reactivity of the method with hydroxyprogesterones was 1% or less. The method had a LOQ of 7.0 nmol l−1. The coefficient of variation (%CV) at 7.69, 18.9 and 28.3 µg l−1 was 4.1, 3.0 and 3.5, respectively, according to published validation data.
The assay of ACTH was performed at the Department of Clinical Chemistry, University Hospital, Uppsala, Sweden, by an ELSA-immunoradiometric assay (CIS-bio international, France). Two monoclonal antibodies were prepared against sterically remote antigenic sites on the ACTH molecule. The first one specific for the N-terminal part of the ACTH radio-labelled with iodine 125 is used as a tracer. The ACTH molecules present in the samples to be tested are ‘sandwiched’ between both antibodies. Following the formation of the coated antibody/antigen/iodinated antibody sandwich, the unbound tracer is easily removed by a washing step. The radioactivity bound to the ELSA is proportional to the concentration of ACTH present in the sample. The method had a LOQ of 5 ng l−1. The coefficient of variation (%CV) at 37.8, 57.3 and 148 ng l−1 was 6.19, 9.59 and 9.62, respectively.
Data analysis
Structural pharmacokinetic model
A one-compartment disposition model was used, which previously had been found adequate for budesonide disposition [4]. The parameters of the model were clearance (CL) and volume of distribution (V). Absorption models tested included instantaneous absorption and first-order absorption denoted with a rate constant (ka). Because systemic availability for budesonide administered via Turbohaler® has been reported as 28% [4], this was used in the final model in which the bioavailability (F) was set to 0.28 and the initial parameter values adjusted.
Structural pharmacodynamic models
Cortisol has in previous modelling been described by endogenous substance models [12–16]. We adopted the same approach for our modelling, distinguishing between two types of models for the production of cortisol/ACTH, one being the sum of cosine functions, the other being described by surges [6]. Endogenous substance models, defined by a production rate constant (kin) and a first-order elimination rate constant (kout) were used for both the cosine and surge-based models. In the cosine model, the circadian rhythm in hormonal production was described by sums of cosine functions [5] and in the surge-based model, a constant zero-order production combined with surges [6]. Furthermore, in the cosine modelling, ACTH and cortisol were analyzed separately, whereas in the surge-based modelling the two were integrated and ACTH was assumed to drive the production of cortisol. Although the action of budesonide was described by an Imax model in both cases, in the surge-based model, it was assumed that it operated solely on the production of ACTH.
The system was initiated at zero for all compartments 24 h before the first observation. Given the short half-lives of the hormones, this was deemed more than sufficient for the system to arrive at pseudo-steady state at the time of the first observation. The cosine model is described by equation 1 where R represents ACTH or cortisol concentration.
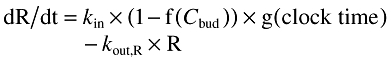 |
(1) |
kin is obtained as the product of the estimated parameters kout and baseline and f(Cbud) is described by a (sigmoid) Emax model and by the parameters Imax and IC50. A sum of cosine functions, each characterized by the amplitude, acrophase and period, was used to define g(clock time). Whereas amplitude and acrophase were estimated parameters, the periods were fixed to 24, 12, 8 and 6 h. The initial model included only a cosine function with a 24 h period. Additional functions were only retained if they improved the description of the data.
The surge-based model is described by equations 2 and 3.
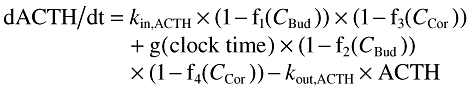 |
(2) |
| (3) |
where kout,ACTH and kout,cortisol are the first order elimination rate constants for ACTH and cortisol, kin,ACTH is the baseline production rate of ACTH in the absence of surges and without any drug, kin,ACTH is obtained as the product of the two estimated parameters kout,ACTH and baselineACTH. f1(CBud) and f2(CBud) are described by Imax models (Equation 4), whereas h(ACTH) is a (sigmoidal) Emax model (equation 5). Negative feedback of cortisol on ACTH production (f3(CCor), f4(CCor)) was introduced in equation 2 using Imax models.
| (4) |
| (5) |
Surges, characterized by the parameters SA (surge amplitude), SW (surge width), T (clock time) and PT (peak time), were used to define the function g(clock time) according to equation 6.
| (6) |
Parameter estimation
The primary aim of the analysis was to develop a PK/PD model for the effect of budesonide on ACTH and cortisol. A sequential approach was used by which the pharmacokinetic model was developed first, and thereafter the individual, model-predicted budesonide concentration–time profiles were used in the analysis of ACTH and cortisol data.
Population parameter estimates were made using nonlinear mixed effect models (NONMEM V and VI beta, level 1.0) [7]. The population analysis was performed with first-order conditional estimation (FOCE) with interaction. Exponential distributions were used to describe interindividual variability in parameters. Covariances between parameters did not improve goodness-of-fit. The starting model for residual variance contained both additive and proportional components. Redundant components of the stochastic models were omitted. Individual empirical Bayesian pharmacokinetic parameters were obtained as part of the output from NONMEM. The decision to adopt a more complex nested model was based on the change of objective function values (OFV). The criteria for change in OFV were set to P < 0.05. For graphical model diagnostics including basic goodness-of-fit plots and individual plots, the S-plus (Insightful Corp, Seattle, WA) based model-building aid Xpose 3.120 was used [8].
Results
Pharmacokinetic modelling of budesonide
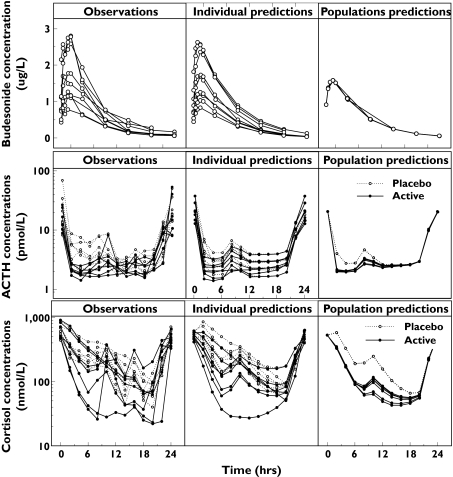
A one-compartment disposition model with first order absorption and linear processes was chosen as the final pharmacokinetic model. A summary of the parameters from the final model is shown in Table 1. In Figure 2 the measured budesonide concentrations and the predictions (individual and population) for the final model are shown.
Table 1.
Population pharmacokinetic parameters for budesonide after a single dose of 1600μg in healthy volunteers using a mixed effects model
| Parameters | Estimate | Relative SE | Brief definition of symbols |
|---|---|---|---|
| CL (l h−1) | 81.5 | Clearance for budesonide | |
| V (l) | 219 | Volume of distribution of budesonide | |
| ka (h−1) | 3.72 | First order absorption rate constant for budesonide | |
| ω CL | 38% | 14% | Interindividual variability for CL (%CV) |
| ωV | 39% | 15% | Interindividual variability for V (%CV) |
| ωka | 25% | 41% | Interindividual variability for ka(%CV) |
| σ | 14% | 2% | Proportional error (%CV) |
| OFV | −216 | Objective function value |
Figure 2.
Plots of observations, individual predictions and population predictions vs. time (h) for budesonide (µg l−1), ACTH baseline, ACTH active treatment (pmol l−1), cortisol baseline and cortisol active treatment (nmol l−1)
Pharmacodynamic modelling
The secretion and serum concentration of ACTH, and consequently cortisol, fluctuate according to a pronounced circadian rhythm with two surges every 24 h, one AM surge and one PM surge. For ACTH, the morning surge was about six times larger than the afternoon surge and for cortisol, about three times larger.
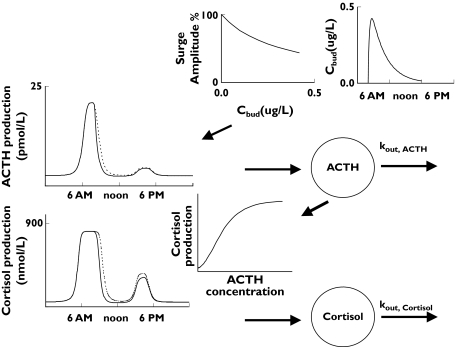
The surge-based approach was chosen as the most appropriate based on goodness-of-fit, the OFVs and the number of parameters. The surge-based model which integrated both ACTH and cortisol data was chosen as the final model. OFVs and the number of parameters for the different models are presented in Table 2. Inter-individual variances in five parameters (baselineACTH, kout,ACTH, IC50, A50 and kout,cortisol) were sufficient to describe the data. Imax for budesonide action on surge amplitude was estimated to a value close to 1 and could be fixed to 1 without decreasing goodness of fit. The same IC50 was used for the impact of budesonide on baseline production and on surges. A negative feedback of cortisol on the production of ACTH did not improve the fit to the data. Figure 1 shows schematically with profiles from a typical individual. The population parameters for the final model are presented in Table 3 and the observed and final model predicted hormonal concentrations are shown in Figure 2. The estimated half-lives of endogenous ACTH and cortisol were 9 and 113 min, respectively.
Table 2.
Objective function values (OFV) and the number of parameters for the different models. The number of θs, ωs and σs are given within the brackets
| Baseline | Active and baseline | |||
|---|---|---|---|---|
| Cosine | Surge | Cosine | Surge | |
| Cortisol | ||||
| OFV | 859 | 871 | 1736 | 1703 |
| Number of parameters | 13 (8, 3, 2) | 7 (4, 2, 1) | 15 (9, 4, 2) | 7 (4, 2, 1) |
| ACTH | ||||
| OFV | 237 | 210 | 384 | 314 |
| Number of parameters | 11 (8, 2, 1) | 10 (6, 2, 2) | 15 (9, 4, 2) | 13 (9, 3, 1) |
| Cortisol and ACTH | ||||
| OFV | 1096 | 1097 | 2120 | 2071 |
| Number of parameters | 24 (16, 5, 3) | 18 (11, 3, 4) | 30 (18, 8, 4) | 20 (13, 4, 3) |
Figure 1.
Schematic illustration of the interaction between budesonide, endogenous ACTH and endogenous cortisol in the surge-based model. The concentration of budesonide (Cbud) suppressed the production of endogenous ACTH thereby decreasing the concentration of ACTH and lowering the peak concentrations (Surge Amplitude). The broken line shows the baseline values for ACTH and the continuous line the values after treatment with budesonide. ACTH governs the production of cortisol
Table 3.
Population parameters for the final model.
| Models and parameters | Estimate | Relative SE | Brief definition of symbols |
|---|---|---|---|
| System model for ACTH | |||
| Amplitude AM surge (pmol h−1) | 87 | 33% | Maximum production rate l−1 of ACTH distribution volume |
| Width AM/PM surge (h) | 1.32 | 13% | Surge width (same for AM and PM) |
| Time AM surge (h:min) | 07:18 | 00:06* | Clock time for morning surge |
| kout ACTH (h−1) | 4.68 | 24% | First order elimination rate constant for ACTH |
| BaselineACTH (pmol l−1) | 2.58 | 7% | Baseline concentration of ACTH in the absence of surges and without drug |
| Amplitude PM surge (pmol l−1) | 10 | 20% | Maximum production rate l−1 of ACTH distribution volume |
| Time PM surge (h:min) | 16:18 | 00:20* | Clock time for afternoon surge |
| ω Baseline | 27% | 24% | Interindividual variability for baseline (%CV) |
| ωkout | 38% | 19% | Interindividual variability for kout (%CV) |
| σ | 32% | 11% | Proportional error (%CV) |
| Drug model | |||
| Imax1 (−) | 0.277 | 21% | Maximum fractional inhibition of ACTH baseline production |
| Imax2 (−) | 1 | fixed | Maximum fractional inhibition of ACTH surge production |
| IC50, 1 and 2 (µg l−1) | 0.325 | 87% | Concentration of drug causing 50% of the maximum suppression |
| ωIC50, 1 and 2 | 170% | 36% | Interindividual variability for IC50,1 and 2 (%CV) |
| System model for cortisol | |||
| Amax (nmol h−1) | 298 | 7.8% | Maximum production rate l−1 of cortisol distribution volume |
| A50 (pmol l−1) | 4.96 | 14% | ACTH concentration where cortisol production is half-maximal |
| γ | 4.10 | 19% | Sigmoidicity factor for ACTH-cortisol production relationship |
| kout,cortisol (h−1) | 0.366 | 24% | First order elimination rate constant for cortisol |
| ωkout,cortisol | 49% | 30% | Interindividual variability for kout,cortisol (%CV) |
| ω A50 | 38% | 19% | Interindividual variability A50 (%CV) |
| σ | 33% | 16% | Proportional error (%CV) |
SE, not relative SE.
Discussion
In order to develop a PK/PD model for the effect of budesonide on ACTH and cortisol, the first step of the data evaluation was to create a pharmacokinetic population model for budesonide. The results of the modelling suggested that the pharmacokinetic model best describing the budesonide plasma concentration data was a one-compartment model, with first order absorption and no lag-time. The absence of lag-time could be explained by the rapid absorption of budesonide from the lung, mainly due to its large surface area. According to the estimates from the chosen model, budesonide was rapidly absorbed with a typical absorption half-life of 11 min and an intersubject variability of approximately 25%. The estimated disposition parameters are in good agreement with previous studies of inhaled budesonide [9–11]. For example, Ryrfeldt et al.[9] reported a plasma half-life (t1/2) of 2.8 (1.1) h compared with our estimate of 2.47 (0.87) h and Minto et al.[10] reported an elimination rate, k, of 0.284 (0.0134) h−1 compared with our estimate of 0.30 (0.06) h−1.
Modelling of cortisol is complex due to the circadian rhythm in the secretion of cortisol and the down regulation effect. Several models for the circadian secretion of cortisol have previously been developed and the subsequent effects of glucocorticoids on cortisol disposition have been studied [12–16]. A comparison of various methods describing the circadian input of cortisol has been published [13]. Circadian rhythms in pharmacodynamic modelling have also been described by sums of cosine functions for other biorhythms such as blood pressure [5]. Although such an empirical approach could describe baseline profiles of individual hormones as well as the more mechanistic models, it did so at the expense of more parameters. In addition, for the simultaneous analysis of both baseline, and treatment profiles, this empirical approach was inferior in terms of goodness-of-fit.
Previous models have mostly focused on the circadian rhythm of cortisol excretion. According to the present pharmacodynamic model estimations, cortisol peaks occurred in pulses at approximately 08.00 h (large peak) and 16.00 h (medium peak). The morning peak corresponds well to results in earlier studies [1, 3], as does the afternoon peak [18, 22]. The amplitude of the peaks varied during the day with a three-fold higher amplitude at 08.00 h compared with 16.00 h. It is generally accepted that measurement of the cortisol concentrations over 24 h provides one of the most sensitive markers of HPA axis function [17].
Exogenous glucocorticoids suppress cortisol production by feed-back mechanisms that act simultaneously on the hypothalamus and pituitary in a manner similar to that of the endogenous glucocorticoids [4]. The modelling described here gives a physiological description of the system with the exception of the negative feed-back regulation exhibited by circulating cortisol molecules along the HPA axis. By adding the feed-back regulation, the modelling could be extended to describe the HPA axis more thoroughly.
The model estimated that the elimination half-life of ACTH was 9 min compared with previously reported estimates of 4 min [19]. The model estimated that the first order elimination rate constant for cortisol, kout,cortisol was 0.366 h−1 compared with a previously reported estimate of 0.405 h−1[16]. The cortisol concentrations (morning and afternoon) presented by Keenan et al.[18] are approximately 500 nmol l−1 and 350 nmol l−1 and are in good agreement with our results. Although this study on budesonide was done in healthy volunteers, results presented are anticipated to be applicable to asthma patients treated with inhaled corticosteroids. Results comparing volunteers and patients are to some degree conflicting and differ between corticosteroids. For budesonide it has been fairly well established that patients have similar systemic bioavailability compared with volunteers [20]. Other PK/PD variables are also essentially comparable [20, 21].
In conclusion, the present surge-based model can serve as a tool for further understanding of the HPA axis. The model may also prove useful in the development of drugs interacting with the axis.
Acknowledgments
Competing interests: None declared.
References
- 1.Donnelly R, Seale JP. Clinical pharmacokinetics of inhaled budesonide. Clin Pharmacokin. 2001;40:427–40. doi: 10.2165/00003088-200140060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lönnebo A, Grahnén A, Jansson B, Brundin RM, Ling-Andersson A, Eckernäs SÅ. An assessment of the systemic effects of single and repeated doses of inhaled fluticasone propionate and inhaled budesonide in healthy volunteers. Eur J Clin Pharmacol. 1996;49:459–63. doi: 10.1007/BF00195931. [DOI] [PubMed] [Google Scholar]
- 3.Chrousos GP, Harris AG. Hypothalamic-pituitary-adrenal axis suppression and inhaled corticosteroid therapy. 1. General principles. Neuroimmunomodulation. 1998;5:277–87. doi: 10.1159/000026348. [DOI] [PubMed] [Google Scholar]
- 4.Dekuijzen PNR, Honour JW. Inhaled corticosteroids and the hypothalamic-pituitary-adrenal (HPA) axis: do we understand their interaction. Respir Med. 2000;94:627–31. doi: 10.1053/rmed.2000.0791. [DOI] [PubMed] [Google Scholar]
- 5.Hempel G, Karlsson MO, de Alwis DP, Toublanc N, McNay J, Schaefer HG. Population pharmacokinetic-pharmacodynamic modeling of moxonidine using 24-hour ambulatory blood pressure measurements. Clin Pharmacol Ther. 1998;64:622–35. doi: 10.1016/S0009-9236(98)90053-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraja N, Pechstein B, Erb K, Klipping C, Hermann R, Locher M, Derendorf H. Pharmacokinetic/pharmacodynamic modeling of luteinizing hormone (LH) suppression and LH surge delay by Cetrorelix after single and multiple doses in healthy postmenopausal women. J Clin Pharmacol. 2003;43:243–51. doi: 10.1177/0091270003251377. [DOI] [PubMed] [Google Scholar]
- 7.Sheiner LB, Beal SL. NONMEM Users Guide. San Francisco: Division of Pharmacology, University of California; 1979. [Google Scholar]
- 8.Jonsson EN, Karlsson MO. Xpose – an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Ryrfeldt A, Edsbacker S, Pauwels R. Kinetics of the epimeric glucocorticoid budesonide. Clin Pharmacol Ther. 1984;35:525–30. doi: 10.1038/clpt.1984.71. [DOI] [PubMed] [Google Scholar]
- 10.Minto C, Li B, Tattam B, Brown K, Seale J, Donnelly R. Pharmacokinetics of epimeric budesonide and fluticasone propionate after repeat dose inhalation – intersubject variability in systemic absorption from the lung. Br J Clin Pharmacol. 2000;50:116–24. doi: 10.1046/j.1365-2125.2000.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorsson L, Edsbäcker S. Lung deposition of budesonide from a pressurized metered-dose inhaler attached to a spacer. Eur Respir J. 1998;6:1340–5. doi: 10.1183/09031936.98.12061340. [DOI] [PubMed] [Google Scholar]
- 12.Möllman H, Wagner M, Meibhom B, Hochhaus G, Barth J, Stöckman R, Krieg M, Weisser H, Falcoz C, Derendorf H. Pharmacokinetic and pharmacodynamic evaluation of fluticasone propionate after inhaled administration. Eur J Clin Pharmacol. 1998;53:459–67. doi: 10.1007/s002280050407. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty A, Krzyzanski W, Jusko WJ. Mathematical modeling of circadian cortisol concentrations in direct response models: comparison of several methods. J Pharmacokinet Biopharm. 1999;27:23–43. doi: 10.1023/a:1020678628317. [DOI] [PubMed] [Google Scholar]
- 14.Meibhom B, Hochhaus G, Möllmann H, Barth J, Wagner M, Krieg M, Stöckmann R, Derendorf H. A pharmacokinetic/pharmacodynamic approach to predict the cumulative cortisol suppression of inhaled corticosteroids. J Pharmacokinet Biopharm. 1999;27:127–47. doi: 10.1023/a:1020670421957. [DOI] [PubMed] [Google Scholar]
- 15.Krzyzanski W, Chakraborty A, Jusko WJ. Algorithm for application of Fourier analysis for biorhythmic baselines of pharmacodynamic indirect response models. Chronobiol Int. 2000;17:77–93. doi: 10.1081/cbi-100101034. [DOI] [PubMed] [Google Scholar]
- 16.Mager DE, Lin SZ, Blum RA, Lates DL, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–27. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 17.Chrousos GP, Harris AG. Hypothalamic-pituitary-adrenal axis suppression and inhaled corticosteroid therapy. 2 Review of the literature. Neuroimmunomodulation. 1998;5:288–308. doi: 10.1159/000026349. [DOI] [PubMed] [Google Scholar]
- 18.Keenan DM, Roelfsema F, Veldhuis JD. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287:E652–E661. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- 19.Gabrielsson J, Jusko WJ, Alari L. Modeling of dose–response-time data: Four examples of estimating the turnover parameters and generating kinetic functions from response profiles. Biopharm Drug Dispos. 2000;21:41–52. doi: 10.1002/1099-081x(200003)21:2<41::aid-bdd217>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Thorsson L, Edsbaacker S, Kallen A, Lofdahl CG. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbohaler. Br J Clin Pharmacol. 2001;52:529–38. doi: 10.1046/j.0306-5251.2001.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison W. Systemic availability of inhaled budesonide and fluticasone propionate: healthy versus asthmatic lungs. Biodrugs. 2001;15:405–11. doi: 10.2165/00063030-200115060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98:4028–33. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]