Abstract
Aims
The novel direct thrombin inhibitor (DTI), dabigatran etexilate (Boehringer Ingelheim Pharma GmbH & Co. KG), shows potential as an oral antithrombotic agent. Two double-blind, randomized trials were undertaken to investigate the pharmacokinetics (PK), pharmacodynamics (PD) and tolerability of orally administered dabigatran etexilate in healthy male subjects.
Methods
Dabigatran etexilate or placebo was administered orally at single doses of 10–400 mg (n = 40) or at multiple doses of 50–400 mg three times daily for 6 days (n = 40). Plasma and urine samples were collected over time to determine the PK profile of dabigatran. PD activity was assessed by its effects on blood coagulation parameters: activated partial thromboplastin time (aPTT), prothrombin time (PT), reported as international normalized ratio (INR), thrombin time (TT), and ecarin clotting time (ECT). All adverse events were recorded.
Results
Dabigatran etexilate was rapidly absorbed with peak plasma concentrations of dabigatran reached within 2 h of administration. This was followed by a rapid distribution/elimination phase and a terminal phase, with associated estimated half-lives of 8–10 h and 14–17 h with single and multiple dose administrations, respectively. Dabigatran exhibited linear PK characteristics with dose-proportional increases observed in maximum plasma concentration and area under the curve. Steady-state conditions were reached within 3 days with multiple dosing. The mean apparent volume of distribution during the terminal phase (Vz/F) of 1860 l (range 1430–2400 l) and the apparent total clearance after oral administration (CLtot/F) of 2031 ml min−1 (range 1480–2430), were dose independent. Time curves for aPTT, INR, TT and ECT paralleled plasma concentration–time curves with values increasing rapidly and in a dose-dependent manner. At the highest dose of 400 mg administered three times daily, maximum prolongations over baseline of 3.1 (aPTT), 3.5 (INR), 29 (TT) and 9.5-fold (ECT) were observed. Dabigatran underwent conjugation with glucuronic acid to form pharmacologically active conjugates that accounted for approximately 20% of total dabigatran in plasma. Overall, variability in PK parameters was low to moderate, with an average interindividual coefficient of variation (CV) of approximately 30% and variability in PD parameters was low, with CV < 10%. Of the four assays, TT and ECT exhibited the greatest sensitivity and precision within the anticipated therapeutic dose range. Bleeding events were few and were mild-to-moderate in intensity, occurring only in the higher, multiple dose groups.
Conclusions
These data suggest that dabigatran etexilate is a promising novel oral DTI with predictable PK and PD characteristics and good tolerability. Further investigation of dabigatran etexilate for the treatment and prophylaxis of patients with arterial and venous thromboembolic disorders, acute coronary syndromes and other medical conditions is warranted.
Keywords: activated partial thromboplastin time, dabigatran, dabigatran etexilate, direct thrombin inhibitor, ecarin clotting time, pharmacodynamics
Introduction
Numerous clinical studies have demonstrated the advantages of thrombin inhibition for the treatment of thromboembolic disorders [1]. Current anticoagulant therapies include heparins (unfractionated and low-molecular-weight heparins), the subcutaneously administered pentasaccharide, fondaparinux, and vitamin K antagonists, such as warfarin, for oral treatment [2]. Despite achieving significant reductions in the incidence of thromboembolic complications, current therapies for the prevention and treatment of venous thromboembolism (VTE) have a number of limitations. In particular, oral anticoagulants have been associated with unpredictable pharmacological profiles due to the potential for multiple drug and food interactions, thus necessitating regular coagulation monitoring [1]. Furthermore, only orally administered warfarin is suitable for long-term use [3].
The need for effective and well tolerated oral antithrombotic agents with more predictable pharmacological properties, combined with an increasing recognition that thrombin is a key enzyme in the blood coagulation cascade and in thrombogenesis, has led to the development of a new class of agents that directly target thrombin [1]. Unlike warfarin and heparin, direct thrombin inhibitors (DTIs) are able to inhibit both free and fibrin-bound thrombin, thus enabling more effective inhibition of coagulation [4]. Importantly, some agents in this drug class have been developed for oral administration [5, 6].
Dabigatran (BIBR 953 ZW) is a small molecule that specifically and reversibly inhibits thrombin and can be orally administered as the prodrug, dabigatran etexilate (BIBR 1048) which differs from dabigatran by an ethyl group at the carboxylic acid and a hexyloxycarbonyl side chain at the amidine. Preclinical pharmacokinetic (PK) studies have demonstrated that dabigatran etexilate can be metabolized efficiently to dabigatran in animal models and in vitro. Furthermore, dabigatran has shown excellent potential as an antithrombotic agent in these studies.
The single dose and multiple dose studies reported here were conducted in order to evaluate the PK and pharmacodynamic (PD) profile and tolerability of dabigatran etexilate in healthy human subjects.
Methods
Study subjects
Healthy male volunteers aged 18–45 years with Broca index values within ±20% of their ideal body weight were enrolled in the studies. Signed, written informed consent was obtained from all subjects and the studies were conducted according to the principles of the Declaration of Helsinki and in accordance with the principles of Good Clinical Practice and local guidelines. Both studies were approved by the ethics committee of the Landesaerztekammer Baden Wuerttemberg, Stuttgart, Germany.
Study design
Two double-blind, randomized, placebo-controlled studies were performed, one a single dose and the second a multiple dose escalating study. Although it is intended to market dabigatran etexilate in a capsule formulation, for the purpose of these studies, dabigatran etexilate was provided as a dry powder in a 100 ml bottle. As an acidic microenvironment has been shown to improve solubility and absorption of dabigatran etexilate, the powder was dissolved in 100 ml tartaric acid drinking solution that was provided in a separate bottle [7]. After dissolution of the powder, the solution was administered to the subject. An additional volume of tap water was used to rinse the bottle and this was also given to the subject resulting in a total volume of administration of 150 ml. The 400 mg dose was given after dissolution in 200 ml tartaric acid solution (provided in two 100 ml bottles). The bottles were each rinsed with 50 ml tap water, and the resulting total volume administered was 300 ml. Details of the two studies are outlined below.
Single dose study
Forty eligible healthy male subjects were enrolled into the single dose escalating, double-blind study. Subjects were randomized to receive a single oral dose of 10, 30, 100, 200 or 400 mg dabigatran etexilate or placebo at approximately 08.00 h following an overnight fast. Subjects were followed for 48 h. For each of the five dabigatran etexilate doses, six subjects were randomized to receive study drug and two to receive placebo. Standardized meals were given during the day at specified meal times (breakfast 09.00 h, lunch 12.00 h, dinner 18.00 h) and subjects were discharged from the study centre 2 h after breakfast (08.00 h) the following day.
Multiple dose study
In the multiple dose, double-blind study, 40 healthy male volunteers were randomized to receive 50, 100, 200 or 400 mg three times daily of dabigatran etexilate or placebo. The first dose was administered at approximately 07.30 h and subsequent dosing was at 8 h intervals (approximately 15.30 h and 23.30 h) for 6 days, and a single dose of study drug was administered on the morning of day 7. For each of the four dabigatran etexilate doses, eight subjects were randomized to receive study drug and two to receive placebo. Standardized meals were given over the 6 day study period at specified meal times (breakfast 08.30 h, lunch 12.00 h, dinner 18.00 h, evening snack 21.00 h). Subjects were discharged from the study centre following breakfast at 07.30 h on day 8 and were followed up for 10 days.
Pharmacokinetic analysis
Venous blood samples (approximately 9 ml) were collected and centrifuged immediately at 4000 g for 10 min. Plasma samples were acidified by the addition of 1 v : v 0.1 n HCl and were then frozen immediately at −18°C. Urine was homogenized and the total volume (or weight) recorded. Following acidification with HCl, a 2.0 ml fraction was frozen until analysis.
Single dose study
Blood samples were collected 0.5 h before drug administration and again at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 48 h post-administration. Urine samples were collected at 0.5 h before drug administration and again from 0 to 4, 4–8, 8–12 and 12–24 h post-administration.
Multiple dose study
Blood samples were taken 0.25 h before the first dose of study drug was administered; 0.5, 1, 1.5, 2, 4, 8 (prior to the second dose), 9.5, 16 (prior to the third dose), 17.5 and 24 h after the first drug administration on day 1; before the first drug administration on the mornings of day 3, day 4 and day 5; before, and 1.5 h after, each of the three drug administrations on day 6; before the final drug administration on day 7, and 0.5, 1, 1.5, 2, 4, 8, 24, 48 and 72 h following the final drug administration.
Plasma samples were collected and analyzed for quantitative determination of dabigatran (BIBR 953 ZW) concentrations using a fully validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The internal standard used was [13C6]-labelled BIBR 953 ZW. The linear calibration curves covered a range from 1–400 ng ml−1 BIBR 953 ZW (single-dose study, extended to 1–500 ng ml−1 for the multiple-dose study). The lower limit of quantification (LOQ) was 1.00 ng ml−1.
Plasma and urine samples were measured directly to determine the fraction of free, nonconjugated dabigatran and after complete alkaline cleavage of dabigatran conjugates for the quantification of free and bound dabigatran.
For the determination of free, nonconjugated dabigatran, 40 µl of internal standard solution ([13C6]-BIBR 953 ZW at 1000 ng ml−1) was added to a 100 µl sample aliquot, then mixed, centrifuged and 10 µl of the supernatant injected onto the high performance LC (HPLC)-MS/MS system. For the determination of total (i.e. free plus conjugated) dabigatran, 40 µl of internal standard solution ([13C6]-BIBR 953 ZW at 1000 ng ml−1) was added to a 100 µl sample aliquot, then mixed, alkalinized with 55 µl of 0.2 n NaOH and incubated at 37°C for 2 h. The incubation period with NaOH was determined to be sufficient to cleave all acylglucuronides. After centrifugation, 10 µl of the supernatant was injected onto the HPLC-MS/MS system. Analytes were extracted by on-line solid phase-extraction (column-switching) on a Bondapak C18 porasil B (20 × 2 mm, 37–75 µm) enrichment column. Chromatography was performed on a Purospher RP-18 E analytical column (60 × 2 mm, 5 µm) with a 20 × 2 mm guard column of the same material. Mobile phases were 0.01 m ammonium formate buffer pH 4.5 (A) and acetonitrile (B) using a gradient from 15 to 50% B at a flow rate of 0.3 ml min−1. Analytes were quantified on a Sciex API 365 triple quadrupole mass spectrometer operated in positive electrospray mode. Transitions from m/z = 472.0 to m/z = 288.9 and from m/z = 478.0 to m/z = 294.9 were recorded for dabigatran and the internal standard, respectively. In addition, the transition from m/z = 648.2 to m/z = 288.9 was monitored for the presence of conjugates.
For the quantification of free, nonconjugated BIBR 953 ZW in plasma, mean assay inaccuracies were between −3.8 and 4.6%. For the analysis of total BIBR 953 ZW in plasma following complete alkaline cleavage of conjugates, mean assay inaccuracies were between −3.5% and 1.1%, respectively.
Mean assay imprecision in the QC samples, presented as CV over the main study periods, was between 2.7 and 14.8% for free BIBR 953 ZW and 2.1–23.0% (including outliers) for total BIBR 953 ZW.
Pharmacokinetic parameters
Monitoring of plasma concentrations was performed in all subjects to assess drug exposure and to determine the PK profile of dabigatran in subjects receiving single dose and multiple dose dabigatran etexilate. All PK parameters were calculated using noncompartmental procedures using the TopFit programme [8].
In both studies, the maximum observed plasma concentration (Cmax, ng ml−1) and the time to first occurrence of Cmax (tmax, h) were determined from the plasma concentration–time curves. The terminal half-life (t1/2, h), apparent volume of distribution during the terminal phase (Vz/F, l) and apparent total clearance after oral administration (CLtot/F, ml min−1) were also calculated in both investigations. The total mean residence time (MRTtot, h) was also calculated. The area under the plasma concentration–time profiles over 12 h (AUC(0,12 h), ng ml−1 h), to infinity (AUC(0,∞)), and at steady-state (AUCss) were determined from the plasma concentration–time curves using the linear trapezoidal rule. In the multiple dose study, tmax at steady-state (tmax,ss, h) and mean residence time at steady-state (MRTss, h) were also assessed.
Pharmacodynamic analysis
The PD effects of dabigatran were assessed by measuring selected coagulation parameters using the clot-based assays described below:
aPTT assay
The aPTT assay targets the intrinsic pathway of the coagulation cascade, involving factors I, II, V, VIII, IX, X, XI and XII. A burst of thrombin is generated in plasma samples after recalcification in the presence of phospholipids and a contact activation trigger. In the presence of anticoagulants, the lag-time until thrombin generation and subsequent clot formation is measured.
PT assay
The PT assay measures clotting times in plasma samples after recalcification in the presence of an excess of tissue factor and procoagulant phospholipids. This represents the clotting time in the extrinsic pathway involving factors I, II, V, VII and X.
TT assay
A certain amount of thrombin is required over a finite period of time in order to convert the fibrinogen in a plasma sample into fibrin and facilitate clot formation. The time to clot formation will depend on the amount of thrombin in the sample and the concentration of thrombin inhibitors. The TT assay directly assesses the activity of thrombin in a plasma sample and provides a direct measure of the activity of DTIs.
ECT assay
The ECT assay uses the snake venom, ecarin, which converts prothrombin into the intermediate meizothrombin. DTIs are able to inhibit the thrombin-like activity of meizothrombin, thereby prolonging the formation of a clot in a plasma sample.
Values were reported as the ratio of aPTT, TT and ECT measured at specific time points following drug administration to the measured blood coagulation times at baseline (predose) and PT was reported as the international normalized ratio (INR).
aPTT, INR, TT and ECT values were determined in two central laboratories according to Good Laboratory Practice regulations. Blood samples were drawn in citrated tubes and plasma was separated. In one central laboratory, aPTT and INR were analyzed with an STA Compact coagulation analyzer (Roche Diagnostics, Basel, Switzerland), and, in another central laboratory, TT and ECT were measured using a Biomatic B10 coagulometer (Desaga, Wiesloch, Germany). For each parameter, maximum effect (Emax) was determined.
Assay precision and accuracy of blood coagulation measurements were monitored by prestudy validation and by analysis of QC samples during analysis. The assay accuracy and precision parameters were within the predefined acceptance limits.
PK/PD correlation
For PK/PD correlations, total dabigatran concentration in the plasma was quantified after complete alkaline cleavage of the glucuronide conjugates present in the samples. The conjugates of dabigatran were shown to exhibit direct thrombin inhibition of the same potency as nonconjugated dabigatran. Therefore, the concentration of dabigatran in the plasma measured after conjugate cleavage represents the total concentration of active thrombin inhibitor in the plasma.
At the given sampling time points, blood plasma concentrations and coagulation times were determined in parallel. The relationship between the PK profile of dabigatran and its PD effects was assessed by pooling all available day 1 data (separately for aPTT prolongation, INR, TT and ECT) from both studies, and plotting the pooled data against respective dabigatran plasma concentrations. Also, all available steady-state data >48 h (aPTT prolongation, INR, TT and ECT) from the multiple-dose study were plotted against respective dabigatran plasma concentrations. For correlation of dabigatran plasma concentrations and aPTT, the square root of the plasma concentration was used for linear regression analysis. Correlations between the PD parameters and plasma concentration were assessed by conducting linear least squares regression analysis using Microsoft® Excel®.
Safety assessments
All adverse events (AEs) that occurred during the course of the study were recorded. Where possible, information on the severity, time of onset and duration of AEs were recorded together with the investigators' assessment of their relationship to treatment. The incidence of AEs was tabulated by treatment using the World HealthOrganization system of body classification. Special attention was paid to the following AEs: nose or gum bleeding, haematuria or blood in the faeces. Routineclinical laboratory tests, haematology, clinical chemistry assessments and vital sign measurements were performed throughout the trials.
Statistical analysis
Drug plasma concentrations and PK parameters were analyzed descriptively in both trials.
The attainment of the steady state was investigated with a repeated-measures anova. The log-transformed trough concentrations of all time-points in the conjectured steady-state-interval were compared with each other. The corresponding confidence intervals (95%, two-sided) and P values, testing the hypothesis that the expected mean differences are equal to 0, were arranged in a table. Comparisons that revealed small P values (<0.05) were inspected to determine whether the differences between the respective time-points were relevant and could have resulted from not yet attaining steady-state.
Dose proportionality was explored using the power model. This was done with an overall regression model applied to the log-transformed data, i.e. a linear regression of the logarithm of the parameter on the logarithm of the dose. Based on the estimate for the slope parameter, a two-sided 95% confidence interval for the slope was computed. Dose proportionality would correspond to a value of 1 for the slope parameter beta in the power model.
Results
Baseline characteristics and laboratory variables
Forty healthy Caucasian male subjects were treated and completed each study, with no withdrawals due to treatment noncompliance or protocol violations. Only one subject in the 50 mg dabigatran etexilate group in the multiple dose study withdrew consent for personal reasons after receiving a total of 200 mg of study drug. The demographic and baseline characteristics of subjects in each dose group within the studies were comparable and no clinically important changes in routine laboratory values were reported (data not shown).
Pharmacokinetic parameters
Single dose study
Pharmacokinetic values after single dose treatment with dabigatran etexilate, at doses of 10–400 mg, are shown in Table 1. Following drug administration, rapid absorption occurred resulting in peak dabigatran plasma concentrations at a median tmax of 1.25–1.5 h. Mean Cmax increased in proportion with dose with mean values of 8, 22, 82, 161 and 344 ng ml−1, following oral dabigatran etexilate doses of 10, 30, 100, 200 and 400 mg, respectively. Cmax and AUC increased in proportion to dose indicating that dabigatran has a linear PK profile. Dose proportionality was explored by investigating a power model. The slope parameters for Cmax and AUC were 0.926 (95% CI 0.84, 1.01) and 0.936 (95% CI 0.85, 1.02), respectively, which indicated dose proportionality of both parameters in the range of 10–400 mg. Due to the limited availability of plasma concentration data after the administration of 10 or 30 mg of dabigatran etexilate, it was impossible to calculate the AUC(0,∞) for the lower doses. Therefore, AUC-derived PK parameters and t1/2 were not determined for dabigatran at these doses. After single dose oral administration of 100, 200 or 400 mg, the t1/2 of dabigatran was approximately 7–9 h. The mean apparent volume of distribution during the terminal phase, Vz/F, of 1860 l (range 1430–2400 l) and the apparent total clearance after oral administration, CLtot/F, 2031 ml min−1 (range 1480–2430 ml min−1), were dose independent. Dabigatran was eliminated mainly unchanged, and the cumulative urinary excretion of dabigatran accounted for less than 5% of the dose. Glucuronide conjugates of dabigatran in urine represented 0.4% of the dabigatran dose.
Table 1.
Arithmetic mean PK parameters of dabigatran after single dose oral administration (10–400 mg dabigatran etexilate) and after multiple dose administration (50–400 mg dabigatran etexilate three times daily)
| Dose of dabigatran etexilate (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Single dose study | Multiple dose study | ||||||||
| Parameter | 10 (n = 6) | 30 (n = 6) | 100 (n = 6) | 200 (n = 6) | 400 (n = 6) | 50 (n = 7–8) | 100 (n = 8) | 200 (n = 8) | 400 (n = 8) |
| Cmax (ng ml−1) | 8.3 | 21.5 | 82.2 | 161.0 | 344.0 | 42.6 | 128.0 | 199.0 | 303.0 |
| (CV%) | (30.1) | (42.7) | (30.0) | (28.1) | (39.4) | (42.1) | (46.2) | (15.6) | (29.6) |
| tmax* (h) | 1.25 | 1.25 | 1.50 | 1.50 | 1.50 | 1.25 | 1.50 | 1.50 | 1.25 |
| Cmax,ss (ng ml−1) | – | – | – | – | – | 64.3 | 191.0 | 359.0 | 697.0 |
| (CV%) | (34.6) | (20.5) | (14.6) | (33.0) | |||||
| Cmin,ss (ng ml−1) | – | – | – | – | – | 18.1 | 56.9 | 105.0 | 224.0 |
| (CV%) | (31.1) | (33.2) | (21.4) | (33.7) | |||||
| tmax,ss (h) | – | – | – | – | – | 1.5 | 1.5 | 1.5 | 1.5 |
| t1/2 (h) | – | – | 7.1 | 8.4 | 8.9 | 7.3 | 14.0 | 17.2 | 16.4 |
| (CV%) | (11.9) | (12.9) | (9.4) | (13.6) | (32.3) | (19.2) | (15.5) | ||
| AUC(0,∞) (ng ml−1 h) | – | – | 548 | 1110 | 2380 | – | – | – | – |
| (CV%) | (31.5) | (26.7) | (40.3) | ||||||
| AUC(0,8 h) (ng ml−1 h) | – | – | – | – | – | 165 | 552 | 821 | 1290 |
| (CV%) | (33.7) | (45.3) | (16.5) | (32.4) | |||||
| AUCss (ng ml−1 h) | – | – | – | – | – | 305 | 904 | 1620 | 3270 |
| (CV%) | (32.6) | (25.5) | (18.5) | (32.3) | |||||
| MRTtot (h) | – | – | 8.7 | 9.6 | 10.3 | – | – | – | – |
| (CV%) | (11.3) | (15.3) | (10.2) | ||||||
| MRTss (h) | – | – | – | – | – | 9.0 | 10.6 | 10.1 | 11.0 |
| (CV%) | (12.8) | (11.0) | (7.67) | (10.7) | |||||
| CLtot/F (ml min−1) | – | – | 2430 | 2390 | 2410 | 2260 | 1480 | 1590 | 1660 |
| (CV%) | (23.7) | (26.6) | (39.2) | (33.9) | (28.4) | (17.4) | (28.8) | ||
| Vz/F (l) | – | – | 1470 | 1720 | 1830 | 1430 | 1840 | 2390 | 2400 |
| (CV%) | (21.7) | (23.9) | (36.8) | (36.1) | (48.1) | (31.7) | (35.1) | ||
Median.
Alkaline cleavage of the glucuronic acid conjugate of dabigatran was only performed in samples from the 100, 200, and 400 mg dose groups because the low concentrations of glucuronides in the 10 and 30 mg samples were impossible to quantify. Areas under the dabigatran plasma concentration–time curves were calculated after cleavage of the glucuronic acid conjugates representing the total AUC of dabigatran in plasma. Additionally, the concentration of nonconjugated dabigatran in plasma was measured and its AUC calculated. The mean fraction of dabigatran conjugates of the total plasma dabigatran was deduced from the difference in total and free dabigatran AUC and was 10%, 19%, and 23% for the 100, 200 and 400 mg doses, respectively. The interindividual variability of the extent of conjugation was low as indicated by a CV of 8–10%. At the lower dose levels, no consistent difference was observed between free and conjugated dabigatran concentrations. Variability in PK parameters was low to moderate, with interindividual CVs of 27–43%.
Multiple dose study
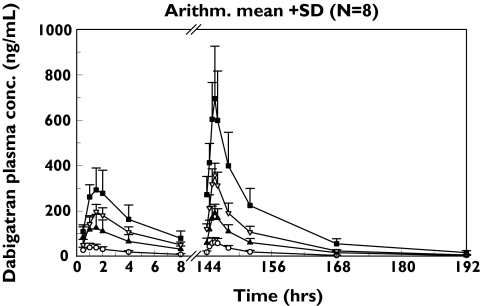
Pharmacokinetic values after multiple dosing with dabigatran etexilate doses of 50–400 mg three times daily are shown in Table 1. After oral administration of dabigatran etexilate in tartaric acid solution, rapid absorption was observed and maximum plasma concentrations were reached with a median tmax of 1.5 h (Figure 1) indicating rapid conversion of the prodrug to its active metabolite. Following day 1 administration of dabigatran etexilate at doses of 50, 100, 200 and 400 mg, mean Cmax values of 43, 128, 199 and 303 ng ml−1, respectively, were observed. At steady state, mean Cmax,ss were 64, 191, 359 and 697 ng ml−1, respectively, for the four doses, reflecting the accumulation of dabigatran in plasma with this three times daily regimen. The t1/2 of dabigatran was approximately 14–17 h and was not dose-dependent. The area under the plasma concentration–time curve associated with the terminal phase represented about 20% of AUC(0,∞) Following administration of multiple 50 mg doses of dabigatran etexilate, a shorter t1/2 of approximately 7.3 h was observed; the limited number of data points available following administration of lower doses of the drug prevented the assessment of the terminal phase and are likely to have resulted in an underestimation of the t1/2. Following three times daily dosing of 50–400 mg dabigatran etexilate in solution, the area under the plasma concentration–time curve at steady state for one dosing interval (8 h, AUCss) and maximum plasma concentrations at steady-state (Cmax,ss) increased in a dose-proportional manner (Table 1). The slope parameters for Cmax,ss and AUCss were 1.00 (95% CI 0.84, 1.15) and 0.99 (95% CI 0.84, 1.15), respectively, which indicated dose proportionality of both parameters in the dose range of 50–400 mg. The attainment of the steady-state was investigated by paired comparisons of the log transformed trough concentrations. P values, testing the hypothesis that the expected mean differences of trough concentrations equals 0, at 48 h were 0.298, 0.168, 0.215 and 0.073 for doses of 50, 100, 200 and 400 mg, respectively, indicating that steady-state was reached on day 3 with three times daily administration. Multiple administration of dabigatran etexilate at 8 h dosing intervals resulted in the accumulation of dabigatran in plasma. The mean Cmax,ss was 359 ng ml−1 and the trough plasma concentration at the end of the 8 h dosing interval (Cmin,ss) was 105 ng ml−1 (Table 1). The AUC after the first dose (AUC(0,8 h)) values were 165, 552, 821 and 1290 ng ml−1 h, respectively, after the administration of 50, 100, 200 and 400 mg three times daily dabigatran etexilate doses. At steady state, the AUCss (day 7) was 305, 904, 1620 and 3270 ng ml−1 h (Table 1). Accumulation ratios at steady state (AUC(0,8 h)ss/AUC(0,8 h)) were 1.8, 1.6, 2.0 and 2.5, respectively, for the four dose groups. As in the single rising-dose study, alkaline cleavage of the glucuronic acid conjugate of dabigatran was performed only in samples from the 100, 200, and 400 mg dose groups as the level of glucuronidation was found to be low in the 50 mg samples. Variations in PK parameters were low to moderate for each dose, with interindividual CVs of 16–46%.
Figure 1.
Arithmetic mean plot (+SD) of dabigatran plasma concentration vs. time following three times daily oral administration of 50 mg (○), 100 mg (▴), 200 mg (▿) or 400 mg (▪) dabigatran etexilate solution for 7 days. (Note n = 7 for the 50 mg dose group as one subject withdrew consent)
Pharmacodynamic parameters
Single dose study
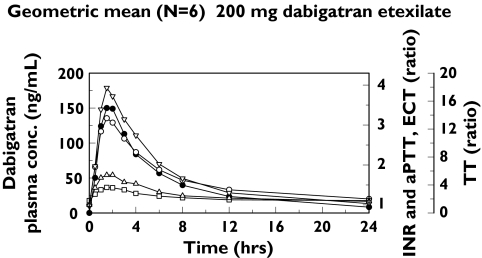
The inhibition of thrombin was determined by assessing prolongation of aPTT, INR, TT and ECT after single dose administration of dabigatran etexilate (Table 2). There were dose-dependent increases in mean aPTT prolongation, INR, TT and ECT values, and a close correlation between prolongation of blood coagulation assays and dabigatran plasma concentrations. Variability in the coagulation parameters was low, with interindividual CV usually below 20%; in the 400 mg dose group, coagulation parameters were more variable, but the CV was still below 30%. The time curves for aPTT, INR, TT and ECT values paralleled the plasma concentration–time curve of dabigatran (Figure 2). The maximum effect (Emax) of dabigatran on clotting parameters occurred at the same time as Cmax, indicating that thrombin inhibition by dabigatran is a direct effect on thrombin located in plasma. The parallel slopes of blood coagulation prolongation and the corresponding plasma concentration-time curves demonstrated that the PD effects of dabigatran have a rapid onset of action without a time delay, as indicated by the absence of a counter-clockwise hysteresis loop (data not shown).
Table 2.
Summary of PD effect (Emax) of dabigatran on aPTT prolongation, INR, TT and ECT in the single- and multiple-dose studies
| aPTT Mean, range | INR Mean, range | TT Mean, range | ECT Mean, range | |||||
|---|---|---|---|---|---|---|---|---|
| Dabigatran etexilate dose (mg) | Emax | CV% | Emax | CV% | Emax | CV% | Emax | CV% |
| 1.25 | 1.19 | |||||||
| Single-dose study | 1.18–1.39 | 7.1% | 1.14–1.25 | 4.1 | ||||
| 10 | 1.16 | 5.5% | 1.14 | 6.8% | 2.77 | 53.9% | 1.39 | 12.8% |
| 1.10–1.25 | 1.09–1.29 | 1.63–5.62 | 1.21–1.68 | |||||
| 30 | 1.25 | 9.2% | 1.22 | 4.5% | 10.5 | 19.9% | 2.42 | 14.0% |
| 1.15–1.46 | 1.17–1.32 | 7.94–13.7 | 1.98–2.94 | |||||
| 100 | 1.58 | 4.6% | 1.30 | 8.9% | 19.3 | 41.5% | 3.28 | 19.8% |
| 1.48–1.69 | 1.11–1.39 | 13.1–35.1 | 2.69–4.49 | |||||
| 200 | 1.81 | 7.3% | 1.47 | 7.6% | 56.5 | 29.1% | 5.30 | 26.1% |
| 1.67–2.03 | 1.31–1.63 | 23.0–64.5 | 3.65–6.87 | |||||
| 400 | 2.08 | 12.1% | 1.88 | 13.4% | ||||
| 1.79–2.41 | 1.61–2.18 | Emax,ss | CV% | Emax,ss | CV% | |||
| Emax,ss | CV% | Emax,ss | CV% | Emax,ss | CV% | Emax,ss | CV% | |
| Multiple-dose study | ||||||||
| 50 | 1.46 | 6.1% | 1.31 | 4.1% | 7.81 | 29.3% | 2.16 | 14.4% |
| 1.40–1.61 | 1.23–1.40 | 4.96–10.3 | 1.80–2.56 | |||||
| 100 | 1.92 | 7.0% | 1.73 | 5.5% | 13.9 | 15.9% | 3.75 | 14.9% |
| 1.80–2.11 | 1.59–1.92 | 10.3–17.4 | 3.05–4.37 | |||||
| 200 | 2.34 | 8.2% | 1.78 | 9.7% | 26.9 | 7.6% | 5.20 | 9.6% |
| 2.00–2.57 | 1.46–2.00 | 23.6–30.1 | 4.49–6.27 | |||||
| 400 | 3.12 | 10.8% | 3.49 | 31.7% | 29.1 | 13.4% | 9.49 | 14.5% |
| 2.77–3.72 | 2.34–5.50 | 24.0–35.9 | 7.87–11.9 | |||||
Figure 2.
Geometric mean plots of aPTT prolongation, INR, TT and ECT together with plasma concentrations of dabigatran vs. time, following a single oral dose of 200 mg dabigatran etexilate in the single dose study. Dabigatran plasma conc. (•) INR (□) ECT ratio (○) aPTT ration (▵) TT ratio (▿)
Multiple dose study
As was observed following single dose administration, aPTT, INR, TT and ECT profiles with three times daily administration closely followed the corresponding plasma concentration–time profiles of dabigatran and increased with increasing doses of dabigatran etexilate. Maximum values were achieved within 2 h of administration, suggesting a rapid onset of action (not shown). Steady-state Emax values of blood coagulation parameters are shown in Table 2.
The effect of dabigatran on the prolongation of blood coagulation was detectable at the end of the 8 h dosing interval except for aPTT and INR at the lowest dose. Mean trough aPTT prolongations at steady state, measured immediately before the administration of the morning dose, increased with dabigatran etexilate doses. At the highest dose of dabigatran etexilate (400 mg three times daily), a 2.5-fold prolongation in aPTT at trough was observed.
Pharmacodynamic effects of dabigatran declined in parallel with declining plasma concentrations of the drug, such that a rapid initial decrease of anticoagulant effects was observed 4–6 h after peak effects followed by a slow terminal phase. Twelve hours after administration, the prolongation of blood coagulation returned to about 50% of the maximum effect. Dabigatran exhibits a t1/2 of 14–17 h. Low plasma concentrations and small residual PD effects were observed 24 h after the final dose administration.
PK/PD correlation
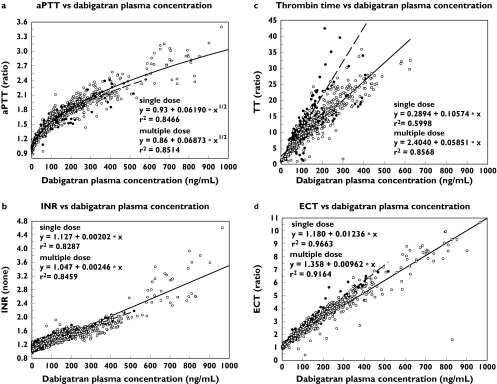
PK/PD analyses suggested notable differences between PD assays in terms of sensitivity and precision at varying dabigatran plasma concentrations (Figure 3). A curvilinear relationship was shown between drug plasma concentrations and aPTT. The data suggest a linear relationship between plasma concentration and INR. The TT assay exhibited a linear relationship with plasma concentration with a high level of sensitivity. The ECT assay displayed a linear relationship with drug plasma concentrations in the clinically relevant drug concentration range and exhibited adequate sensitivity and precision.
Figure 3.
PK/PD correlation for aPTT prolongation (a), INR (b), TT (c) and ECT (d). Day 1 data were pooled from the single and multiple dose studies; steady-state data are from the multiple dose study. Individual single dose (•) Individual data multiple dose (○) Correlation single dose (––) Correlation multiple dose (—)
Safety assessments
Single dose study
Dabigatran was well tolerated after administration of single oral doses of 10–400 mg dabigatran etexilate. Seven subjects reported an AE, all of mild to moderate severity; the onset of AEs occurred before treatment in one subject and during treatment in six subjects, one of whom received placebo. The only bleeding event, haematoma at a venepuncture site, occurred approximately 30 min before treatment and did not increase in size following treatment. The most frequently reported AE was headache, but in no case was this considered related to treatment with the study drug. There were no deaths, no serious AEs, and no AEs leading to withdrawal during the study. Laboratory variables were out of the normal range in only a few cases and these were without clinical relevance. There was no hepatotoxicity identified in any of the subjects treated with dabigatran etexilate.
Multiple dose study
Dabigatran was well tolerated after oral three times daily administration for 6 days at doses up to 200 mg dabigatran etexilate, representing a total daily dose of 600 mg. Fourteen subjects reported at least one AE; all AEs were mild-to-moderate in severity, except for one case of severe headache of 10 h duration. The onset of AEs occurred during treatment in 13 subjects and during or post-treatment in three subjects. Eight subjects who received dabigatran etexilate (two in the 200 mg group and six in the 400 mg group) experienced bleeding events, which included haematoma (n = 5; one at venepuncture site), gingival bleeding (n = 4) and prolonged bleeding at the venepuncture site (n = 4). All bleeding events occurred between day 1 and day 9 and were mild-to-moderate in intensity. All were considered treatment-related, due to the mechanism of action of dabigatran and the study procedure. One subject who received placebo developed a haematoma at a venepuncture site, which was also classified as treatment-related. The most commonly reported nonbleeding AE was headache (n = 5). In addition, gastrointestinal disorders were reported in three subjects, and herpes simplex infection, weakness plus dizziness, polyuria, and pain were each reported in one subject. However, none of these AEs was considered treatment-related. There were no deaths and no serious or otherwise significant AEs during the course of the study. Laboratory variables were out of the normal range in only a few cases, and these were without clinical relevance. Vital signs and other physical assessments reflected individual variations, without indication of any treatment effect.
Discussion
Most anticoagulants used for the prevention and treatment of thromboembolism have a narrow therapeutic window, necessitating careful monitoring; furthermore, dosing can be problematic because of potential multiple food and drug interactions [9]. These limitations highlight the need for oral therapies with more predictable effects. The studies presented here were designed to investigate the PK, PD and tolerability profiles of single and multiple rising doses of the novel oral DTI, dabigatran etexilate.
Dabigatran exhibited a favourable PK profile in healthy volunteers, with rapid absorption following oral administration (Cmax reached within 2 h), a bi-exponential distribution phase and a t1/2 of 14–17 h after multiple dosing. Dose-proportionality was observed, with Cmax and AUC increasing linearly with dose; steady-state conditions were reached within 3 days with three times daily administration. Importantly, variations in plasma concentrations and PK parameters were low to moderate (overall interindividual CV: ∼30%), indicating PK predictability.
Due to rapid initial distribution and elimination, dabigatran plasma concentrations declined to <25% of Cmax within 12 h of single dosing, and to ∼30% of Cmax 8 h after attaining steady-state. Since the PD effects of dabigatran have been shown to decline in parallel with plasma concentration, these data suggest that, in patients requiring surgery, a wait of only ∼12 h would be required following administration of the last dabigatran etexilate dose. Slow terminal elimination maintained low plasma concentrations for 24 h following dosing, indicating the potential for dabigatran to maintain therapeutic concentrations throughout a 24 h period, whilst retaining sufficient reversibility.
Elimination of dabigatran occurs predominantly (up to 80%) via the renal pathway. In the single-dose study, urinary excretion accounted for <5% of dose during the 24 h following administration, indicating the low oral bioavailability of dabigatran etexilate (∼6.5%). Renal clearance of dabigatran amounted to 50–100 ml min−1. The absorbed prodrug is rapidly and completely converted to active dabigatran in the plasma, resulting in effective and rapid anticoagulation.
Dabigatran undergoes conjugation with glucuronic acid to form acylglucuronides. Administration of ≥100 mg dabigatran etexilate resulted in measurable concentrations of plasma acylglucuronides, accounting for 10–23% and 15–24% of total plasma dabigatran after single and multiple dose administration, respectively. These conjugates were shown to be pharmacologically active, demonstrating almost identical PD properties to free, nonconjugated dabigatran.
The single dose study demonstrated that aPTT was prolonged, and INR, TT and ECT increased with single rising doses and these effects were still present ≥8 h post-administration for doses >50 mg. The effect-time curve for all parameters paralleled the plasma concentration–time profile of dabigatran, with no lag time between peak plasma concentrations and maximum effect, suggesting a direct effect in the central compartment, consistent with the localization of thrombin in plasma. Maximum effects were observed within 2 h of initial administration, indicating a rapid onset of action.
In the multiple dose study, parallel increases in blood coagulation times and dabigatran plasma concentrations were also observed at steady state. Peak concentrations and maximum PD effects were attained approximately 2 h after dosing, followed by a rapid distribution and elimination phase. On average, aPTT prolongation declined to ∼46% of maximum within 8 h of dosing.
Twenty-four hours after cessation of drug administration, an aPTT prolongation of 14–20% of maximum was observed for the 100–400 mg doses; with the 50 mg dose, no further aPTT prolongation was seen. ECT prolongation declined to ∼27% of maximum after 8 h, and to <10% of maximum after 24 h. The small residual effects observable ≥24 h after cessation of treatment, in conjunction with the initial rapid decline in prolongation of blood coagulation, suggest the safety of this agent should bleeding occur in the absence of an available antidote or reversal agent.
In both studies, aPTT, INR, TT and ECT increased with dose. Mean trough and maximum aPTT values, for example, increased two- and three-fold, respectively, with 400 mg three times daily administration in the multiple dose study. aPTT prolongations of this magnitude have been shown to exceed the aPTT effect required for effective prevention of DVT, as demonstrated in the BISTRO II dose-range finding trial [10].
aPTT has long been used to monitor the anticoagulant effects of heparin [11] and has also been employed with DTIs, such as hirudin [12], argatroban [13] and melagatran [14]. Therapeutic ranges for aPTT have been established empirically for heparin in various indications [15]; however, appropriate aPTT ranges for dabigatran must be determined in further randomized controlled clinical trials.
INR, TT and ECT increased in direct proportion to dabigatran plasma concentration, whereas aPTT prolongation was linearly related to the square root of the plasma concentration. Similar curvilinear aPTT relationships have been reported for other DTIs [16–18], suggesting that although aPTT may provide a qualitative indication of anticoagulant activity, it may not be suitable for precise quantification of effect [18]. Problems with nonlinearity are exacerbated by lack of standardization for aPTT assays [19, 20]. INR response to dabigatran was linear, but the sensitivity of this assay was low. Therefore, INR is not considered a suitable tool for monitoring the anticoagulant effects of DTIs. Although the TT assay provided a linear response, it is not readily available in clinical practice and, as with aPTT, lacks standardization. In addition, TT may be too sensitive for the clinically relevant plasma concentration range of dabigatran. There were marked discrepancies between the single-dose and multiple-dose TT data and considerable variability. Such variability was not observed in later studies which showed a much smaller imprecision comparable to that observed with ECT. Therefore, the discrepancy between the single-dose and multiple-dose–response and the imprecision of the TT response might possibly be due to poor sample handling and assay performance. TT might be too sensitive to dabigatran plasma concentrations in the clinically relevant plasma concentration range. At dabigatran concentrations greater than 600 ng ml−1, the thrombin clotting time frequently exceeded the maximum measurement time of the coagulometer. The ECT response was linear over the full range of concentrations, and was more sensitive than, for example, aPTT, as evidenced by an 8-fold maximum ECT prolongation, compared with a 2.5-fold maximum aPTT prolongation with multiple 400 mg doses. ECT is also a more precise parameter than aPTT, as indicated by the smaller scatter of data in Figure 3. Overall, these findings suggest that ECT may provide a more accurate measurement of DTI anticoagulation than the other parameters, in agreement with previous findings [17, 19].
There was no evidence of dose-dependent AEs, with the exception of bleeding events in the multiple-dose study. These episodes were mild-to-moderate in intensity and increased with dose, showing that the drug exhibited its known pharmacological activity in humans [21]. Based on these data, 200 mg three times daily was considered to be the maximum tolerable dose in this group of healthy subjects. The dabigatran plasma concentrations and the prolongation of blood coagulation parameters attained with 200 mg three times daily dose were beyond those required for effective and safe thromboprophylaxis [10]. Thus, this study established a maximum tolerable multiple dose for future studies in healthy volunteers, while the low incidence of AEs observed, even with higher dose three times daily regimens, indicates a favourable safety profile.
By displaying rapid conversion to its active form, predictable and reproducible anticoagulant effects, and complete and effective inhibition of thrombin, dabigatran etexilate exhibits many features consistent with the profile of an ideal anticoagulant [1, 9]. Therefore, dabigatran etexilate has the potential to offer physicians and patients the convenience of a simple, oral dosing regimen, without the burden of coagulation monitoring.
In summary, dabigatran etexilate is a small molecule that specifically and reversibly inhibits thrombin. It is a promising novel, oral DTI with predictable PK characteristics.
The authors gratefully acknowledge the expert technical assistance of Ms Karin Hörmann and Isolde Holzschuh.
References
- 1.Haas S. Oral direct thrombin inhibition: an effective and novel approach for venous thromboembolism. Drugs. 2004;64(Suppl 1):7–16. doi: 10.2165/00003495-200464001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hyers TM. Management of venous thromboembolism: past, present and future. Arch Intern Med. 2003;163:759–68. doi: 10.1001/archinte.163.7.759. [DOI] [PubMed] [Google Scholar]
- 3.Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Sixth ACCP Consensus Conference on Antithrombotic therapy. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(Suppl):8S–21S. doi: 10.1378/chest.119.1_suppl.8s. [DOI] [PubMed] [Google Scholar]
- 4.Nutescu EA, Helgason CM, Briller J, Schwertz DW. New blood thinner offers first potential alternative in 50 years: ximelagatran. J Cardiovasc Nurs. 2004;19:374–83. doi: 10.1097/00005082-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G. Clinical potential of oral direct thrombin inhibitors in the prevention and treatment of venous thromboembolism. Drugs. 2004;64(Suppl 1):47–52. doi: 10.2165/00003495-200464001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Francis CW, Davidson BL, Berkowitz SD, Lotke PA, Ginsberg JS, Lieberman JR, Webster AK, Whipple JP, Peters GR, Colwell CW. JR. Ximelagatran versus warfarin for the prevention of venous thromboembolism after total knee arthroplasty. A randomized, double-blind trial. Ann Intern Med. 2002;137:648–55. doi: 10.7326/0003-4819-137-8-200210150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stähle H, Rathgen K, Svärd R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–63. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- 8.Tanswell P, Koup J. TopFit: a PC-based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993;31:514–20. [PubMed] [Google Scholar]
- 9.Weitz J. Orally active direct thrombin inhibitors. Sem Cardiovasc Med. 2003;3:131–7. doi: 10.1055/s-2003-40671. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson BI, Dahl OE, Buller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kalebo P, Reilly P, Bistro II Study Group. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–11. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 11.Kher A, Al Dieri R, Hemker HC, Beguin S. Laboratory assessment of antithrombotic therapy: what tests and if so why? Haemostasis. 1997;27:211–8. doi: 10.1159/000217459. [DOI] [PubMed] [Google Scholar]
- 12.Conrad KA. Clinical pharmacology and drug safety: lessons from hirudin. Clin Pharmacol Ther. 1995;58:123–6. doi: 10.1016/0009-9236(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 13.Clarke RJ, Mayo G, Fitzgerald GA, Fitzgerald DJ. Combined administration of aspirin and a specific thrombin inhibitor in man. Circulation. 1991;83:1510–8. doi: 10.1161/01.cir.83.5.1510. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson D, Elg M. The pharmacodynamics and pharmacokinetics of the oral direct thrombin inhibitor ximegalatran and its active metabolite megalatran: a mini-review. Thromb Res. 2003;109(Suppl 1):S9–S15. doi: 10.1016/s0049-3848(03)00249-4. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh J. Heparin. N Engl J Med. 1991;324:1565–74. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 16.Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, a thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol. 2001;51:71–9. doi: 10.1046/j.0306-5251.2001.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak G. Clinical monitoring of hirudin and direct thrombin inhibitors. Semin Thromb Hemost. 2001;27:537–41. doi: 10.1055/s-2001-17964. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson SC, Mattsson C, Eriksson UG, Sarich TC, Wahlander K, Eliasson A, Karlson BW, Sheth SB, Held P. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res. 2005;115:9–18. doi: 10.1016/j.thromres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Fenyvesi T, Jorg I, Harenberg J. Monitoring of anticoagulant effects of direct thrombin inhibitors. Semin Thromb Hemost. 2002;28:361–8. doi: 10.1055/s-2002-34305. [DOI] [PubMed] [Google Scholar]
- 20.Gosselin RC, King JH, Janatpour KA, Dager WE, Larkin EC, Owings JT. Comparing direct thrombin inhibitors using aPTT, ecarin clotting times, and thrombin inhibitor management testing. Ann Pharmacother. 2004;38:1383–8. doi: 10.1345/aph.1D565. [DOI] [PubMed] [Google Scholar]
- 21.Stangier J, Rathgen K, Gansser D, Kohlbrenner V, Stassen JM. Pharmacokinetics of BIBR953ZW, a novel low molecular weight direct thrombin inhibitor in healthy volunteers. Thromb Haemost. 2001;(Suppl):Abstract. OC2347. [Google Scholar]