Abstract
What is already known about this subject
Recent concerns about lack of cardiovascular safety have led to restrictions in the use of venlafaxine in the UK.
Venlafaxine may predispose to arrhythmia in high-risk individuals.
What this study adds
Venlafaxine ingestion gave rise to sympathomimetic cardiovascular features and QTc prolongation in previously healthy young adults.
These findings suggest plausible mechanisms by which venlafaxine might predispose to arrhythmia, and require further consideration.
Aims
Venlafaxine may increase the risk of arrhythmia in certain patients. We sought to characterize the cardiovascular effects of venlafaxine overdose in adults.
Methods
A retrospective casenote review of patients admitted to the Royal Infirmary of Edinburgh between January 2000 and June 2006. Haemodynamic and electrocardiographic data were examined in the whole group and a subset that ingested venlafaxine alone.
Results
Two hundred and thirty-five patients (65 men) with median (interquartile range) age 34 years (27–43 years) had ingested venlafaxine 1500 mg (919–2800 mg). Tachycardia (40.0%), high blood pressure (28.4%) and mydriasis (36.6%) were common. Corrected QT >450 ms occurred in seven men (11.1%) and 17 women (10.5%) and transient arrhythmia in three patients. There was a positive correlation between stated quantity of venlafaxine ingested and heart rate [ρ = 0.195, 95% confidence interval (CI) 0.054, 0.328] and QTc (ρ = 0.314, 95% CI 0.089, 0.509).
Conclusions
Venlafaxine overdose is associated with sympathomimetic cardiovascular effects and prolonged QTc, irrespective of coingested drugs. These mechanisms might pose an increased risk of arrhythmia and require further exploration.
Keywords: arrhythmia, cardiotoxicity, drug overdose, electrocardiograph, poisoning, safety monitoring
Introduction
Venlafaxine is a phenylethylamine derivative whose pharmacological mechanisms of action are related to inhibition of serotonin and norepinephrine neuronal reuptake [1]. Utilization of venlafaxine has increased progressively since it was introduced in 1994, for example in patients who do not respond adequately to selective serotonin reuptake inhibitors (SSRIs) [2]. It is associated with a number of predictable adverse effects that are generally mild, including tachycardia, increasedblood pressure, fatigue, headache, dizziness and dry mouth [3]. The potential long-term consequences of the modest increase in systemic blood pressure have not been evaluated, although venlafaxine is a recognized cause of hypertensive crisis [4]. In healthy young adults, venlafaxine is capable of prolonging the QT interval, particularly at doses >200 mg daily [5]. The effects of high doses, as might be encountered in self-poisoning, have not been fully characterized, but tachycardia, high blood pressure, seizures and serotonin syndrome have been described [6, 7]. One series of venlafaxine poisoning cases found a higher prevalence of seizures and arrhythmia than other antidepressants, associated with increased risk of fatalities [8].
Data reported by coroners in England and Wales between 1998 and 2000 have implicated venlafaxine in 12.7 deaths per million prescriptions, which was substantially higher than for SSRIs and similar to tricyclic antidepressants (1.9 and 12.6 deaths per million prescriptions, respectively) [9]. Census data show the number of venlafaxine-related deaths per million prescriptions have been consistently higher than those related to SSRIs, e.g. 13.2 vs. 1.6 in England, Wales and Scotland between 1993 and 1999, and 8.5 vs. 0.9 in England and Wales between 1993 and 2002 [10, 11]. However, these epidemiological data do not take depression severity into account, and baseline suicidal behaviour may be an important confounding factor. In view of concerns about cardiovascular toxicity, the Medicines and Healthcare products Regulatory Agency (MHRA) amended the licence for venlafaxine use within the UK in 2004. Proposed contraindications to venlafaxine were pre-existing cardiac disease, hypertension and arrhythmia, in order to minimize risk in these patient groups. These recommendations have recently been revised and a warning has been introduced regarding patients with heart disease. Venlafaxine is contraindicated in patients at high risk of arrhythmia or who have uncontrolled hypertension, and initiation of doses of ≥300 mg should happen only under specialist supervision [12]. The MHRA have suggested venlafaxine be reserved as second-line treatment after SSRIs. In light of the potential for cardiotoxic effects, we wished to establish the potential for cardiovascular effects in patients with acute venlafaxine poisoning. Therefore, we examined the haemodynamic and electrocardiographic characteristics of adults who presented to hospital after deliberate venlafaxine overdose.
Methods
The study was a retrospective review of casenotes of patients admitted to the Royal Infirmary of Edinburgh due to venlafaxine poisoning between 1 January 2000 and 30 June 2006. Our standard practice is that patients who present to the Emergency Department may be admitted to the Toxicology Unit, or the High Dependency Unit if non-invasive ventilatory support is likely to be required, or the Intensive Treatment Unit if invasive ventilation may be required. Patients in all these clinical areas were included. The study fulfilled locally agreed criteria for clinical audit, so that formal approval by the regional research ethics committee was not sought.
Data collection
A standardized data collection sheet was used to record patient age, gender, stated date and time of overdose, quantity ingested, type and quantity of any coingested drugs and alcohol and any documented clinical history of heart disease. The interval between drug ingestion and assessment was determined, and primary outcome variables were heart rate, systolic and diastolic blood pressures, PR interval, QRS duration, QT interval and QTc (QT interval corrected by Bazett's formula, QTc = QT/vRR). Tachycardia was defined as resting heart rate >100 min−1, high systolic and diastolic blood pressures by values >140 mmHg and >85 mmHg, respectively, and QT prolongation by QT or QTc interval >450 ms [13]. Mydriasis was defined by pupil diameter >6 mm on inspection.
Data analyses
Variables were examined across the whole group, and also the subset that ingested venlafaxine alone, and compared between men and women. Data were not expected to be normally distributed and therefore presented as median and interquartile ranges. Mann–Whitney tests were used to compare unpaired data and two-tailed Yates' corrected χ2 tests used to compare proportions. Abnormal variables were identified as those outside normal 95% population reference values and statistical significance determined by binomial tests. Correlations were sought between the stated venlafaxine dose and haemodynamic and electrocardiographic variables using Spearman's coefficient of rank correlation (ρ) and 95% confidence limits for ρ. Statistical tests were performed using MedCalc (version 9.0.1.1; MedCalc, Mariakerke, Belgium) and P-values <0.05 were accepted as statistically significant.
Results
There were 260 cases of venlafaxine poisoning during the study period. Casenotes were unavailable for 25 and therefore 235 cases were included. These involved women in 170 cases (72.3%) and the median (interquartile range) population age was 34 years (27–43 years). The stated quantity of ingested venlafaxine was 1500 mg (919–2800 mg). In 154 cases (65.5%), additional drugs were coingested; these were paracetamol (16.6%), benzodiazepines (13.6%), other antidepressants (10.6%), nonsteroidal anti-inflammatory drugs (8.9%), antipsychotics (8.9%) and others (17.8%).
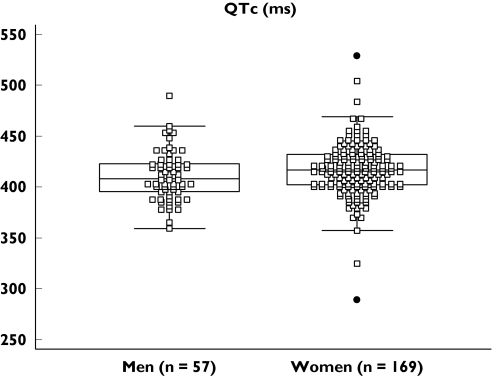
Haemodynamic and electrocardiographic variables are summarized in Table 1 and the prevalence of abnormalities is summarized in Table 2. Tachycardia, high blood pressure, mydriasis and QTc prolongation were highly prevalent in both men and women, irrespective of whether venlafaxine was coingested or ingested alone. The distribution of QTc values is represented in Figure 1. A positive correlation was found between the stated quantity of venlafaxine ingested and heart rate [ρ = 0.195, 95% confidence interval (CI) 0.054, 0.328, P = 0.0073] and QTc (ρ = 0.314, 95% CI 0.089, 0.509, P = 0.0081), in the population who had ingested venlafaxine alone. No significant correlation was found between dose and systolic or diastolic blood pressure, or other electrographic variables.
Table 1.
Clinical and electrocardiographic variables after venlafaxine overdose, in the whole group (n = 170 women, 65 men) and subset that ingested only venlafaxine (n = 60 women, 21 men), presented as median and interquartile range
| Whole group | Venlafaxine alone | ||
|---|---|---|---|
| Age | Women | 34 (27–41) | 34 (26–41) |
| Men | 37 (30–45) | 34 (27–43) | |
| Both | 34 (27–43) | 34 (27–42) | |
| Venlafaxine dose (mg) | Women | 1500 (900–2700) | 1575 (1088–3000) |
| Men | 1538 (984–3000) | 1838 (1119–3000) | |
| Both | 1500 (919–2800) | 1800 (1113–3000) | |
| Interval to assessment (h) | Women | 3.0 (1.9–5.6) | 2.8 (1.8–5.5) |
| Men | 4.1 (2.1–11.2) | 2.9 (1.9–12.3) | |
| Both | 3.1 (2.0–7.6) | 2.9 (1.8–7.1) | |
| Heart rate (min−1) | Women | 99 (84–117) | 106 (89–120) |
| Men | 93 (81–108) | 89 (79–105) | |
| Both | 98 (83–112) | 100 (82–117) | |
| Systolic BP (mmHg) | Women | 126 (116–139) | 132 (121–141) |
| Men | 138 (126–148) | 144 (135–155) | |
| Both | 130 (118–142) | 134 (123–145) | |
| Diastolic BP (mmHg) | Women | 76 (66–85) | 80 (71–93) |
| Men | 80 (71–90) | 81 (74–91) | |
| Both | 77 (67–86) | 80 (71–92) | |
| PR (ms) | Women | 151 (137–161) | 148 (141–161) |
| Men | 150 (138–165) | 153 (140–165) | |
| Both | 151 (137–162) | 148 (140–162) | |
| QRS (ms) | Women | 76 (35–89) | 75 (35–87) |
| Men | 83 (58–97) | 82 (49–95) | |
| Both | 79 (40–91) | 77 (40–88) | |
| QT (ms) | Women | 337 (310–364) | 337 (310–352) |
| Men | 337 (323–368) | 332 (324–373) | |
| Both | 337 (314–364) | 336 (313–355) | |
| QTc (ms) | Women | 420 (403–430) | 415 (401–430) |
| Men | 417 (398–431) | 417 (390–436) | |
| Both | 419 (402–433) | 415 (401–432) |
Table 2.
Clinical and electrocardiographic abnormalities after venlafaxine overdose, within the whole group (n = 235) and subset that ingested only venlafaxine (n = 81)
| Whole group | Venlafaxine alone | ||
|---|---|---|---|
| Mydriasis (>6 mm) | Women | 65 (38.2%)** | 29 (48.3%)** |
| Men | 21 (32.3%)** | 11 (52.4%)** | |
| Both | 86 (36.6%)** | 40 (49.4%)** | |
| Heart rate >100 min−1 | Women | 71 (43.6%)** | 41 (70.7%)**† |
| Men | 23 (36.5%)** | 7 (35.0%)** | |
| Both | 94 (40.0%)** | 38 (48.7%)** | |
| Systolic BP >140 mmHg | Women | 37 (22.7%)**†† | 15 (12.9%)**†† |
| Men | 27 (43.5%)** | 12 (60.0%)** | |
| Both | 64 (28.4%)** | 27 (34.6%)** | |
| Diastolic BP >85 mmHg | Women | 40 (24.5%)** | 20 (34.5%)** |
| Men | 20 (32.3%)** | 8 (40.0%)** | |
| Both | 60 (26.7%)** | 28 (35.9%)** | |
| PR >200 ms | Women | 0 (–) | 0 (–) |
| Men | 0 (–) | 0 (–) | |
| Both | 0 (–) | 0 (–) | |
| QRSd >120 ms | Women | 7 (5.3%) | 1 (2.7%) |
| Men | 2 (3.6%) | 1 (5.0%) | |
| Both | 9 (4.8%) | 2 (2.9%) | |
| QT >450 ms | Women | 0 (–) | 0 (–) |
| Men | 0 (–) | 0 (–) | |
| Both | 0 (–) | 0 (–) | |
| QTc >450 ms | Women | 17 (10.5%)** | 4 (7.0%)* |
| Men | 7 (11.1%)** | 4 (19.0%)** | |
| Both | 24 (10.7%)** | 8 (10.3%)** |
Data presented as the absolute number and percentage of evaluable data in parentheses.
P < 0.05
P < 0.005 by binomial testing, assuming 0.025% population chance.
P < 0.01
P < 0.005 between men and women by two-tailed proportional χ2 test with Yates' correction.
Figure 1.
QTc in men and women after venlafaxine overdose; QTc = QT interval corrected by Bazett's formula = QT√RR
Arrhythmia was documented in three patients. Supraventricular tachycardia occurred in one 36-year-old woman 6 h after ingesting venlafaxine 3 g alone, which resolved shortly after administration of intravenous sodium bicarbonate. Atrial fibrillation occurred in a 30-year-old woman who had ingested venlafaxine 3.25 g and 3.2 g ibuprofen, which resolved spontaneously. Frequent ventricular ectopic beats and short salvos of ventricular tachycardia occurred in a 55-year-old man after ingestion of unknown quantities of venlafaxine and paroxetine, which resolved spontaneously. Nonspecific electrocardiograph abnormalities were identified in nine other cases, including left and right bundle branch block patterns, T-wave inversion and ST depression.
No venlafaxine-related fatalities occurred, including those patients whose casenotes were unavailable for analysis.
Discussion
The key observations were the dose-dependent relationships between venlafaxine ingestion and tachycardia and prolonged QTc. Haemodynamic effects associated with venlafaxine were more prevalent than anticipated, as reported elsewhere [3, 8]. None of the patients had a medical history of cardiovascular disease and the observations were not attributable to the effect of coingested drugs. Moreover, the effects on heart rate and blood pressure are consistent with potentiation of norepinephrine within the cardiovascular system by venlafaxine [14]. The QT interval is inversely related to heart rate, although the extent of this relationship varies considerably between individuals. A variety of techniques have been adopted to minimize the effects of heart rate on interpretation of the QT interval, so-called ‘QT correction’ methods. In some situations, these involve establishing the specific relationship between QT and RR interval from a range of values in individuals. More commonly, however, universal formulae are applied. For example, the present study used Bazett's formula, which allows more reliable assessment than QT interval alone in the setting of drug-induced tachycardia [15]. This method was selected because it is very widely reported and commonly used as a means of QT correction in a clinical setting, albeit that it is unable to correct fully for the influence of heart rate [16]. Other methods include Fridericia's correction (= QT/(RR)1/3) formula, which may allow more accurate appraisal of QT in the setting of extreme heart rate values [17]. Whilst application of a universal formula might allow more accurate interpretation of QT, each of the methods possess inherent strengths and weaknesses and none adequately takes account of interindividual variability. Modelling of the relationship between heart rate (or the inverse R–R interval) and QT interval in individuals has previously been used to examine the time-course of QT effects in the setting of antidepressant drug overdose [18].
QTc prolongation >450 ms occurred in a significantly higher than expected proportion of men and women. Positive correlation between the stated venlafaxine dose and QTc, in the absence of coingested drugs, suggests a causal relationship. QTc prolongation is important because it may herald an increased risk of life-threatening arrhythmia [19]. Although torsades de pointes was not encountered, other arrhythmias did occur in patients who did not have underlying cardiac disease. Consistent with this, an earlier report has described ventricular tachycardia as a consequence of acute venlafaxine and lamotrigine coingestion [20]. The mechanisms by which high doses of venlafaxine are capable of prolonging the QT interval require further exploration. Stimulation of cardiac sympathetic activity by venlafaxine might be sufficient to cause tachyarrhythmia in predisposed individuals [21]. Other pathways might also be important, including blockade of cardiac sodium channels by venlafaxine [22]. Whilst the significance of this effect is unclear, a similar mechanism has been closely linked to arrhythmia and seizures after tricyclic antidepressant poisoning [23]. Additional work is also required to explore whether the cardiac effects of venlafaxine poisoning are directly applicable to therapeutic doses. Conventional doses of venlafaxine have been shown capable of suppressing heart rate variability to a greater extent than other antidepressants, although the mechanisms are uncertain [24].
A key limitation is that this research is observational, including collection and analysis of uncontrolled data. Lack of cardiovascular data prior to hospital attendance means that we are unable to comment on the magnitude of changes from baseline as a consequence of drug ingestion. Only cardiovascular manifestations of toxicity are considered here, whereas other features, e.g. seizures, are also important [8]. Importantly, the data are weakened by lack of a comparator group, for example patients who had ingested an alternative antidepressant. Selection bias may also be introduced by the fact that only patients presenting to hospital after overdose were included. Nonetheless, these data were collected in a realistic setting and are therefore applicable to other patients with venlafaxine poisoning who might be encountered in everyday clinical practice. A further potential limitation is that the quantity of venlafaxine ingested was based on self-reporting. Nonetheless, this appears valid, because a positive correlation exists between the stated amount ingested and plasma drug concentrations in self-poisoned patients [25].
In summary, venlafaxine poisoning is associated with an unexpectedly high prevalence of cardiovascular adverse events. Tachycardia and QTc prolongation are dose-dependent effects which may be linked to development of arrhythmia in certain patients and require further evaluation.
Acknowledgments
We thank Lisa Galloway of the Royal Infirmary of Edinburgh for assistance with identification and retrieval of casenotes for this study.
References
- 1.Holliday SM, Benfield P. Venlafaxine. A review of its pharmacology and therapeutic potential in depression. Drugs. 1995;4:280–2. doi: 10.2165/00003495-199549020-00010. [DOI] [PubMed] [Google Scholar]
- 2.Ciuna A, Andretta M, Corbari L, Levi D, Mirandola M, Sorio A, Barbui C. Are we going to increase the use of antidepressants up to that of benzodiazepines? Eur J Clin Pharmacol. 2004;60:629–34. doi: 10.1007/s00228-004-0810-8. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EM, Whyte E, Mulsant BH, Pollock BG, Weber E, Begley AE, Reynolds CF. Cardiovascular changes associated with venlafaxine in the treatment of late-life depression. Am J Geriatr Psychiatry. 2006;14:796–802. doi: 10.1097/01.JGP.0000204328.50105.b3. [DOI] [PubMed] [Google Scholar]
- 4.Khurana RN, Baudendistel TE. Hypertensive crisis associated with venlafaxine. Am J Med. 2003;115:676–7. doi: 10.1016/s0002-9343(03)00472-8. [DOI] [PubMed] [Google Scholar]
- 5.Letsas K, Korantzopoulos P, Pappas L, Evangelou D, Efremidis M, Kardaras F. QT interval prolongation associated with venlafaxine administration. Int J Cardiol. 2006;109:116–7. doi: 10.1016/j.ijcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN. Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol. 2004;42:67–71. doi: 10.1081/clt-120028747. [DOI] [PubMed] [Google Scholar]
- 7.Kolecki P. Isolated venlafaxine–induced serotonin syndrome. J Emerg Med. 1997;15:491–3. doi: 10.1016/s0736-4679(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 8.Whyte IM, Dawson AH, Buckley NA. Relative toxicity of venlafaxine and selective serotonin reuptake inhibitors in overdose compared to tricyclic antidepressants. QJM. 2003;96:369–74. doi: 10.1093/qjmed/hcg062. [DOI] [PubMed] [Google Scholar]
- 9.Cheeta S, Schifano F, Oyefeso A, Webb L, Ghodse AH. Antidepressant-related deaths and antidepressant prescriptions in England and Wales, 1998–2000. Br J Psychiatry. 2004;184:41–7. doi: 10.1192/bjp.184.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Buckley NA, McManus PR. Fatal toxicity of serotoninergic and other antidepressant drugs: analysis of United Kingdom mortality data. BMJ. 2002;325:1332–3. doi: 10.1136/bmj.325.7376.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan O, Griffiths C, Baker A, Majeed A. Fatal toxicity of antidepressants in England and Wales, 1993–2002. Health Stat Q. 2004;23:18–24. [PubMed] [Google Scholar]
- 12.Medicines and Healthcare products Regulatory Authority (MHRA). Updated Prescribing Advice for Venlafaxine (Effexor/Effexor XL): Information for Healthcare Professionals. 31 May 2006. Available in Safety information section of http://www.mhra.gov.uk Last accessed October 2006.
- 13.US Food and Drug Administration (FDA). Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Available at http://www.fda.gov/cder/guidance/6922fnl.htm Last accessed October 2006. Guidance for Industry E14.
- 14.Abdelmawla AH, Langley RW, Szabadi E, Bradshaw CM. Comparison of the effects of venlafaxine, desipramine, and paroxetine on noradrenaline- and methoxamine-evoked constriction of the dorsal hand vein. Br J Clin Pharmacol. 1999;48:345–54. doi: 10.1046/j.1365-2125.1999.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Extramiana F, Maison-Blanche P, Cabanis MJ, Ortemann-Renon C, Beaufils P, Leenhardt A. Clinical assessment of drug-induced QT prolongation in association with heart rate changes. Clin Pharmacol Ther. 2005;77:247–58. doi: 10.1016/j.clpt.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl.):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 17.Fossa AA, Wisialowski T, Magnano A, Wolfgang E, Winslow R, Gorczyca W, Crimin K, Raunig DL. Dynamic beat-to-beat modeling of the QT–RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a-go-go-related gene inhibition. J Pharmacol Exp Ther. 2005;312:1–11. doi: 10.1124/jpet.104.073288. [DOI] [PubMed] [Google Scholar]
- 18.Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic–pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol. 2006;61:177–90. doi: 10.1111/j.1365-2125.2005.02546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sala M, Coppa F, Cappucciati C, Brambilla P, d'Allio G, Caverzasi E, Barale F, De Ferrari GM. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Invest Drugs. 2006;7:256–63. [PubMed] [Google Scholar]
- 20.Peano C, Leikin JB, Hanashiro PK. Seizures, ventricular tachycardia, and rhabdomyolysis as a result of ingestion of venlafaxine and lamotrigine. Ann Emerg Med. 1997;30:704–8. doi: 10.1016/s0196-0644(97)70093-3. [DOI] [PubMed] [Google Scholar]
- 21.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Rev. 2005;49:555–65. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Khalifa M, Daleau P, Turgeon J. Mechanism of sodium channel blockade by venlafaxine in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 1999;291:280–4. [PubMed] [Google Scholar]
- 23.Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev. 2005;24:205–14. doi: 10.2165/00139709-200524030-00013. [DOI] [PubMed] [Google Scholar]
- 24.Davidson J, Watkins L, Owens M, Krulewicz S, Connor K, Carpenter D, Krishnan R, Nemeroff C. Effects of paroxetine and venlafaxine XR on heart rate variability in depression. J Clin Psychopharmacol. 2005;25:480–4. doi: 10.1097/01.jcp.0000177547.28961.03. [DOI] [PubMed] [Google Scholar]
- 25.Balit CR, Isbister GK, Hackett LP, Whyte IM. Quetiapine poisoning: a case series. Ann Emerg Med. 2003;42:751–8. doi: 10.1016/s0196-0644(03)00600-0. [DOI] [PubMed] [Google Scholar]