Abstract
What is already known about this subject
Both oral clearance as well as delivery of pravastatin to its molecular targets in hepatoctyes are greatly influenced by the organic anion transporting polypeptide 1B1 (OATP1B1), encoded by SLCO1B1.
The role of genetic factors that determine the marked interindividual variability in lipid-lowering efficacy of pravastatin in Chinese patients is not known.
The present study was designed to evaluate the impact of a common functional genetic polymorphism in SLCO1B1 (521T→C: Val174Ala) on pravastatin efficacy in Chinese patients with coronary heart disease.
What this study adds
521T→C functional genetic polymorphism of SLCO1B1 is significantly associated with an attenuated total cholesterol-lowering efficacy of pravastatin in Chinese patients with coronary heart disease.
Aims
Pravastatin is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, which is widely used both in primary and secondary prevention of coronary heart disease (CHD). Pravastatin is not subject to metabolism by cytochrome P450s, but it is actively transported from blood into target tissues (e.g. hepatocytes in the liver) by the organic anion transporting polypeptide 1B1 (OATP1B1), encoded by SLCO1B1. The aim of the present study was to evaluate the impact of SLCO1B1 521T→C (Val174Ala) functional genetic polymorphism on the lipid-lowering efficacy of multiple-dose pravastatin in Chinese patients with CHD.
Methods
Forty-five hospitalized patients with CHD prospectively received pravastatin as a single-agent therapy (20 mg day−1 p.o.) for 30 days. Serum triglycerides, total cholesterol, low-density lipoprotein-cholesterol and high-density lipoprotein-cholesterol concentrations were determined before and after pravastatin treatment.
Results
Pravastatin treatment significantly decreased plasma lipids in all patients (P< 0.001). Importantly, we showed an attenuated pravastatin pharmacodynamic effect on total cholesterol in patients with 521TC heterozygote genotype (from 5.52 ± 0.51 mmol l−1 to 4.70 ± 0.35 mmol l−1, % change −14.5 ± 6.6%, N = 9) compared with 521TT homozygote genotype (from 5.47 ± 1.15 mmol l−1 to 4.21 ± 0.89 mmol l−1, % change −22.4 ± 10.3%, N = 36) (mean ± SD, P = 0.03, two-tailed test with α set at 5%). SLCO1B1 521T→C functional polymorphism did not significantly influence pravastatin pharmacodynamics on other plasma lipids (P > 0.05).
Conclusions
The 521T→C polymorphism of SLCO1B1 appears to modulate significantly the total cholesterol-lowering efficacy of pravastatin in Chinese patients with CHD. Further studies are warranted to determine the extent to which SLCO1B1 genetic variation may contribute to resistance to pravastatin in Asian patients treated with standard doses of pravastatin.
Keywords: coronary heart disease, functional polymorphism, interethnic variation, pharmacogenetic variation, pravastatin efficacy, SLCO1B1
Introduction
Pravastatin, one of the 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors (statins), is widely used in the treatment of hypercholesterolaemia. Pravastatin is prescribed both for the primary and secondary prevention of coronary heart disease (CHD) [1–3]. However, there is marked interindividual variability in therapeutic response to pravastatin. A deeper understanding of the mechanisms mediating attenuated response or treatment failure [4] associated with pravastatin therapy in different human populations is essential for individualization of preventive antilipidaemic interventions and reducing the global public health burden of CHD.
After an oral dose, pravastatin is rapidly absorbed from the small intestine and then taken up rapidly and selectively from the portal circulation into the liver through a sodium-independent bile acid transporter, the organic anion transporting polypeptide 1B1 (OATP1B1), encoded by SLCO1B1[5–7]. OATP1B1 plays an important role in the hepatic uptake of many endogenous and foreign chemicals as well as drugs such as pravastatin, rosuvastatin, pitavastatin, atorvastatin, atrasentan and repaglinide [8–12]. SLCO1B1 gene displays a number of single nucleotide polymorphisms (SNPs) that cause impaired transporter activity [13–16]. For example, clinical pharmacokinetic investigations in vivo have determined that SLCO1B1*15 haplotype is associated with a significant reduction in oral clearance of a single dose of pravastatin [13]. Consistent with this, SLCO1B1*5 (521T→C), *15 (388A→G and 521T→C) and *17 (−11187G→A, 388A→G and 521T→C) haplotypes result in increased systemic exposure of pravastatin [14–16]. Importantly, the 521T→C genetic variation is the predominant and shared key SNP in determining the functional impact of SLCO1B1*5, *15 and *17 haplotypes on OATP1B1 transporter function [13–16].
Because the uptake and delivery of pravastatin into hepatocytes by OATP1B1 is a crucial prerequisite step for its therapeutic efficacy, it is reasonable to postulate that interindividual differences in intracellular pravastatin exposure in hepatocytes can modulate, in part, its cholesterol-lowering efficacy. Therefore, subjects who carry the key functional 521T→C SNP (associated with impaired transporter activity) in SLCO1B1 may exhibit decreased intracellular pravastatin concentration and, by extension, an attenuated pharmacodynamic effect on cholesterol levels. The aim of the present study was to evaluate the role of SLCO1B1 521T→C genetic variation in relation to the lipid-lowering efficacy of multiple-dose pravastatin in Chinese patients with CHD.
Materials and methods
Subjects
The study was approved by the Research Ethics Committee of the Xiangya School of Medicine, Central South University. All subjects provided written, informed consent in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). A total of 45 stable CHD inpatients with or without hyperlipidaemia were enrolled (17 females, 28 males; age range 41–78 years). They had initial coronary angiograms and significant coronary stenosis (>40% luminal narrowing) as defined by coronary angiography. None of the subjects had taken HMG-CoA reductase inhibitors (statins) previously and all strictly abstained from smoking, alcohol and caffeine during treatment in hospital. Subjects with diabetes mellitus, liver or renal failure, dropsical nephritis, liver or kidney transplantation were excluded from participation in the study. To prevent the potential confounding effect of concomitant medications on plasma lipid concentrations or OATP1B1 transporter activity, patients who had taken any other lipid-lowering drugs, ciclosporin, rifampicin, methotrexate, fexofenadine, caspofungin, irinotecan or flavanoids in the previous 2 months were also excluded.
Study design
Investigators and participants were blind to genotype data during collection of the phenotypic data. Similarly, genotyping was performed blind to the phenotypic data. All subjects were asked to take low-fat diet and prospectively treated with an oral 20-mg daily dose of pravastatin at 21.00 h before bedtime for 30 consecutive days. Blood samples were collected at baseline before the first dose of pravastatin (study day 0) and on study day 30, prior to breakfast.
Genotyping for SLCO1B 1521T→C functional polymorphism
Genomic DNA was extracted by a standard manual chloroform–phenol extraction procedure. An amplification refractory mutation system (ARMS-PCR) was used for 521T→C genotype analysis as previously described [17].
Measurement of plasma lipid levels
Measurement of plasma lipids concentrations was performed in a hospital laboratory. Total and high-density lipoprotein (HDL)-cholesterol and triglyceride concentrations in the plasma were determined by use of standard, automated methods (Hitachi 747, Tokyo, Japan). The low-density lipoprotein (LDL)-cholesterol was calculated by the Friedewald formula [18].
Statistical analysis
Statistical analyses were performed with SPSS software (version 11.0 for Windows; SPSS Inc., Chicago, IL, USA). Hardy–Weinberg equilibrium was assessed with a χ2 test of goodness-of-fit in the study sample. Paired Student's t-test was performed for the lipid concentrations before and after pravastatin treatment. An unpaired Student's t-test was used to compare the differences in the degree of reduction in plasma concentrations between the two SLCO1B1 genotypic groups. A two-sided test with type error level (α) set at 5% was used in all statistical analyses. Data were presented as mean ± SD. A two-tailed P-value < 0.05 was considered to be statistically significant for all analyses.
Results
Pravastatin was well tolerated by all patients. No subject showed any elevation of aminotransferase or creatine phosphokinase after treatment, and no subjects reported skeletal muscle abnormalities or other notable safety concerns during the study. After SLCO1B1 521T→C genotyping, nine patients were identified with 521T→C heterozygote genotype with the remaining of the 36 subjects displaying the 521TT homozygous genotype. The allele frequency of the 521C allele was 10% in our sample of Chinese individuals. The genotype frequencies were in Hardy–Weinberg equilibrium. Baseline characteristics of the two SLCO1B1 genotypic panels are presented in Table 1.
Table 1.
Baseline characteristics of study subjects before pravastatin treatment
| Characteristic | SLCO1B1521TT group(n = 36) | SLCO1B1521TC group(n = 9) | P-value |
|---|---|---|---|
| Age (years) | 65.8 ± 11.2 | 66.3 ± 8.81 | 0.70 |
| Sex (M/F) | 22/14 | 6/3 | – |
| Body mass index (kg m−2) | 25.49 ± 3.45 | 24.78 ± 2.65 | 0.54 |
| Medication | |||
| Aspirin | 29 (81%) | 7 (78%) | 0.85 |
| β-blocker | 25 (69%) | 6 (67%) | 0.87 |
| Calcium antagonists | 17 (47%) | 5 (56%) | 0.66 |
| Angiotensin-converting enzyme inhibitors | 22 (61%) | 5 (56%) | 0.76 |
| Nitrates | 20 (56%) | 6 (67%) | 0.55 |
| Clopidogrel | 15 (42%) | 3 (33%) | 0.65 |
| Total cholesterol (mmol l−1) | 5.47 ± 1.15 | 5.52 ± 0.51 | 0.11 |
| LDL-cholesterol (mmol l−1) | 3.67 ± 0.91 | 3.63 ± 0.15 | 0.64 |
| HDL-cholesterol (mmol l−1) | 1.15 ± 0.37 | 1.01 ± 0.35 | 0.76 |
| Triglycerides (mmol l−1) | 1.96 ± 0.85 | 2.34 ± 1.66 | 0.12 |
Data are shown as mean±SD. LDL, Low-density lipoprotein; HDL, high-density lipoprotein.
After pravastatin treatment for 30 days, total cholesterol, LDL-cholesterol and triglyceride concentrations were decreased from baseline, on average, by 20.9 ± 10.1%, 27.5 ± 14.4% and 18.1 ± 22.6%, respectively (Figure 1) (P< 0.001). HDL-cholesterol was not significantly affected after pravastatin treatment (Figure 1).
Figure 1.
Total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C) and triglyceride (TG) concentrations (mmol l−1) at baseline (▪) and after treatment with 20 mg pravastatin daily for 30 days (□) in 45 patients with coronary heart disease. Data are shown as mean ± SE. ***P-value < 0.05
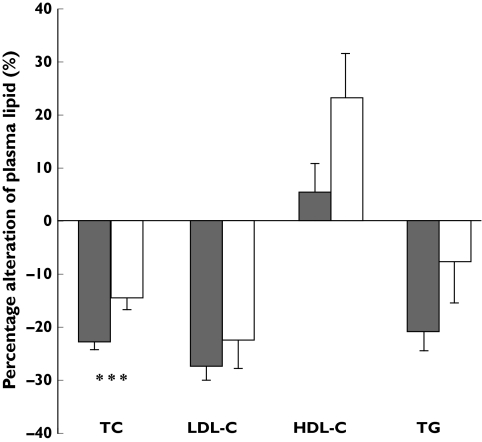
We found a significant influence of SLCO1B1 genotype on percentage reduction in total cholesterol from baseline after pravastatin treatment. The group of patients with a 521TC heterozygous genotype showed, on average, a 14.5 ± 6.6% (SD) reduction in total cholesterol, whereas patients with 521TT homozygous genotype displayed a 22.4 ± 10.3% reduction from baseline (P= 0.03) (Figure 2 and Table 2). On the other hand, the degree of change in plasma LDL-cholesterol, HDL-cholesterol and triglyceride concentrations did not show a significant difference between 521TT and 521TC genotypes (Figure 2 and Table 2).
Figure 2.
Comparison of percentage changes from baseline in total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C) and triglyceride (TG) between the SLCO1B1 reference genotype group (521TT, ▪) and those who carry the 521C allele (521TC genotype, □). Data are shown as mean ± SE. ***P-value < 0.05
Table 2.
Comparison of changes from baseline in total cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride between SLCO1B1 521TT and 521TC genotypic groups after treatment with 20 mg pravastatin daily for 30 days
| Genotype group (N) | Baseline (mmol l−1) | After pravastatin (mmol l−1) | Mean % change (95% CI) | P | |
|---|---|---|---|---|---|
| Total cholesterol | TT (36) | 5.47 ± 1.15 | 4.21 ± 0.89 | −22.4 (−25.9, −19.0) | 0.03 |
| TC (9) | 5.52 ± 0.51 | 4.70 ± 0.35 | −14.5 (−19.6, −9.4) | ||
| LDL-cholesterol | TT (36) | 3.67 ± 0.91 | 2.60 ± 0.74 | −27.5 (−31.9, −23.2) | 0.33 |
| TC (9) | 3.63 ± 1.05 | 2.72 ± 0.67 | −22.3 (−34.1, −10.6) | ||
| HDL-cholesterol | TT (36) | 1.15 ± 0.37 | 1.15 ± 0.37 | 5.3 (−6.3, 16.9) | 0.15 |
| TC (9) | 1.01 ± 0.35 | 1.19 ± 0.25 | 23.1 (3.8, 42.4) | ||
| Triglyceride | TT (36) | 1.96 ± 0.85 | 1.51 ± 0.66 | −20.7 (−28.2, −13.3) | 0.12 |
| TC (9) | 2.34 ± 1.66 | 1.98 ± 1.17 | −7.7 (−25.3, 9.8) |
N, Sample size in each genotypic group; 95% CI, 95% confidence interval. Data are shown as mean±SD. P-value, Significant difference of lipid-lowering effect (% change) of pravastatin in 521T→C variants by unpaired t-test. LDL, Low-density lipoprotein; HDL, high-density lipoprotein.
Discussion
Cholesterol-lowering therapy is a crucial step in the primary and secondary prevention of CHD and other cardiovascular diseases. Although pravastatin is a widely prescribed HMG-CoA reductase inhibitor for the treatment of hypercholesterolaemia, it displays marked interindividual variability in lipid-lowering efficacy [19]. The mechanism(s) of individual differences in pravastatin pharmacodynamics remains largely unknown.
The dosage of pravastatin used in clinical practice ranges from 10 mg day−1 to 80 mg day−1. Previous studies have indicated that 10 mg day−1 cannot attain sufficient lipid-lowering efficacy and is seldom used in lipid-lowering treatment. Meanwhile, there is little difference in the lipid response to pravastatin between daily doses of 20 mg and 40 mg [20, 21]. Moreover, it has been reported that pravastatin therapy for 4 weeks in patients with hyperlipidaemia is sufficient to decrease the total cholesterol level by 17–24% and LDL-cholesterol levels by 23–35% [22]. For the above reasons, in this study all patients had taken pravastatin 20 mg day−1 for 30 days.
Pravastatin is not subject to metabolism by cytochrome P450s. The disposition of pravastatin is significantly influenced by OATP1B1 expressed in the basolateral (sinusoidal) membrane of hepatocytes [23, 24]. After oral administration, pravastatin is actively transported by OATP1B1 from blood into hepatocytes and subsequently excreted into the bile as an unchanged compound. Hence, it is conceivable that genetic and/or environmental factors that impair pravastatin delivery to its intracellular drug targets (e.g. HMG-CoA reductase) in hepatocytes may explain treatment failure or partial response to pravastatin in some patients. Consistent with this postulation, we found in our study that the SLCO1B1 521T→C polymorphism carriers (521TC genotype) showed attenuated total cholesterol-lowering effect compared with those with the 521TT genotype. Presumably, this may be a consequence of a decreased intracellular concentration of pravastatin. However, the percentage reduction of LDL-cholesterol was not found significantly different between the groups. Because the subjects were recruited without knowing their genotypes, and after genotype assay, the TT homozygotes (n = 36) were four times more prevalent than the CT heterozygotes (n = 9). This affected sample power, which is only 80%, which presumably explains the significant positive result only with total cholesterol but not LDL-cholesterol.
The prospective design of the present study and exclusion of subjects with concomitant medications that may interfere with OATP1B1 activity reduced the risk for confounding by environmental factors and drug-drug interactions, which lend further support to the association observed with the functional SLCO1B1 polymorphism. This finding is also consistent with previous reports on pravastatin pharmacokinetics. For example, 521T→C variant, the predominant SNP shared by SLCO1B1*5 and SLCO1B1*15 haplotypes, was associated with an increased pravastatin plasma concentration [13–15]. This is probably due to impaired uptake of pravastatin into hepatocytes by OATP1B1 encoded by carriers of the 521C allele. In a previous study by our group, we have shown that the SLCO1B1 521C allele may result in increased plasma concentrations of another OATP1B1 substrate (antidiabetic agent nateglinide) in healthy Chinese volunteers [17].
In a multiple-dose clinical pharmacokinetic study of healthy subjects, there was a 110% increase in pravastatin AUC in subjects with the SLCO1B1 variant haplotype carrying the 521C allele [23]. Moreover, there was a nonsignificant finding for a SLCO1B1 genotype effect on the lipid-lowering efficacy of pravastatin (40 mg day−1) after 3 weeks: the average percentage decrease in total cholesterol concentration was 13.1 ± 9.1% and 19.1 ± 8.3% in the variant and control groups, respectively (P= 0.19). It is noteworthy that the attenuated lipid-lowering efficacy of pravastatin in the presence of 521C allele may be less discernible in healthy subjects compared with CHD patients who have high plasma cholesterol. SLCO1B1 genotype effect on plasma lipids may also require pravastatin treatment for >3 weeks. In the same study, treatment with pravastatin for 3 weeks was associated with a 27.7% decrease in LDL-cholesterol in volunteers with impaired OATP1B1 activity (carriers of 521C). The sample size was small in this study, but it indicates that SLCO1B1 polymorphism may not necessarily be the major determinant in nonresponse to pravastatin [23]. In a retrospective study conducted in another Asian population, 66 Japanese hypercholesterolaemia patients under treatment with OATP1B1 substrate drugs pravastatin, simvastatin or atorvastatin, SLCO1B1 521TT genotype was associated with a higher total cholesterol-lowering effect [25]. This is in accordance with the findings of the present prospective study in Chinese patients with CHD.
In summary, the 521T→C polymorphism of SLCO1B1 appears to modulate the total cholesterol-lowering efficacy of pravastatin treatment in Chinese CHD patients. We caution that the finding of an attenuated cholesterol-lowering efficacy in patients who carry the SLCO1B1 521C allele needs to be replicated in larger prospective cohorts of patients in China and other Asian populations. Moreover, it remains to be determined whether and to what extent SLCO1B1 521T→C polymorphism influences pravastatin efficacy on plasma lipids other than cholesterol during longer term (>1 month) treatment at different dose ranges. On the other hand, cardiovascular diseases continue to impose a large global public health burden. Genetic testing that is helpful in identifying subpopulations at risk for treatment failure to antilipidaemic agents may open an avenue for early therapeutic interventions in the form of genotype-guided individualized dosing of pravastatin or presumably, genotype-directed coadministration ofOATP1B1 inducers to augment pravastatin delivery to target tissues.
This work was supported by research grants from the National Natural Science Foundation of China (30528026, 30300428, 30672497, 30000211 and 30200346), and by the China Medical Board of New York (grants 99-697 and 01-755).
References
- 1.Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397–412. doi: 10.2165/00003088-200039060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Gotto AM. Lipid lowering, regression, and coronary events – a review of the interdisciplinary council on lipids and cardiovascular risk intervention, Seventh council meeting. Circulation. 1993;87:1781–91. doi: 10.1161/01.cir.92.3.646. [DOI] [PubMed] [Google Scholar]
- 4.Pazzucconi F, Dorigotti F, Gianfranceschi G, Campagnoli G, Sirtori M, Franceschini G, Sirtori CR. Therapy with HMG CoA reductase inhibitors: characteristics of the long-term permanence of hypocholesterolemic activity. Atherosclerosis. 1995;117:189–98. doi: 10.1016/0021-9150(95)05571-d. [DOI] [PubMed] [Google Scholar]
- 5.Hatanaka T, Honda S, Sasaki S, Katayama K, Koizumi T. Pharmacokinetic and pharmacodynamic evaluation for tissue-selective inhibition of cholesterol synthesis by pravastatin. J Pharmacokinet Biopharm. 1998;26:329–47. doi: 10.1023/a:1023237510458. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki M, Suzuki H, Hanano M, Tokui T, Komai T, Sugiyama Y. Na+-independent multispecific anion transporter mediates active transport of pravastatin into rat liver. Am J Physiol. 1993;264:G36–44. doi: 10.1152/ajpgi.1993.264.1.G36. [DOI] [PubMed] [Google Scholar]
- 7.Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, OATP2. Pharm Res. 1999;16:904–8. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- 8.Richard B. Kim, 3-Hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors (statins) and genetic variability (single nucleotide polymorphisms) in a hepatic drug uptake transporter: what's it all about? Clin Pharmacol Ther. 2004;75:381–5. doi: 10.1016/j.clpt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, Lim KS, Moon KH, Shin SG, Jang IJ. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–50. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Lau YY, Okochi H, Huang Y, Benet LZ. Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: application of in vitro and ex situ systems. J Pharmacol Exp Ther. 2006;316:762–71. doi: 10.1124/jpet.105.093088. [DOI] [PubMed] [Google Scholar]
- 11.Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M, Daly AK, Eichelbaum M, Kivisto KT, Neuvonen PJ. Polymorphic organic anion transporting polypeptide 1B1 is a major determinant of repaglinide pharmacokinetics. Clin Pharmacol Ther. 2005;77:468–78. doi: 10.1016/j.clpt.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Katz DA, Carr R, Grimm DR, Xiong H, Holley-Shanks R, Mueller T, Leake B, Wang Q, Han L, Wang PG, Edeki T, Sahelijo L, Doan T, Allen A, Spear BB, Kim RB. Organic anion transporting polypeptide 1B1 activity classified by SLCO1B1 genotype influences atrasentan pharmacokinetics. Clin Pharmacol Ther. 2006;79:186–96. doi: 10.1016/j.clpt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–75. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 14.Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, Takane H, Irie S, Kusuhara H, Urasaki Y, Urae A, Higuchi S, Otsubo K, Sugiyama Y. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–65. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 15.Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) *5 and *1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther. 2004;75:415–21. doi: 10.1016/j.clpt.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ, Eichelbaum M, Kivisto KT. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–40. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, He YJ, Han CT, Liu ZQ, Li Q, Fan L, Tan ZR, Zhang WX, Yu BN, Wang D, Hu DL, Zhou HH. Effect of SLCO1B1 genetic polymorphism on the pharmacokinetics of nateglinide. Br J Clin Pharmacol. 2006;62:567–72. doi: 10.1111/j.1365-2125.2006.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Pazzucconi F, Dorigotti F, Gianfranceschi G, Campagnoli G, Sirtori M, Franceschini G, Sirtori CR. Therapy with HMG CoA reductase inhibitors: characteristics of the long term permanence of hypocholesterolemic activity. Atherosclerosis. 1995;117:189–98. doi: 10.1016/0021-9150(95)05571-d. [DOI] [PubMed] [Google Scholar]
- 20.Jones PH, Farmer JA, Cressman MD, McKenney JM, Wright JT, Proctor JD, Berkson DM, Farnham DJ, Wolfson PM, Colfer HT, et al. Once-daily pravastatin in patients with primary hypercholesterolemia: a dose response study. Clin Cardiol. 1991;14:146–51. doi: 10.1002/clc.4960140211. [DOI] [PubMed] [Google Scholar]
- 21.Illingworth DR, Erkelens DW, Keller U, Thompson GR, Tikkanen MJ. Defined daily doses in relation to hypolipidaemic efficacy of lovastatin, pravastatin, and simvastatin. Lancet. 1994;343:1554–5. doi: 10.1016/s0140-6736(94)92945-9. [DOI] [PubMed] [Google Scholar]
- 22.Pan HY, DeVault AR, Swites BJ, Whigan D, Ivashkiv E, Willard DA, Brescia D. Pharmacokinetics and pharmacodynamics of pravastatin alone and with cholestyramine in hypercholesterolemia. Clin Pharmacol Ther. 1990;48:201–7. doi: 10.1038/clpt.1990.136. [DOI] [PubMed] [Google Scholar]
- 23.Igel M, Arnold KA, Niemi M, Hofmann U, Schwab M, Lutjohann D, von Bergmann K, Eichelbaum M, Kivisto KT. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther. 2006;79:419–26. doi: 10.1016/j.clpt.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–77. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, Tokunaga K, Kondo I, Sugiyama Y, Miki T. Effect of genetic polymorphism of OATP-C (SLCO1B1) on the lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokin. 2004;19:375–80. doi: 10.2133/dmpk.19.375. [DOI] [PubMed] [Google Scholar]