Abstract
What is already known about this subject
Flavonoids are largely recognized as potential inhibitors of platelet function, through nonspecific mechanisms such as antioxidant activity and/or inhibition of several enzymes and signalling proteins.
In addition, we, and few others, have shown that certain antiaggregant flavonoids may behave as specific TXA2 receptor (TP) ligands in platelets.
Whether flavonoids interact with TP isoforms in other cell types is not known, and direct evidence that flavonoid–TP interaction inhibits signalling downstream TP has not been shown.
What this study adds
This study first demonstrates that certain flavonoids behave as ligands for both TP isoforms, not only in platelets, but also in human myometrium and in TP-transfected HEK 293T cells.
Differences in the effect of certain flavonoids in platelet signalling, induced by either U46619 or thrombin, suggest that abrogation of downstream TP signalling is related to their specific blockage of the TP, rather than to a nonspecific effect on tyrosine kinases or other signalling proteins.
Aims
Flavonoids may affect platelet function by several mechanisms, including antagonism of TxA2 receptors (TP). These TP are present in many tissues and modulate different signalling cascades. We explored whether flavonoids affect platelet TP signalling, and if they bind to TP expressed in other cell types.
Methods
Platelets were treated with flavonoids, or other selected inhibitors, and then stimulated with U46619. Similar assays were performed in aspirinized platelets activated with thrombin. Effects on calcium release were analysed by fluorometry and changes in whole protein tyrosine phosphorylation and activation of ERK 1/2 by Western blot analysis. The binding of flavonoids to TP in platelets, human myometrium and TPα- and TPβ-transfected HEK 293T cells was explored using binding assays and the TP antagonist 3H-SQ29548.
Results
Apigenin, genistein, luteolin and quercetin impaired U46619-induced calcium mobilization in a concentration-dependent manner (IC50 10–30 µm). These flavonoids caused a significant impairment of U46619-induced platelet tyrosine phosphorylation and of ERK 1/2 activation. By contrast, in aspirin-treated platelets all these flavonoids, except quercetin, displayed minor effects on thrombin-induced calcium mobilization, ERK 1/2 and total tyrosine phosphorylation. Finally, apigenin, genistein and luteolin inhibited by >50% 3H-SQ29548 binding to different cell types.
Conclusions
These data further suggest that flavonoids may inhibit platelet function by binding to TP and by subsequent abrogation of downstream signalling. Binding of these compounds to TP occurs in human myometrium and in TP-transfected HEK 293T cells and suggests that antagonism of TP might mediate the effects of flavonoids in different tissues.
Keywords: flavonoid, HEK 293T cells, platelets, smooth muscle cells, TP signalling
Introduction
The central involvement of platelets in the development of atherothrombosis [1], the leading cause of death in developed countries, raises interest in the potential prevention of platelet hyperactivity by dietary components, including flavonoids. These are a heterogeneous group of plant polyphenolic compounds, all sharing a primary chemical structure of two benzene rings linked through a pyrone ring, that are believed to have anticarcinogenic, anti-inflammatory and antithrombotic activity [2, 3].
Classically viewed as natural antioxidants, research into the mechanisms by which flavonoids affect cell function increasingly reveals that these compounds do not act solely through a conventional hydrogen-donating activity, but may also exert selective actions at enzymes, receptors, components of kinase signalling cascades and gene regulators [4–6]. It is likely that the specific aspects of flavonoid structure, such as number and substitution of hydroxyl groups, degree of unsaturation or glycosylation status, will largely influence its mechanism of action on target cells. With regard to platelets, many dietary flavonoids have been long shown to inhibit platelet function, but the underlying mechanisms are far from clear [7–12].
Thromboxane A2 (TxA2) is a powerful unstable inducer of platelet activation and aggregation produced by sequential arachidonic acid metabolism by cyclooxygenase and thromboxane synthase, upon activation of platelets with agonists such as adenosine diphosphate, thrombin or collagen. Once generated, TxA2 acts in an autocrine and paracrine manner, increasing activation and recruitment of the surrounding platelets to the site of vascular damage [13]. The major involvement of TxA2 in the pathogenesis of atherothrombosis is demonstrated by the established clinical benefits of treating risk patients with aspirin, an irreversible inhibitor of cyclooxygenase [14]. However, aspirin is neither efficient in all patients nor free of an increased risk of bleeding or other undesirable complications [15, 16]. Thus, the search for other TxA2 pathway modulators that could be not only selective and effective, but also safe and well tolerated, is of great interest [17].
TxA2 effects on platelets, and on other target cells, are mediated via interaction with specific seven-transmembrane G-protein-coupled receptors (GPCR). The TxA2 receptor (TP) is encoded by a single gene that is alternatively spliced in the carboxyl terminus resulting in two variants, TPα (343 residues) and TPβ (407 residues), that share the first 328 amino acids [18, 19]. While platelets express the message for both TPα and TPβ, the former is the predominant isoform in these cells [20]. The TP is functionally coupled to distinct heterotrimeric G proteins, notably Gαq and G12/13, and participates in the activation of multiple signalling cascades [21, 22]. Consistent with the important role of TP signalling in platelet function and haemostasis, mice lacking this receptor have prolonged bleeding times and impaired aggregation response to TxA2 analogues [23]. Several studies have shown that flavonoids impair agonist-induced TxA2 formation through the inhibition of arachidonic acid liberation and metabolism by cyclooxygenase and TxA2 synthase activities [9, 24–26]. Moreover, studies using TxA2 analogues indicate that certain flavonoids may behave as TP antagonists [27, 28]. However, direct interaction of flavonoids with TP and their interference on the TP signalling pathway have not been investigated in detail [29, 30].
We have recently observed that certain flavonoids that behave as ligands for I-BOP (a TP agonist) binding sites inhibit platelet secretion and aggregation induced by collagen and arachidonic acid, but not by thrombin [31]. Here, we show that these flavonoids specifically impair signal transduction events downstream of the platelet TP, namely TxA2-induced Ca2+ mobilization and protein phosphorylation. In addition, we further demonstrate by competitive radioligand binding assays the interaction of these flavonoids with TP, not only in platelets but also in human myometrium and in a cellular model expressing the TP isoforms.
Materials and methods
Reagents
Apigenin, quercetin and rutin were kindly provided by Furfural Español (Murcia, Spain). Genistein and luteolin were from Sigma-Aldrich Química (Madrid, Spain). The TP agonist U46619 (9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid) and human thrombin were from Calbiochem-Novabiochem AG (Lucerne, Switzerland). The TP agonist I-BOP ([1S-[1α,2α(Z),3β(1E,3S*),4α]]-7-[3-[3-hydroxy-4-(4-iodophenoxy)-1-butenyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) and the stable synthetic TP antagonist SQ29548 ([1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid) were purchased from Cayman Chemical (Ann Arbor, MI, USA).
The tritium-labelled antagonist 3H-SQ29548 (specific activity 48.2 Ci mmol−1) was from PerkinElmer (Boston, MA, USA). Oregon Green 488 BAPTA-1 AM was from Molecular Probes (Eugene, OR, USA). Protease inhibitor and phosphatase inhibitor cocktail II were from Sigma-Aldrich Química. The Src kinase inhibitor PP2 and the MEK/ERK inhibitor U0126 were obtained from Calbiochem-Novabiochem. Monoclonal antiphosphotyrosine PY20 purified mouse immunoglobulin was from Sigma-Aldrich and monoclonal antiphospho ERK 1/2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibody SAP.4G5 to βI tubulin was from Abcam Ltd (Cambridge, UK). Antimouse IgG horseradish peroxidase antibody and the ECL detection system were purchased from Amersham Biosciences Europe GmbH (Barcelona, Spain). Lipofectamine were from Invitrogen SA (Barcelona, Spain). All other chemicals and solvents were of the highest analytical grade commercially available.
Isolation of platelets
Blood samples were collected from healthy volunteers in 13 mm trisodium citrate (final concentration) supplemented with prostaglandin E1 (0.14 µm) and apyrase (100 U l−1). Platelets were isolated and washed free of plasma components by the Mustard procedure [32] and resuspended in the appropriate assay buffer. The platelet concentration was determined using a Coulter counter (STKS; Coulter Electronics, Hialeah, FL, USA) and adjusted to an appropriate count for the experiment to be performed. In all cases, platelets were left to rest at 37°C for 30 min prior to experiments.
Measurement of intraplatelet free calcium concentration ([Ca2+]i)
Washed platelets (600 × 109 l−1) in calcium-free Krebs–HEPES buffer (118 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 1.2 mm KH2PO4, 4.2 mm NaHCO3, 11.7 mm glucose, 10 mm HEPES, pH 7.4) were incubated with 3 µm Oregon green BAPTA-1 AM for 45 min at 37°C [33]. Excess of fluorochrome was removed by two steps of centrifugation of platelets at 800 g, 8 min and washing in modified Tyrode's buffer (137 mm NaCl, 2.9 mm KCl, 12 mm NaHCO3, 0.42 mm Na2HPO4, 2 mm MgCl2, 5.5 mm glucose, 10 mm HEPES, pH 6.5) and then resuspended at 200 × 109 l−1 in Krebs–HEPES buffer. To establish the concentration of flavonoid necessary to obtain half-maximal inhibition of [Ca2+]i mobilization (IC50), platelets were incubated with flavonoids at different concentrations, or with equivalent volume of dimethylsulphoxide, in the presence of 2 mm ethylene glycol tetraacetic acid (EGTA), before U46619 (2 µm)-induced [Ca2+]i mobilization. To investigate the selective effect of flavonoids on the inhibition of [Ca2+]i mobilization, platelets or aspirin-treated platelets (1 mm aspirin for 10 min at room temperature) were incubated with flavonoids (50 µm) and stimulated with 2 µm U46619 or 0.5 U ml−1 thrombin (aspirin-treated platelets).
Fluorescence was recorded using a FLUOstar fluorometer (BMG Laboratory Technologies GmbH, Offenburg, Germany) with excitation and emission wavelengths of 488 nm and 520 nm, respectively [33]. At the end of each experiment, fluorescence intensities were calibrated for determination of [Ca2+]i values by permeabilizing cells with 0.5% Triton X-100 in the presence of 2 mm CaCl2 to release all the dye (Fmax) and subsequent chelating with 10 mm EGTA (Fmin). Calcium concentrations were calculated using the equation [Ca2+]i = Kd × (F − Fmin)/(Fmax − F). An equilibrium dissociation constant (Kd) of 170 nm was used for Oregon Green 488 BAPTA-1 AM. The dose-dependent inhibition curve achieved for each flavonoid was analysed with a nonlinear curve-fitting package (Ultrafit; Biosoft, Cambridge, UK) to establish the concentration of flavonoid necessary to obtain half-maximal inhibition of [Ca2+]i mobilization of (IC50).
Analysis of protein phosphorylation
Washed platelets (1 × 1012 l−1) were resuspended in calcium-free Tyrode's buffer pH 7.35, also containing 1 mm EGTA to prevent signalling events dependent on aggregation and secretion [11]. Platelets, or aspirin-treated platelets (1 mm aspirin for 10 min at room temperature) if specifically stated, were incubated either with flavonoids or with other compounds and were then stimulated with agonists at 37°C for 2 min under stirring. Incubations were terminated by adding 2% sodium dodecyl sulphate (SDS) Tyrode's buffer also containing 1 : 50 volume of protease and phosphatase inhibitor cocktails. Proteins were resolved by SDS–polyacrylamide gel electrophoresis under reducing conditions. Immunodetection of total tyrosine phosphorylated proteins and phosphorylated ERK 1/2 was performed using PY20 and antiphospho-ERK 1/2 (both at a 1 : 1000 dilution), respectively, followed by appropriate peroxidase-labelled secondary antibodies. Intra-assay control of protein loading was assessed by immunodetection of βI tubulin. Blots were exposed to enhanced chemiluminiscence reagents and to preflashed photographic film. Densitometric measurements were made using a Chemi-Doc densitometer and Quantity One software (Bio-Rad, Segrate, Italy) and the intensities of phosphorylation were expressed as arbitrary units. Comparisons were made with phosphorylation levels achieved by platelets activated with agonists in the absence of flavonoids or other inhibitors.
Transfection of TPα and TPβ cDNA into HEK 293 T cells
HEK 293T cells, kindly provided by C. Jimenez-Cervantes (University of Murcia, Spain), were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, and 2 × 106 cells were plated in 60-mm culture dishes 24 h before transfection. Cells were transiently transfected with the purified pcDNA3 plasmid expressing α or β isoforms of TP, kindly provided by J-L. Parent (University of Sherbrooke, Québec, Canada). Transfections were performed with lipofectamine according to the manufacturer's recommendations. Cells were harvested 24 h after transfection, washed twice in DMEM medium and resuspended in phosphate-buffered saline.
Myometrium preparation
Samples of human myometrium, free from endometrium and serosa, were obtained from the corpus uteri of three hysterectomies performed for benign gynaecological disorders, with approval of a local Research Ethics Committee. The tissues were collected in saline, washed and frozen for a maximum period of 1 week before processing. For binding experiments, a crude membrane fraction was prepared by the method described by Chiang and Tai [34]. Isolated longitudinal and circular muscles were cut into small pieces and mechanically homogenized (Polytron; Brinckman Instruments, Westbury, NY, USA) in 10 mm Tris–HCl buffer (pH 7.4) containing 10 µm indomethacin and 0.29 µm phenylmethyl sulphonyl fluoride. The homogenate was centrifuged at 2000 g for 30 min at 4°C and the supernatant was then centrifuged twice at 40 000 g (for 30 min at 4°C). The resulting pellets were washed twice and suspended in SQ buffer (10 mm Tris–HCl, 120 mm NaCl, 5 mm glucose, 0.8 µm indomethacin, pH 7.4). The protein concentration was determined using a Bradford assay kit (Bio-Rad, Richmond, CA, USA) and homogenates were frozen for a maximum period of 2 weeks before use in radioligand binding studies.
Radioligand binding studies
The myometrial membrane fractions (100 µg per tube), platelets (100 × 106 per tube) or HEK 293T cells (1 × 106 per tube) in SQ buffer were incubated in duplicate with 5 nm3H-SQ29548 alone or in the presence of increasing concentrations of unlabelled SQ29548, in a final volume of 0.5 ml SQ buffer containing 2 mm ethylenediamine tetraacetic acid. Nonspecific binding was determined in the presence of 10 µm cold SQ29548. After incubation at room temperature for 45 min, the incubation mixture was filtered through a Millipore GF/C glass-fibre filter (Millipore Ibérica, Madrid, Spain) using a vacuum filtration device (1225 Sampling Manifold; Millipore Ibérica). After washing out three times, the filters were placed in glass vials, 5 ml scintillation liquid (OptiSolv; FSA Laboratory, Loughborough, UK) was added and the filter bound radioactivity was counted (Wallac 1409 counter; AG & G, Turku, Finland). The equilibrium binding data for 3H-SQ29548 in TP-transfected HEK 293T cells, human myometrium and platelets were fitted to a single class of sites, using the ‘Cold’ option of the computer program LIGAND (Kell-Biosoft, Cambridge, UK). This computer analysis provides both the number of binding sites (Bmax) and the affinity constant of the ligand for these sites (Kd).
To assess the ability of flavonoids to bind to TP, binding assays were performed, essentially as above, by incubating platelets, HEK cells and myometrium samples, with 5 nm3H-SQ29548 either alone or in the presence of 100 µm flavonoids. According to our previous work [31], this concentration is expected to reduce significantly the ligand binding to TP.
Statistical analysis
Results are reported as mean ± SD from at least three experiments conducted on different platelet, tissue or cell samples. Statistical comparisons of untreated samples and those treated with the flavonoids or with other test compounds were achieved by two-tailed paired t-test using Prism for Windows version 4.0 (GraphPad Inc., San Diego, CA, USA). Differences were considered significant when P < 0.05.
Results
Effect of flavonoids in TP-dependent calcium mobilization
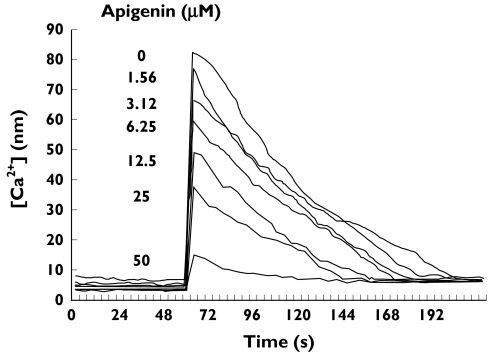
To investigate whether flavonoids interfere in platelet TxA2 signalling pathways, we first assessed their effect on calcium mobilization after selective stimulation of platelet TP. As illustrated in Figure 1, stimulation of Oregon Green-loaded platelets with 2 µm U46619, in the presence of 2 mm EGTA to prevent aggregation and the influx of extracellular calcium, resulted in a fast 10-fold increase in the intraplatelet calcium concentration. As shown, the flavone apigenin impaired this U46619-induced [Ca2+]i mobilization in a concentration-dependent manner. Under these experimental conditions, other tested flavonoids such as genistein, luteolin and quercetin also behaved as inhibitors of U46619-induced calcium flux, with concentrations between 10 and 30 µm displaying half-maximal impairment (Table 1). By comparison with these compounds, rutin, the glycosylated counterpart of quercetin, exhibited a negligible effect even at very high concentration, while SQ29548, a recognized TP antagonist, also inhibited U46619-induced [Ca2+]i (Table 1).
Figure 1.
Inhibition of [Ca2+]i mobilization by apigenin. A representative example of interference of [Ca2+]i mobilization by some flavonoids. Oregon Green BAPTA-1 AM-loaded platelets were stimulated with 2 µm U46619 in the absence or in the presence of increasing concentrations of apigenin and [Ca2+]i mobilization was recorded vs. time as stated in Materials and methods. The arrow indicates the addition of agonist
Table 1.
Dose-dependent inhibition of U46619-induced [Ca2+]i mobilization by flavonoids and by SQ29548
| Flavonoid | IC50 (µmol l−1) |
|---|---|
| Apigenin | 16.6 ± 6.1 |
| Genistein | 15.0 ± 6.1 |
| Luteolin | 10.5 ± 3.4 |
| Quercetin | 26.4 ± 2.2 |
| Rutin | >500 |
| SQ29548 | 0.007 ± 0.003 |
Oregon green BAPTA-1 AM-loaded platelets (200 ×109 l−1) were incubated with increasing concentrations of flavonoids or SQ29548, before U46619 (2 µm) induced [ Ca2+]i mobilization. Dose-dependent inhibition curves were analysed with Ultrafit software to calculate the concentration of flavonoid necessary to obtain half-maximal inhibition of [ Ca2+]i mobilization (IC50). Results are mean ± SD values from three experiments with different platelets.
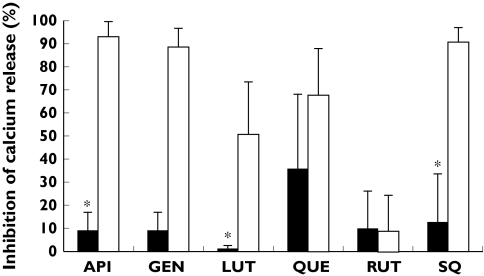
To analyse further the specificity of such inhibition, we investigated the effect of flavonoids on thrombin-induced [Ca2+]i mobilization in aspirin-treated platelets. Consistent with the previous data, when flavonoids were added at a concentration intended to inhibit the U46619-induced [Ca2+]i mobilization (all flavonoids except for rutin), a strong inhibition of U46619-induced [Ca2+]i mobilization was observed by all tested compounds but rutin, as well as by the TP antagonist SQ29548. However, these compounds caused a minor inhibitory effect on [Ca2+]i mobilization upon thrombin stimulation (Figure 2).
Figure 2.
Effect of flavonoids on U46619- and thrombin-induced [Ca2+]i mobilization. Oregon green BAPTA-1 AM-loaded platelets, or aspirin-treated platelets, were incubated with dimethylsulphoxide (DMSO), flavonoids (50 µm) or with the TP antagonist SQ29548 (1 µm) and stimulated with 2 µm U46619 or 0.5 U ml−1 thrombin. Data represent mean percentage of inhibition compared with mean peak calcium concentration with DMSO [n = 3; *indicates significant differences (P< 0.05) in [Ca2+]i mobilization upon activation with U46619 or thrombin]. Open bars represent U46619-activated platelets; black bars indicate thrombin-stimulation of aspirin-treated platelets. API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin; SQ, SQ29548
Effects of flavonoids on TP-dependent whole platelet tyrosine phosphorylation and on ERK 1/2 phosphorylation
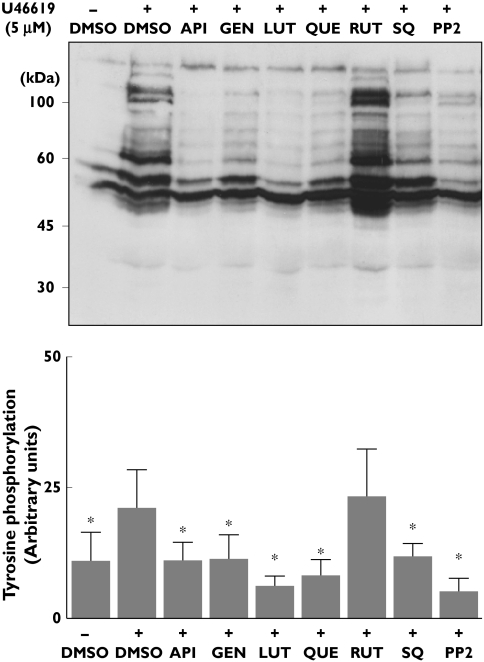
Since it is known that signalling through TP is accompanied by tyrosine phosphorylation of multiple proteins [21, 22], we investigated the effect of flavonoids on U46619-induced whole protein tyrosine phosphorylation. These experiments were carried out in platelets stimulated under non-aggregating conditions due to the inclusion of 1 mm EGTA, thus attempting the study of TP-dependent primary signalling events, rather than secondary events following secretion and aggregation. Because this experimental approach reduces levels of positive feedback signalling as a result of calcium chelation, a high concentration of U46619 (5 µm) was used in order to ensure a substantial increase in protein tyrosine phosphorylation. Consequently, in these assays the flavonoids were also tested at increased concentrations (4–10-fold; 100 µm) when compared with the IC50 values for platelet calcium mobilization (Table 1). As illustrated in Figure 3, we confirmed that U46619 stimulation increased platelet phosphotyrosine content in several proteins, the most notable with MW of 120–130 kDa, 100–105 kDa, 60–80 kDa and smaller proteins of 50 and 40 kDa. Figure 3 also shows that pretreatment of platelets with either apigenin, genistein, luteolin or quercetin (100 µm)) resulted in a sharp inhibition of U46619-induced increased level of protein tyrosine phosphorylation, which remained similar to levels found in nonstimulated platelets. In this respect, these flavonoids resembled the effect of the TP antagonist SQ29548 or that of the Src kinase inhibitor PP2. The glycosylated flavonoid rutin, which, as shown above, displayed negligible effect on [Ca2+]i mobilization, also caused no significant impairment in U46619-induced rise of phosphotyrosine content (Figure 3).
Figure 3.
Effect of flavonoids on U46619-induced tyrosine phosphorylation of platelet proteins. Washed platelets, in the presence of ethylene glycol tetraacetic acid (1 mm), were incubated with vehicle (dimethylsulphoxide), flavonoids (100 µm), the TP antagonist SQ29548 (10 µm) or with the Src kinase inhibitor PP2 (10 µm) and stimulated with 5 µm U46619 for 2 min. Platelets were then lysed and phosphotyrosine-containing proteins were identified by Western blot using PY20 antibody. A representative blot is shown in the upper panel. In each case, tyrosine phosphorylation was quantified by densitometry using Quantity One software. The lower panel shows comparative results of tyrosine phosphorylation achieved with platelets stimulated with U46619 in the absence or presence of flavonoids, SQ29548 or PP2. Data are mean ± SD from four separate experiments. API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin; SQ, SQ29548. *P < 0.05 compared with phosphotyrosine content in U46619-stimulated platelets in the absence of inhibitors
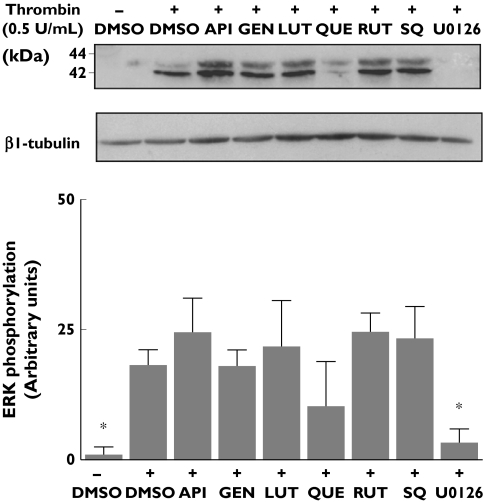
Phosphorylation of the extracellular signal-regulated kinase (ERK) isoforms of mitogen-activated protein (MAP) kinases has been previously implicated in cell signalling downstream TP in platelets and other cells [21, 35]. Consistently, we found that stimulation of platelets under our experimental conditions with U46619 (5 µm) resulted in marked phosphorylation of ERK 1/2, which was inhibited by the TP antagonist SQ29548 and by the specific MEK inhibitor U0126 (Figure 4). Platelet treatment either with apigenin, genistein, luteolin or quercetin also resulted in a sharp inhibition of U46619-induced phosphorylation of ERK 1/2. In contrast, treatment of platelets with rutin resulted in no change in the phospho-ERK content compared with that found in platelets stimulated in the absence of flavonoid (Figure 4).
Figure 4.
Inhibition of U46619-induced phosphorylation of ERK 1/2 by flavonoids. Washed platelets, containing ethylene glycol tetraacetic acid (1 mm), were incubated with vehicle [dimethylsulphoxide (DMSO)], flavonoids (100 µm), SQ29548 (10 µm) or the MEK inhibitor U0126 (10 µm), before activation with 5 µm U46619. Platelet lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and Western blotting with a specific phospho-p44/42 ERK monoclonal antibody. Equal protein loading was verified using antibody against βI tubulin. The upper plot is a representative example from six separate experiments with distinct platelet samples. The lower panel compares the densitometric quantification, using Quantity One software, of U46619-promoted ERK phosphorylation achieved in presence or absence of flavonoids, or U0126 (mean ± SD, n = 6). API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin; SQ, SQ29548. *P < 0.05 compared with ERK phosphorylation detected in stimulated platelets in the absence of inhibitors (+DMSO)
Effects of flavonoids on whole platelet tyrosine phosphorylation and on ERK 1/2 phosphorylation induced by thrombin
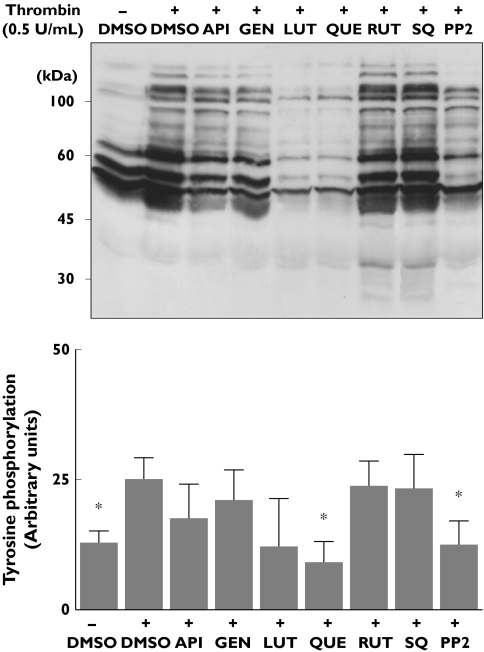
The above observations are consistent with the antagonism of TP receptors by some flavonoids. This may, however, also be explained by less selective inhibition of kinases, as has been reported in a number of studies, antioxidant potential or a combination of each of these. Experiments were therefore performed to assess whether the deleterious effect of apigenin, genistein, luteolin or quercetin, but not rutin, in the U46619-promoted increase in phosphotyrosine protein content and on ERK 1/2 phosphorylation was related to selective impairment of the TP signalling, or a consequence of other mechanisms. We examined the effect of these flavonoids on thrombin-induced phosphorylation events in aspirin-treated platelets. In these platelets thrombin elevated phosphotyrosine protein content, and this increase was significantly impaired by the Src kinase inhibitor PP2 (Figure 5). In contrast, the TP receptor antagonist SQ29548 had a negligible effect on this thrombin-induced response, indicating that it is a TxA2-independent process. Under these experimental conditions, quercetin and, to a lesser extent, luteolin behaved as efficient inhibitors of thrombin-induced whole protein tyrosine phosphorylation, while rutin, genistein and apigenin displayed a minor inhibitory trend (which did not reach significance, Figure 5).
Figure 5.
Effect of flavonoids on thrombin-induced protein tyrosine phosphorylation in aspirinized platelets. Washed platelets treated with 1 mm aspirin were incubated with flavonoids (100 µm), SQ29548 (10 µm) or with the Src kinase inhibitor PP2 (10 µm) and then stimulated for 2 min with thrombin (0.5 U ml−1). Phosphotyrosine proteins were identified by Western blot as described in Figure 3 and quantified by densitromety using Quantity One software. The upper plot is a representative blot and the lower panel shows comparative results of the densitometric analysis (mean ± SD, n = 4). API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin; SQ, SQ29548. *P < 0.05 compared with platelet phosphotyrosine content detected upon thrombin activation in the absence of inhibitors (+dimethylsulphoxide)
As can be seen from Figure 6, thrombin also promoted ERK 1/2 phosphorylation in aspirinized platelets, and this effect was sharply reduced by the specific MEK inhibitor U0126. In contrast, this thrombin activation response was not significantly impaired by either the TP antagonist SQ29548 or any of the tested flavonoids (Figure 6).
Figure 6.
Effect of flavonoids on thrombin-induced phosphorylation of ERK 1/2 in aspirinized platelets. In vitro aspirinized platelets were incubated with flavonoids (100 µm), SQ29548 (10 µm) or the MEK inhibitor U0126 (10 µm) and then stimulated for 2 min with thrombin (0.5 U ml−1). Phospho-p44/42 ERK in platelet lysates were identified by Western blot as described in Figure 4. The upper plot is a representative example of phosho-ERK detection and the lower panel shows comparative results of the densitometric quantification performed with Quantity One software (mean ± SD, n = 4). API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin; SQ, SQ29548. *P < 0.05 compared with ERK phosphorylation detected in the absence of inhibitors (+dimethylsulphoxide)
Effect of flavonoids on 3H-SQ29548 binding to platelets and other cells
The above findings are consistent with the hypothesis that certain flavonoids behave as selective TP antagonists. In order to further confirm the interaction of these compounds with TP, binding assays using 3H-SQ29548 as a TP tracer were carried out in washed platelets, in myometrium tissue and in a model of TPα- or TPβ-transfected HEK 293T cells. Under our experimental conditions, 3H-SQ29548 binding to these four types of cells was best fitted by Scatchard plots to a single class of binding sites, and binding parameters (Kd and Bmax values) were calculated with LIGAND software. Transfected HEK 293T cells displayed overexpression of 3H-SQ29548 binding sites with an affinity comparable to that of platelets and myometrium (Table 2). Non-specific binding of 3H-SQ29548 to nontransfected HEK 293T cells was negligible.
Table 2.
Parameters of binding of 3H-SQ29548 to platelets, human myometrium and HEK 293T cells
| Kd (nm) | Bmax | |
|---|---|---|
| Platelets | 8.7 ± 10.5 | 1240 ± 1268 |
| Myometrium | 32.7 ± 24.5 | 439 ± 189 |
| HEK 293T, TPα | 16.2 ± 4.7 | 95 1951 ± 42 2276 |
| HEK 293T, TPβ | 13.2 ± 1.3 | 37 7774 ± 19 4787 |
Washed platelets, human myometrium homogenates or HEK 293T cells were incubated as described in Materials and methods with3H-SQ29548 (5 nm) in the presence of increasing concentrations of SQ29548. Displacement curves of specific binding were analysed with LIGAND, to achieve the dissociation constant (Kd) and the maximum number of binding sites (Bmax; molecules/platelet; molecules/HEK 293T cell; fmol mg−1protein for myometrium). Results are mean ± SD values from three experiments with different cells or tissues.
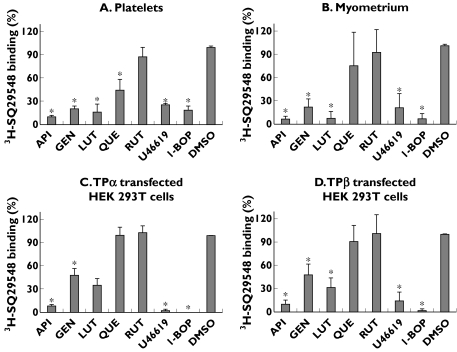
As illustrated in Figure 7, established TP ligands such as U46619 and I-BOP, but also high doses of apigenin, genistein and luteolin behaved as inhibitors of 3H-SQ29548 binding to the different cell types analysed. By comparison, quercetin reduced less effectively 3H-SQ29548 binding to TPα- and TPβ-transfected HEK 293T cells and to myometrium, than to platelets (Figure 7), and glycosylated rutin displayed negligible interference in 3H-SQ29548 binding to either cells.
Figure 7.
Effect of flavonoids on 3H-SQ29548 binding to (A) platelets, (B) myometrium, (C) TPα-transfected HEK 293T cells and (D) TPβ-transfected HEK 293T cells. Washed cells or tissue were incubated with 5 nm3H-SQ29548 in the absence or in the presence of U46619 (5 µm), I-BOP (5 µm) or flavonoids (100 µm) as competitors. Nonspecific binding was determined as the residual binding of 3H-SQ29548 in the presence of 10 µm SQ29548. Data represent percentage of 3H-SQ29548-specific binding, considering 100% of binding as that achieved in the absence of competitor [dimethylsulphoxide (DMSO)]. API, Apigenin; GEN, genistein; LUT, luteolin; QUE, quercetin; RUT, rutin. Data are mean ± SD from three different experiments. *P < 0.05 compared with binding in samples without competitor (+DMSO)
Discussion
Diet is recognized as one of the environmental factors that influences haemostasis and may alter the predisposition to thrombosis [36], and the dietary components flavonoids have been found in epidemiological studies to have beneficial effects on cardiovascular diseases [37]. To date, an increasing number of in vitro and ex vivo studies strongly suggest that flavonoids may exert this beneficial effect by modulating platelet hyperreactivity, which plays a major role in atherothrombosis [1]. Consequently, a major effort is focusing on unravelling the mechanisms underlying the effect of flavonoids on platelet function [7–12, 24–31].
In this investigation we have provided evidence that some flavonoids, namely apigenin, genistein, luteolin and quercetin, inhibit TP-mediated signalling events. First, we have shown that these flavonoids impair U46619-induced release of Ca2+ from intracellular stores in a dose-dependent manner, with IC50 values between 10 and 25 µm. These results are in close agreement with previous data from McNicol [30] also showing, under rather different experimental conditions, that genistein inhibits U46619-induced release of Ca2+, and with other reports that reveal the ability of quercetin to inhibit [Ca2+]i mobilization induced by chilling or collagen [11, 38]. In our study glycosylated rutin, up to 500 µm, displayed a negligible effect on U46619-induced [Ca2+]i release. This is in contrast to the reported impairment by rutin of collagen-induced [Ca2+]i mobilization [25] and may suggest a selective action of this flavonoid in collagen signalling. Some of these flavonoids might be modulating platelet Ca2+ levels through alternative mechanisms such as inhibition of protein kinases or blockage of calcium channels [11, 25, 39]. However, the minor effect of apigenin, genistein, luteolin and, to a lesser extent, quercetin on thrombin-induced [Ca2+]i mobilization in aspirin-treated platelets, together with our binding data, suggest that the effect of those flavonoids on U46619-induced [Ca2+]i mobilization may involve antagonism of TP.
Activation of numerous downstream kinases, including tyrosine and MAP kinases, is another well-established consequence of TxA2/TP ligation [21, 22], also confirmed in this study under conditions of calcium chelation to prevent feedback signalling events depending on aggregation and secretion. Apigenin, genistein, luteolin and quercetin, similarly to SQ29548, and in contrast to rutin, inhibited substantially U46619-induced increase in protein tyrosine phosphorylation. Some of these flavonoids, most notably genistein, have long been considered to act as nonselective inhibitors of tyrosine kinases [40]. However, this potential mode of action would be unlikely to account solely for the impairment of U46619-promoted increase in phosphotyrosine protein content by flavonoids seen in our study, since at the same concentration they were significantly less efficacious, with the exception of quercetin, in reducing thrombin-induced elevation in protein tyrosine phosphorylation in aspirin-treated platelets. Thus, our results further support the concept that isoflavone genistein is not a particularly good inhibitor of tyrosine kinases in human platelets following stimulation with thrombin [29, 30], and for this purpose the flavonol quercetin is a better choice.
Human platelets contain several MAP kinases, including ERK-1 (or p44-MAP) and ERK-2 (or p42-MAP), p38-MAP, and MEK 1 and MEK 2 kinases [41]. The ERKs are phosphorylated, and presumably activated, by various agonists such as thrombin, collagen and TxA2 analogues, but their role and relevance in platelet function remain unclear [35, 42–44]. Despite recent interest in the effect of flavonoids on the ERK pathway in several cell types and mainly in the cancer setting [4, 6], the effect of flavonoids on ERK 1/2 activation in human platelets has not been previously investigated.
This study further confirms ERK 1/2 activation in response to platelet stimulation with either U46619 or thrombin. Moreover, we have demonstrated that apigenin, genistein, luteolin and quercetin are efficient inhibitors of ERK 1/2 phosphorylation induced by TP agonists. This inhibitory effect could involve blockage of signalling downstream TP, since these flavonoids have a minor effect on TxA2-independent thrombin-induced ERK phosphorylation. Under our conditions, thrombin-induced ERK phosphorylation was sharply inhibited by the MEK inhibitor U0126, but not by the Src-inhibitor PP2 (data not shown), thus raising doubts on whether ERK phosphorylation proceeds in a Src kinase-dependent manner [43].
In addition to platelets, TP are abundantly expressed in other tissues, such as vascular and uterine smooth muscle, placental tissue, endothelium, epithelium, thymus, liver and small intestine, with TPα expression predominating over TPβ in most tissues [45]. In platelets, the above data and our recent work [31] strongly suggest that the inhibitory effect of certain flavonoids is related to their ability to antagonize TP. Flavonoids may use the same mechanism to modulate TP signalling and TxA2-mediated actions in other target tissues, such as contractile activity in the uterus [46–48]. In support of this hypothesis, we found that apigenin, genistein and luteolin, but not rutin, behave as TP antagonists, not only in platelets but also in human myometrium and in TPα- and TPβ-transfected HEK 293T cells. The fact that these compounds bind to both TPα- and TPβ-transfected HEK 293 T cells suggests that flavonoids might modulate cell-signalling events downstream of both TP isoforms.
In summary, we have provided evidence of impairment by certain flavonoids of platelet signalling events downstream of TP, which is likely to be related to their interference with TxA2–TP interaction. Remarkably, binding of certain flavonoids to TP also occurs in human myometrium and in transiently transfected TPα and TPβ HEK 293T cells. These findings raise the question of the role of dietary manipulation or pharmacological supplementation with flavonoids in situations such as the prevention of atherothrombosis, or for the prevention and/or treatment of hypertensive pregnancy complications due to overproduction of TxA2.
Acknowledgments
This study was supported in part by a research grant from the Ministerio de Educación, Ciencia y Tecnología (SAF 2004-07535). J.A.G. is a postdoctoral fellow of Ministerio de Educación y Ciencia (EX-2006-0429). L.N-N. is a fellow of Ministerio de Educación y Ciencia (BES-2005-7496). C.M. is a Ramón y Cajal investigator from the University of Murcia.
References
- 1.Nieswandt B, Aktas B, Moers A, Sach UJH. Platelets in aterothrombosis: lessons from mouse models. J Thromb Haemost. 2005;3:1725–36. doi: 10.1111/j.1538-7836.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- 2.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–38. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 4.Williams RJ, Spencer JPE, Rice-Evans C. Flavonoids: antioxidants or signaling molecules? Free Radic Biol Med. 2004;36:838–49. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar FH, Li Y. Cell signalling pathways altered by chemoprotective agents. Mut Res. 2004;555:53–64. doi: 10.1016/j.mrfmmm.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Pignatelli P, Pulcinelli FM, Celestini A, Lenti L, Ghiselli A, Gazzaniga PP, Violi F. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr. 2000;72:1150–5. doi: 10.1093/ajcn/72.5.1150. [DOI] [PubMed] [Google Scholar]
- 8.Freedman JE, Parker C, 3, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–8. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 9.Dutta-Roy AK. Dietary components and human platelet activity. Platelets. 2002;13:67–75. doi: 10.1080/09537100120111540. [DOI] [PubMed] [Google Scholar]
- 10.Bucki R, Pastore JJ, Giraud F, Sulpice JC, Janmey PA. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J Thromb Haemost. 2003;1:1820–8. doi: 10.1046/j.1538-7836.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard GP, Stevens JM, Cicmil M, Sage T, Jordan PA, Williams CM, Lovegrove JA, Gibbins JM. Quercetin inhibits collagen-stimulated platelet activation through inhibition of multiple components of the glycoprotein VI signaling pathway. J Thromb Haemost. 2003;1:1079–88. doi: 10.1046/j.1538-7836.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 12.Pearson DA, Hiolt RR, Rein D, Paglieroni T, Schmitz HH, Keen CL. Flavanols and platelet reactivity. Clin Dev Immunol. 2005;12:1–9. doi: 10.1080/10446670410001722140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arita H, Nakano T, Hanasaki K. Thromboxane A2: its generation and role in platelet activation. Prog Lipid Res. 1989;28:273–301. doi: 10.1016/0163-7827(89)90002-7. [DOI] [PubMed] [Google Scholar]
- 14.Patrono C, García Rodriguez LA, Landolfi R, Bigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–83. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 15.Szczeklik A, Musiat J, Undas A, Sanak M. Aspirin resistance. J Thromb Haemost. 2005;3:1655–62. doi: 10.1111/j.1538-7836.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- 16.Patrono C, Coller B, Fitzgerald GA, Hirsh J, Roth G. Platelet-active drugs: the relationship among dose, effectiveness, and side effect: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl.):234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 17.Dogne JM, Hanson J, Pratico D. Thromboxane, prostacyclin and isoprostanes: therapeutic target in atherogenesis. Trends Pharmacol Sci. 2005;26:639–44. doi: 10.1016/j.tips.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Naruyima S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–20. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 19.Raychowdhury MK, Yukawa M, Collins LJ, McGrail SH, Kent KC, Ware JA. Alternative splicing produces a divergent cytoplasmic tail in the human endothelial thromboxane A2 receptor. J Biol Chem. 1994;269:19256–61. [PubMed] [Google Scholar]
- 20.Habib A, FitzGerald GA, Maclouf J. Phosphorylation of the thromboxane receptor α, the predominant isoform expressed in human platelets. J Biol Chem. 1999;274:2645–51. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- 21.Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signalling through thromboxane A2 receptors. Cell Signal. 2004;16:521–33. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Boss CL, Richel DJ, Ritsema T, Peppelenbosch MP, Verteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Thomas DW, Mannon RB, Mannin PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landolfi R, Mowere RL, Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids. Structure–activity relations. Biochem Pharmacol. 1984;33:1525–30. doi: 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- 25.Sheu JR, Hsiao C, Chou PH, Shen MY, Chou DS. Mechanisms involved in the antiplatelet activity of rutin, a glycoside of the flavonol quercetin, in human platelets. J Agric Food Chem. 2004;52:4414–8. doi: 10.1021/jf040059f. [DOI] [PubMed] [Google Scholar]
- 26.Son DJ, Cho MR, Jin YR, Kim SY, Park YH, Lee SH, Akiba S, Sato T, Yun YP. Antiplatelet effect of green tea catechins: a possible mechanism through arachidonic acid pathway. Prostaglandins Leukot Essent Fatty Acids. 2004;71:25–31. doi: 10.1016/j.plefa.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Tzeng SH, Ko WC, Ko FN, Teng CM. Inhibition of platelet aggregation by some flavonoids. Thromb Res. 1991;64:91–100. doi: 10.1016/0049-3848(91)90208-e. [DOI] [PubMed] [Google Scholar]
- 28.Guglielmone HA, Agnese AM, Nunez Montoya SC, Cabrera JL. Inhibitory effects of sulphated flavonoids isolated from Flaveria bidentis on platelet aggregation. Thromb Res. 2005;115:495–502. doi: 10.1016/j.thromres.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima KT, Joite T, Nozawa Y. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol Pharmacol. 1991;39:475–80. [PubMed] [Google Scholar]
- 30.McNicol A. The effects of genistein on platelet function are due to thromboxane receptor antagonism rather than inhibition of tyrosine kinase. Prostaglandins Leukot Essent Fatty Acids. 1993;48:379–84. doi: 10.1016/0952-3278(93)90118-g. [DOI] [PubMed] [Google Scholar]
- 31.Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J Thromb Haemost. 2005;3:369–76. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- 32.Mustard JF, Perry DW, Ardlie NG, Packham MA. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972;22:192–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin K, Sadée W, Qullan JM. Rapid measurement of intracellular calcium using a fluorescence plate reader. Biotechniques. 1999;26:318–22. doi: 10.2144/99262rr02. [DOI] [PubMed] [Google Scholar]
- 34.Chiang N, Tai HH. The role of N-glycosylation of human thromboxane A2 receptor in ligand binding. Arch Biochem Biophys. 1998;352:207–13. doi: 10.1006/abbi.1998.0620. [DOI] [PubMed] [Google Scholar]
- 35.Roger S, Pawlowski M, Habib A, Jandrot-Perrus M, Rosa JP, Bryckaert M. Costimulation of the Gi-coupled ADP receptor and the Gq-coupled TXA2 receptor is required for ERK2 activation in collagen-induced platelet aggregation. FEBS Lett. 2004;556:227–35. doi: 10.1016/s0014-5793(03)01430-3. [DOI] [PubMed] [Google Scholar]
- 36.Allman-Farinelli MA, Dawson B. Diet and aging: bearing on thrombosis and hemostasis. Semin Thromb Hemost. 2005;31:111–7. doi: 10.1055/s-2005-863813. [DOI] [PubMed] [Google Scholar]
- 37.Art ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(Suppl):317S–25S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 38.Pastore JJ, Funaki M, Janmey PA, Bucki R. Flavonoid-mediated inhibition of actin polymerization in cold-activated platelets. Platelets. 2005;16:362–7. doi: 10.1080/09537100500124442. [DOI] [PubMed] [Google Scholar]
- 39.Dobrydneva Y, Williams RL, Morris GZ, Blackmore PF. Dietary phytoestrogens and their synthetic structural analogues as calcium channel blockers in human platelets. J Cardiovasc Pharmacol. 2002;40:399–410. doi: 10.1097/00005344-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 41.Börsch-Haubold AG, Ghomashchi F, Pasquet S, Goedert M, Cohen P, Gelb MH, Watson SP. Phosphorylation of cytosolic phospholipase A2 in platelets is mediated by multiple stress-activated protein kinase pathways. Eur J Biochem. 1999;265:195–203. doi: 10.1046/j.1432-1327.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- 42.McNicol A, Jackson ECG. Inhibition of MEK/ERK pathway has no effect on agonist-induced aggregation of human platelets. Biochem Pharmacol. 2003;65:1243–50. doi: 10.1016/s0006-2952(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 43.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelet. Blood. 2005;106:3410–4. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosado JA, Sage SO. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J Biol Chem. 2001;276:15659–65. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- 45.Miggin SM, Kinsella BT. Expression and tissue distribution of the mRNAs encoding the human thromboxane A2 receptor (TP) alpha and beta isoforms. Biochim Biophys Acta. 1998;1425:543–59. doi: 10.1016/s0304-4165(98)00109-3. [DOI] [PubMed] [Google Scholar]
- 46.Picherit C, Dalle M, Neliat G, Lebecque P, Davicco MJ, Barlet JP, Coxam V. Genistein and daidzein modulate in vitro rat uterine contratile activity. J Steroid Biochem Mol Biol. 2000;75:201–8. doi: 10.1016/s0960-0760(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 47.Mooro F, Asboth G, Lopez BA. Thromboxane receptor signalling in human myometrial cells. Prostaglandins Other Lipid Med. 2002;67:31–47. doi: 10.1016/s0090-6980(01)00169-1. [DOI] [PubMed] [Google Scholar]
- 48.Griffiths AL, Marshall KM, Senior J, Fleming C, Woodward DF. Effect of the oestrous cycle, preganancy, and uterine region on the responsiveness of the isolated mouse uterus to prostaglandin F2{alpha} and the thromboxane mimetic U46619. J Endocrinol. 2006;188:569–77. doi: 10.1677/joe.1.06466. [DOI] [PubMed] [Google Scholar]