For any business a key strategy is to sell a product to the widest possible market. The pharmaceutical industry is no different – the profit of a company will be significantly increased if new markets can be opened up for existing products or market size increased for new medications. In a free market for a non-essential item this strategy is commonplace and regarded as ‘good business’. However, problems arise when the marketplace is a cash-limited health service such as the NHS and the product is as essential and emotive as healthcare. A recent series of papers published by the Public Library of Science (PLoS) has expressed concerns about how the pharmaceutical industry is actively expanding drug markets, this practice being given the label ‘disease mongering’[1–8]. Disease mongering has been defined as ‘widening boundaries of treatable illness in order to expand markets for those who profit from treatments’[9].
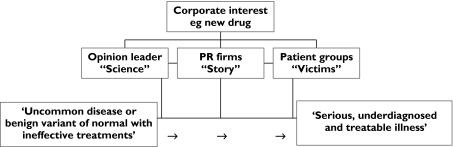
A number of the following examples have occurred outside the UK in North America or Australia. However, they share a common model (Figure 1), which is broadly applicable to drug promotion in the UK. Indeed, recently, the manufacturer of a treatment for restless legs syndrome (see below) was ruled to have breached the UK pharmaceutical industry's marketing code [10].
Figure 1.
Marketing model for drug market expansion. A party with a financial interest in a disease (most commonly a pharmaceutical company with a new medication or old medication seeking a new market) will find key opinion leaders to support the company's medication; approach and often support patient awareness groups to raise the disease profile; and employ public relations firms to combine the ‘science’ with the disease to make the public and health professionals aware of a serious, under diagnosed but now treatable illness. In some instances this will be beneficial to public health but when the disease is actually benign, uncommon or untreatable then ‘disease mongering’ can have adverse consequences
To increase the market for drug therapy one approach is to repackage a condition considered normal as a disease. Male-pattern baldness was linked in the media with serious emotional consequences and a risk of unemployment at the time a new medication was licensed [9]. These stories originated from the new drug's manufacturer but no reference was made to the source in the media. Another approach commonly used is to inflate the prevalence of a disease and encourage self-diagnosis. This was used to increase the market for a new drug to treat restless legs syndrome (RLS) [8]. Stories appeared in the media stating a disease prevalence of 1 in 10 Americans (based on questionable data), suggesting that most doctors fail to diagnose RLS and encouraging self-diagnosis via the RLS patient support group website. This website was sponsored by the manufacturer of a new therapy for the condition and the source for the media stories was the same drug manufacturer. A different approach was taken by the manufacturer of sildenafil, who increased the market for their drug by packaging it as a ‘lifestyle’ drug for any man with any degree of erectile dysfunction rather than strictly for treating the consequences of organic disease such as diabetes and cancer [4]. Risk factors are often repackaged as diseases to increase the public perception that expensive treatment is essential. In some instances this has been beneficial to public health (e.g. statin use in hyperlipidaemia), but often selling risk factors as diseases that are easy and essential to treat is misleading, expensive and not necessarily in the patient's best interests (for example, some areas of the treatment of osteoporosis) [9].
Why is this practice bad and who is to blame? This aggressive marketing of disease forces healthy people to consider themselves sick and exposes people to drug side-effects without balancing benefit. The increased, unnecessary prescribing is also potentially very expensive for any state-funded health service and could result in significant opportunity costs with more cost-effective treatments not being funded. However, to lay the blameat the door of the pharmaceutical industry (who are ultimately accountable to their shareholders to provide profit) would be to miss the complexity of the problem. Almost everyone involved in healthcare has to take responsibility.
Doctors can have a very uncomfortable relationship with pharmaceutical companies and become too closely linked to provide unbiased information to their patients. This is a problem especially for the key opinion leaders so critical to successful drug marketing. While a complete separation of physicians from involvement with industry may be unrealistic, transparency is essential. A significant translational block would be created if NHS and university-employed physicians could not work with industry in taking discoveries from ‘bench to bedside’, but it is essential that all involvement is clearly stated when opinions are quoted in the media and papers published in medical journals. Authors and editors must also be careful not to overstate and extrapolate the significance of clinical trial results. Suggesting that a new drug may have uses beyond those supported by robust data encourages the spurious creation of new drug markets.
Patients, as the consumers in this market, also have a responsibility. Specifically, we would encourage distance between disease awareness groups and the pharmaceutical industry to prevent the advice of these groups being compromised. The media also play a central role. Too often, medical stories appear in the media (and not always just the lay media) without any mention of the source of the information (e.g. a PR firm employed by a pharmaceutical company) and without questioning the statements regarding disease prevalence, drug benefit or potential side-effects.
So are pharmaceutical companies behaving unethically or is this just good business? Guidance on this subject can be obtained from the Association of the British Pharmaceutical Industry (ABPI) Code of Practice, which was updated this year [11]. This code governs the promotion of prescription medications in the UK. When the practices described above are compared with guidelines there are a number of areas of concern.
The Code highlights the need for pharmaceutical firms to take ‘ownership’ of promotional material, but the information presented in the media often does not quote a source. The use of PR firms may avoid actually breaching the Code but is not consistent with its ‘spirit’.
The Code prohibits direct-to-consumer advertising for prescription-only medications. If a company raises awareness of a certain disease and then refers patients to a company-sponsored website, this is only a ‘stone’s throw' from advertising directly and may warrant mention in the Code.
The Code allows pharmaceutical companies to sponsor patient groups. However, we and others [12, 13] encourage patient groups to be clearer on their websites about commercial links and prominently display their relationship with their sponsors.
‘Disease mongering’ or actively expanding drug markets is always going to exist and forms a logical business strategy. However, it can be detrimental to patient care and it is the responsibility of everyone to limit its adverse effects.
Acknowledgments
Competing interests: None declared.
References
- 1.Applbaum K. Pharmaceutical marketing and the invention of the medical consumer. PLoS Med. 2006;3:e189. doi: 10.1371/journal.pmed.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath I. Combating disease mongering: daunting but nonetheless essential. PLoS Med. 2006;3:e146. doi: 10.1371/journal.pmed.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar CJ, Deoker A, Kumar A, Kumar A, Hegde BM. Awareness and attitudes about disease mongering among medical and pharmaceutical students. PLoS Med. 2006;3:e213. doi: 10.1371/journal.pmed.0030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lexchin J. Bigger and better: how Pfizer redefined erectile dysfunction. PLoS Med. 2006;3:e132. doi: 10.1371/journal.pmed.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mintzes B. Disease mongering in drug promotion: do governments have a regulatory role? PLoS Med. 2006;3:e198. doi: 10.1371/journal.pmed.0030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moynihan R, Henry D. The fight against disease mongering: generating knowledge for action. PLoS Med. 2006;3:e191. doi: 10.1371/journal.pmed.0030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiefer L. Female sexual dysfunction: a case study of disease mongering and activist resistance. PLoS Med. 2006;3:e178. doi: 10.1371/journal.pmed.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woloshin S, Schwartz LM. Giving legs to restless legs: a case study of how the media helps make people sick. PLoS Med. 2006;3:e170. doi: 10.1371/journal.pmed.0030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moynihan R, Heath I, Henry D. Selling sickness: the pharmaceutical industry and disease mongering. BMJ. 2002;324:886–91. doi: 10.1136/bmj.324.7342.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyer O. GSK breached marketing code. BMJ. 2006;333:368. doi: 10.1136/bmj.333.7564.368-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidance on the Code of PracticeControls on the Promotion of Prescription Medicines in the UK. Available at http://www.abpi.org.uk/publications/pdfs/PMPCA.pdf (last accessed: 26 June 2006).
- 12.Funding patient groups. Lancet. 2006;368:2. [Google Scholar]
- 13.Jack A. Too close for comfort? BMJ. 2006;333:13. doi: 10.1136/bmj.333.7557.13. [DOI] [PMC free article] [PubMed] [Google Scholar]