Abstract
What is already known about this subject
Nonclinical studies have shown that exenatide is primarily cleared by the renal system.
It was not known to what degree the clinical pharmacokinetics and tolerability would be affected by increasing renal impairment (RI).
What this study adds
Patients with mild to moderate RI adequately tolerate current therapeutic doses of exenatide.
However, exenatide is not recommended in patients with severe RI or end-stage renal disease.
Aims
To evaluate the pharmacokinetics (PK), safety and tolerability of a single exenatide dose in patients with renal impairment (RI).
Methods
Exenatide (5 or 10 µg) was injected subcutaneously in 31 subjects (one with Type 2 diabetes) stratified by renal function [Cockcroft–Gault creatinine clearance (CrCL), number of subjects]: normal (>80 ml min−1, n = 8), mild RI (51–80 ml min−1, n = 8), moderate RI (31–50 ml min−1, n = 7) or end-stage renal disease (ESRD) requiring haemodialysis (n = 8). PK data were combined with four previous single-dose studies in patients with Type 2 diabetes to explore the relationship of exenatide clearance (CLp/F) and CrCL.
Results
Mean half-life for healthy, mild RI, moderate RI and ESRD groups were 1.5, 2.1, 3.2 and 6.0 h, respectively. After combining data from multiple studies, least squares geometric means for CLp/F in subjects with normal renal function, mild RI, moderate RI and ESRD were 8.14, 5.19, 7.11 and 1.3 l h−1, respectively. Exenatide was generally well tolerated in the mild and moderate RI groups, but not in subjects with ESRD due to nausea and vomiting. Simulations of exenatide plasma concentrations also suggest patients with ESRD should have a propensity for poor tolerability at the lowest available therapeutic dosage (5 µg q.d.).
Conclusions
Since tolerability and PK changes were considered clinically acceptable in patients with mild to moderate RI, it would be appropriate to administer exenatide to these patients without dosage adjustment. However, poor tolerability and significant changes in PK make the currently available therapeutic doses (5 and 10 µg) unsuitable in severe RI or ESRD.
Keywords: end-stage renal disease, exenatide, incretin mimetic, pharmacokinetics, renal impairment, Type 2 diabetes
Introduction
Exenatide (exendin-4) is a 39-amino acid peptide approved by the US Food and Drug Administration as an adjunctive treatment of Type 2 diabetes in patients unable to achieve adequate control using metformin and/or a sulphonylurea [1]. Exenatide, injected subcutaneously, at doses of 5 and 10 µg bid significantly lowers HbA1c and reduces body weight in patients with Type 2 diabetes, as shown by three 30-week placebo-controlled trials [2–4]. It shares several glucoregulatory actions with the endogenous incretin hormone, glucagon-like peptide-1 (GLP-1) and has been shown in vitro to be an agonist of the human islet GLP-1 receptor [5]. Exenatide thus belongs to a new class of antidiabetic agents known as incretin mimetics. GLP-1 is released in response to meal-derived glucose and leads to glucose-dependent enhancement of insulin secretion [6] and suppression of inappropriately elevated glucagon secretion [7]. GLP-1 also slows the rate of gastric emptying [8].
Exenatide reaches peak plasma concentrations in approximately 2 h, has a terminal half-life of approximately 2.5 h in human subjects with normal renal function [19] and is predominantly eliminated by renal mechanisms. In anaesthetized pigs, extraction from kidney, liver, lungs, intestine and leg tissue detected exenatide only in the kidney, and by an amount equivalent to that accounted for by glomerular filtration [10]. Also, in nephrectomized rats, clearance was reduced fourfold compared with control rats [11], indicating a major role of the kidneys in exenatide elimination. After renal filtration, exenatide is enzymatically degraded in the kidney tubules with negligible amounts of intact exenatide being detected in the urine of rats [12].
Renal dysfunction is widely prevalent in patients with diabetes. In the UKPDS 64 study, approximately 25% of patients with Type 2 diabetes had microalbuminuria and 5% had macroalbuminuria by 10 years following diagnosis of diabetes [13]. Diabetic nephropathy represents the most common cause of end-stage renal failure, accounting for about 40% of all new cases of end-stage renal disease (ESRD) in the USA [14]. Studies have shown that glycaemic control is important to prevent development of diabetic nephropathy and microvascular complications [15, 16]. As renal dysfunction develops, therapeutic options for patients with Type 2 diabetes become more complicated. Impaired renal function can reduce the clearance of drugs that are extensively cleared by the kidneys, thereby increasing the incidence of exposure-dependent side-effects. Hence, it is important to explore the effects of renal impairment (RI) on novel antidiabetic medications such as exenatide.
This study evaluated the pharmacokinetics, safety and tolerability of a single subcutaneous administration of exenatide in patients with mild or moderate RI or ESRD compared with subjects with healthy renal function. Pharmacokinetic simulations based on those results were used to predict the steady-state profiles for the moderate-RI and ESRD groups. In addition, a combined analysis with four previous pharmacokinetic studies in patients with Type 2 diabetes was performed to quantify further the relationship between exenatide clearance and creatinine clearance. These complementary analyses were the basis for exenatide dosage recommendations for patients with various stages of RI.
Methods
Subjects and study groups
Male and female subjects, between 25 and 80 years of age and with a body mass index (BMI) of between 19 and 40 kg m−2, were eligible to enter this open-label study. Thirty-one eligible subjects were stratified into four parallel study groups based on their renal function (Cockcroft–Gault creatinine clearance, CrCL) at screening. The groups were classified as having normal renal function (control group CrCL > 80 ml min−1, n = 8), mild RI (CrCL 51–80 ml min−1, n = 8), moderate RI (CrCL 31–50 ml min−1, n = 7) at screening or ESRD (on haemodialysis for at least 1 month before screening, n = 8). Subjects in the healthy control group were, as practically possible, age and gender matched to subjects in the RI groups. Subjects with impaired renal function were allowed to continue their concomitant medication for the treatment of renal disease. Control subjects were not permitted concomitant medications, apart from hormonal contraception. RI groups could include subjects with Type 2 diabetes controlled by diet alone and/or by sulphonylureas.
The study protocol and informed-consent document were approved by the ethical review boards at the City Hospitals of Antwerp, General St Jean Hospital of Brussels and the Academic Hospital of the Free University of Brussels. The study was conducted at three sites in Belgium according to good clinical practices, all local laws and regulations and the Declaration of Helsinki. Informed consent was obtained from all study subjects before participation.
Drug administration, dosing and sample collection
Subjects were admitted to the study centre either on the day of dosing (day 1) or the previous evening, and remained resident in the unit until day 2, approximately 24 h postdose. A single subcutaneous injection of 5 or 10 µg exenatide was administered into the abdomen approximately 15 min prior to a standardized breakfast on day 1. Subjects were provided lunch, afternoon snack and evening meal at specified times following the dose.
To minimize the potential safety and tolerability risk posed by increased drug accumulation with RI, dosing was to commence with the mild RI group. Each subject in this group received a single subcutaneous dose of 10 µg exenatide and safety and tolerability data from at least three subjects were evaluated prior to the dosing of subjects with moderate RI. Subjects with moderate RI or ESRD could be given a lower dose if deemed clinically necessary. Similarly, dosing of subjects with ESRD was based on evaluation of pharmacokinetic, safety and tolerability data from at least three subjects with moderate renal dysfunction. Dosing in subjects with ESRD occurred during the longest weekly interval between dialysis sessions to allow for adequate pharmacokinetic sampling prior to the next dialysis. The effects of dialysis in patients with ESRD were to be evaluated in this study by measuring plasma exenatide concentrations before (48 h postdose) and after a dialysis cycle.
To accommodate longer half-lives in the renal impairment groups, sampling durations were prolonged relative to the healthy group. Serial blood samples (3 ml) for the measurement of exenatide plasma concentrations were taken postdose up to 12 h (normal renal function), 18 h (mild and moderate RI) and 48 h (ESRD). Plasma samples were analysed for exenatide concentration using a validated immunoenzymetric assay over the range 10–500 pg ml−1 as previously described [17]. The overall relative standard deviation, which is an expression of the precision, ranged between 3.7% and 16.2%. The overall relative error, which is an expression of the accuracy, ranged between −11.0% and +17.3% for all concentrations.
Safety assessment
Supine vital signs (blood pressure and heart rate) and 12-lead ECG were assessed for all subjects at scheduled intervals from predose to 24 h postdose. In addition, standing vital signs were assessed for the control group. Blood samples for the measurement of plasma glucose concentrations were also taken during the study at scheduled intervals and if hypoglycaemia was suspected. Adverse events were recorded as they occurred during the study. Physical examinations were conducted for each subject at 24 h and at 4–15 days after dosing.
Pharmacokinetic analyses
Exenatide plasma concentrations were evaluated by standard noncompartmental methods to determine key pharmacokinetic parameters. The maximum concentration (Cmax) and the time of maximum concentration (tmax) were identified from the observed data. The area under the plasma exenatide concentration–time curve (AUC0–∞) was calculated using the log linear trapezoidal rule. The dose-weight normalized Cmax and AUC0–∞ were log transformed and evaluated by analysis of variance (anova). One subject with moderate RI was excluded as an outlier from statistical analyses for having unusually low exenatide concentrations compared with the rest of the group. Based on predefined criteria, plasma concentrations for this subject were deemed to be outliers because they were less than three times the SD of the mean of the rest of the subjects. The differences in least square (LS) geometric means between each of the RI groups and the healthy group were transformed back to the original scale to yield the ratio of the geometric means and the corresponding 90% confidence interval (CI) for each comparison. WinNonLin Professional 3.1 (Pharsight, Cary, NC, USA) and SAS 8.2 for Windows (SAS Inc., Cary, NC, USA) software were used for the pharmacokinetic and statistical analyses, respectively.
Combined analysis of renal study with previous studies
Exenatide pharmacokinetic parameters derived from this study were combined with those from four previous single-dose studies [18–20] and stratified by renal function. These previous studies were conducted in patients with Type 2 diabetes. In contrast, the renal study included only one subject with Type 2 diabetes in the mild RI group. Nonetheless, this combination is justified from a pharmacokinetic perspective because exenatide clearance in patients with Type 2 diabetes has been shown to be similar to that of healthy subjects [9] and the same bioanalytical assay was used in all studies from which the data were combined. Of the previous trials, Studies ‘A’ and ‘B’ were single-blind, placebo-controlled studies: the former was dose-rising over 0.1–0.4 µg kg−1, the latter conducted at 0.1 µg kg−1. Study ‘C’ was a double-blind, placebo-controlled, single-dose rising study over 0.05–0.2 µg kg−1. Study ‘D’ was a randomized, open-label, single-dose crossover study using 10 µg exenatide. As these previous studies had crossover designs, they included subjects who were dosed on more than one occasion and multiple pharmacokinetic (PK) parameter estimates could therefore be derived for each subject. A linear mixed-effects model was used for statistical analysis where subject was a random effect and RI group was a fixed effect to take into account the correlation among multiple observations from the same subject. The ratios of the LS geometric means and the corresponding 90% CI were calculated for AUC0–∞ and Cmax estimates from each of the RI groups compared with the normal renal-function group of the combined analysis dataset. Furthermore, the relationship between exenatide clearance and creatinine clearance was evaluated by fitting a linear mixed-effects model. Clearance for the severe RI group was estimated by interpolation from the linear regression line over a creatinine clearance range of 10–30 ml min−1.
Nonparametric simulation of single dose pharmacokinetic data
Steady-state concentration–time profiles were projected by nonparametric simulations of single-dose plasma concentration–time profiles from this renal study using WinNonLin Professional 3.1 (Pharsight). Simulations were conducted on the mean, lowest and highest single-dose concentration–time profiles for RI groups that showed a statistically significant increase in exposure compared with the control group. Projected steady-state profiles were compared with a range of plasma exenatide concentrations that are known to be efficacious and tolerated (50–350 pg ml−1).
Results
Study groups
Table 1 provides demographic characteristics including mean creatinine clearance, gender, age, weight and BMI for the four study groups. The groups were comparable with respect to gender and BMI, but age varied more because of the higher age in the moderately impaired group compared with the control group. All subjects were nondiabetic, except for one subject in the mild RI group who had Type 2 diabetes controlled by diet and exercise. Subjects with normal renal function and mild RI received a 10-µg exenatide dose. Following review of the PK, safety and tolerability in the mild RI group, it was decided to reduce the dose to 5 µg in the moderate group. Two subjects with moderate RI were administered 10 µg of exenatide inadvertently, but did not experience any adverse events. Similarly, based on evaluation of data in the moderate group, subjects with ESRD were administered 5 µg of exenatide.
Table 1.
Study group descriptions
| Study groups† | Mean creatinine clearance (range), ml min−1 | n | Exenatide dose, µg | Gender distribution, male : female | Age, mean±SD, years | Body weight±SD, kg | BMI, mean±SD, kg m−2 |
|---|---|---|---|---|---|---|---|
| Healthy (control) | 111 (83–156) | 8 | 10 | 3 : 5 | 46 ± 5.5 | 73.3 ± 10.9 | 25.7 ± 4.08 |
| Mild RI | 68 (60–78) | 8 | 10 | 5 : 3 | 56 ± 9.9 | 76.9 ± 13.3 | 25.5 ± 2.77 |
| Moderate RI | 45 (34–50) | 7 | 5 or 10‡ | 5 : 2 | 64 ± 9.6* | 76.1 ± 12.6 | 27.2 ± 3.02 |
| ESRD | N/A | 8 | 5 | 3 : 5 | 52 ± 18.3 | 64.1 ± 13.5 | 23.7 ± 3.29 |
P < 0.05, compared with normal renal function group. BMI, Body mass index; RI, renal impairment; ESRD, end-stage renal disease.
Inclusion criteria for study groups based on Cockcroft–Gault creatinine-clearance (CrCL): normal renal function: CrCL>80 ml min−1; Mild RI: CrCL 51–80 ml min−1; Moderate RI: CrCL 31–50 ml min−1; ESRD: haemodialysis for at least 1 month at screening.
Five patients received 5 µg and two patients received 10 µg exenatide.
Safety and tolerability
Table 2 displays the frequency of adverse events reported by subjects during the study. The most common treatment-emergent adverse events were vomiting, nausea and headache. Most episodes occurred within approximately 2.5 h of dosing. The nature and frequency of adverse events were comparable between the control (10 µg), mild RI (10 µg) and ESRD groups (5 µg). No adverse events were observed for the group with moderate RI (5 and 10 µg exenatide). Antiemetic concomitant medications (mostly metoclopramide) were administered to seven subjects with ESRD, one with mild RI and one in the healthy group because of more severe or longer-duration nausea or vomiting. No subjects discontinued the study due to adverse events.
Table 2.
Treatment-emergent adverse events reported by subjects
| Number of adverse events [number of subjects with adverse event] | ||||
|---|---|---|---|---|
| MedDRA preferred term | Healthy subjects (10 µg exenatide) (n = 8) | Mild RI (10 µg exenatide) (n = 8) | Moderate RI (5 µg exenatide)* (n = 7) | ESRD (5 µg exenatide) (n = 8) |
| Vomiting NOS | 9 [6] | 6 [3] | 0 | 10 [7] |
| Nausea | 8 [8] | 1 [1] | 0 | 8 [7] |
| Headache† | 7 [5] | 2 [2] | 0 | 6 [5] |
| Dizziness | 1 [1] | 3 [2] | 0 | 0 |
| Malaise | 0 | 0 | 0 | 2 [2] |
| Tachycardia | 1 [1]‡ | 0 | 0 | 1 [1]§ |
| Other¶ | 2 [1] | 5 [2] | 0 | 1 [1] |
| Total | 28 [8] | 17 [5] | 0 | 28 [7] |
Two subjects in this group received 10 µg exenatide.
Five headache events were probably not related to exenatide.
Medical Dictionary for Regulatory Activities (MedDRA) term: Tachycardia NOS (NOS = not otherwise specified).
MedDRA term: Sinus tachycardia.
Dyspepsia, cold sweat and increased sweating (not related to hypoglycaemia), asthenia (twice), dry throat, abdominal pain upper, dyspnoea. Each reported by one subject.
Three adverse events for two subjects were rated severe. One control subject experienced severe nausea that began 40 min postdose, lasted approximately 3 h and was treated with oral administration of metoclopramide. This subject had a peak plasma exenatide concentration of 1295 pg ml−1, 1 h after administration of 10 µg. One subject with ESRD had two severe episodes of vomiting. The first episode began approximately 1 h postdose, lasted 30 min and was treated with intravenous administration of metoclopramide. The second episode began approximately 4 h postdose, lasted 10 min and was treated with intravenous administration of alizapride. This subject reached a peak exenatide plasma concentration of 494 pg ml−1, 2 h after administration of 5 µg. Both subjects had exenatide plasma concentrations similar to others within their treatment group.
Transient increases in supine systolic and diastolic blood pressure were observed 1–3 h after exenatide dosing. Mean peak increases from predose baselines for the treatment groups ranged from 11 to 18 mmHg for systolic and 6 to 12 mmHg for diastolic blood pressures. In addition, transient increases in supine heart rate were apparent 3–8 h postdose, with the mean peak increases from baselines ranging from 10 to 17 beats min−1 among the groups.
Two subjects each experienced one adverse event of mild tachycardia. One (healthy subject) began approximately 3 h postdose and lasted 21 h, although it was not apparent in the 12-lead ECG assessed at 3, 6 and 24 h postdose. The other (ESRD subject) occurred approximately 1 h postdose, lasted 7 h and was considered clinically significant by ECG assessment at 3 h postdose. Exenatide exposures (Cmax and AUC0–∞) for these two subjects were below their respective group means.
Transient reductions in plasma glucose concentrations were observed for most subjects 0.5–2 h following exenatide dosing, consistent with exenatide's glucose-lowering actions. However, no subjects had symptoms of hypoglycaemia.
Exenatide pharmacokinetics
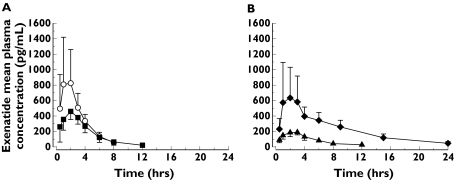
Mean plasma exenatide concentration–time profiles and PK parameters are shown by study group in Figure 1 and Table 3, respectively. Mean exenatide clearance (CLp/F) was substantially reduced in subjects with ESRD (0.9 l h−1) compared with the control group (3.4 l h−1). Consistent with the change in clearance, mean half-life increased as renal function decreased from normal renal function (1.5 h) to mild (2.1 h) and moderate RI (3.2 h). The longest half-life occurred with ESRD (6 h), which was four times that of subjects with normal renal function. The effect of dialysis could not be evaluated in patients with ESRD because exenatide concentrations were below the quantification limit 48 h postdose, prior to the subsequent dialysis cycle.
Figure 1.
Mean (SD) plasma exenatide concentration–time profiles for subjects with (A) normal renal function and mild renal impairment (RI) following a 10-µg dose; (B) moderate RI and end-stage renal disease (ESRD) following a 5-µg dose. Two subjects in the moderate RI group who received 10 µg exenatide are not shown. ( , Healthy (10 µg);
, Healthy (10 µg);  , Mild (10 µg);
, Mild (10 µg);  , Moderate (5 µg);
, Moderate (5 µg);  , ESRD (5 µg))
, ESRD (5 µg))
Table 3.
Exenatide pharmacokinetic parameters by renal group in the single study
| Geometric mean (CV%) | |||||
|---|---|---|---|---|---|
| Parameter (units) | Healthy | Mild RI | Moderate RI * | ESRD | |
| n | 8 | 8 | 5 | 1 | 8 |
| Dose (µg) | 10 | 10 | 5 | 10 | 5 |
| Cmax (pg ml−1) | 821 (61.0) | 470 (24.6) | 202 (19.9) | 353 | 601 (69.4) |
| AUC0–∞ (pg h ml−1) | 2930 (31.4) | 2080 (17.4) | 1150 (15.2) | 2090 | 5380 (42.2) |
| CLp/F (l h−1) | 3.4 (31.4) | 4.8 (17.4) | 4.4 (14.2) | 0.9 (42.2) | |
| Vz/F (l) | 7.1 (40.2) | 14.7 (21.9) | 20.2 (45.1) | 8 (43.9) | |
| t1/2 (h)† | 1.5 (0.9–2.0) | 2.1 (1.6–3.4) | 3.2 (1.8–7.0) | 6 (4.3–7.6) | |
| Tmax (h)† | 2.0 (1.0–3.0) | 2.0 (0.5–3.0) | 2.50 (1.0–3.0) | 2.0 (1.0–4.0) | |
One subject in the 10-µg group was excluded as an outlier from all statistical evaluations.
t1/2and Tmaxrepresented as mean (range) and median (range), respectively.
The statistical comparison of exenatide clearance and dose–weight normalized exenatide exposure between each renal impairment group and the normal renal group (Table 4) showed mild to moderate RI was not significantly different with respect to clearance or AUC0–∞, but yielded a mean Cmax approximately 32–35% lower than the healthy control group. For the ESRD group, the clearance was approximately one-quarter of the healthy control group (P < 0.001) and dose–weight normalized AUC0–∞ and Cmax were 3.37 (P< 0.001) and 1.38 (P= 0.09) times higher, respectively, than the healthy control group.
Table 4.
Comparison of exenatide clearance and dose–weight normalized AUC0–∞ and Cmax between each renal impairment group (mild, moderate and ESRD) and the healthy control group
| Renal function group | n | LS geometric mean | LS geometric mean ratio renal group/healthy (90% CI) | P-value | |
|---|---|---|---|---|---|
| AUC0–∞ (pg h ml−1)/(µg kg−1) | Healthy | 8 | 19 917 | – | |
| Mild RI | 8 | 16 036 | 0.81 (0.66, 0.98) | 0.066 | |
| Moderate RI* | 6 | 19 258 | 0.97 (0.77, 1.21) | 0.801 | |
| ESRD | 8 | 67 102 | 3.37 (2.80, 4.06) | <0.001 | |
| Cmax (pg ml−1)/(µg kg−1) | Healthy | 8 | 5 392 | – | |
| Mild RI | 8 | 3 650 | 0.68 (0.49, 0.93) | 0.047 | |
| Moderate RI* | 6 | 3 507 | 0.65 (0.45, 0.94) | 0.060 | |
| ESRD | 8 | 7 434 | 1.38 (1.01, 1.88) | 0.088 | |
| CLp/F (l h−1) | Healthy | 8 | 3.64 | – | – |
| Mild RI | 8 | 4.72 | 1.30 (1.02, 1.66) | 0.08 | |
| Moderate RIa | 6 | 4.07 | 1.12 (0.84, 1.49) | 0.52 | |
| ESRD | 8 | 0.94 | 0.26 (0.20, 0.33) | ≤0.001 |
One subject receiving 10 µg was excluded as an outlier due to atypically low concentrations. ESRD, End-stage renal disease; RI, renal impairment.
Combined analysis of renal study with previous studies
Four of the eight subjects in the control group exhibited exenatide plasma concentrations substantially higher (Cmax 960–1996 pg ml−1) than expected due to exenatide clearance being substantially lower (CLp/F 2.13–3.16 l h−1) than expected. The mean peak exenatide plasma concentration and apparent clearance typically observed following a 10-µg subcutaneous dose in subjects with normal renal function is approximately 200 pg ml−1 and 9 l h−1, respectively [1]. Therefore, to provide a more robust control sample, results from this renal study were combined with previously available data from four single-dose crossover studies. The previous studies included 67 subjects with Type 2 diabetes (50 male, 17 female); mean ± SD for the key demographics were: age 52.3 ± 8.77 years, BMI 33 ± 4.71 kg m−2, weight 97.1 ± 18 kg. The majority of subjects in the previous studies had normal renal function [CrCL 140 ± 47.5 ml min−1 (n = 63)]; however, four subjects exhibited mild RI (CrCL 71.5 ± 5.62 ml min−1).
LS geometric mean clearances in the normal renal function, mild RI, moderate RI and ESRD groups in the combined-studies analysis were 8.14, 7.11, 5.19 and 1.3 l h−1, respectively (Table 5). These clearance estimates differ from those in Table 3 because of the lower than usual clearance estimates for some subjects in the renal study control group. Results of the combined analysis showed that exenatide clearance was not significantly different between the normal renal function and mild renal impairment groups (P= 0.26), but was significantly reduced by 36% in the moderate renal impairment group (P= 0.008) and 84% in the ESRD group (P< 0.001) compared with the normal control group (Table 5).
Table 5.
Comparison of exenatide clearance and dose–weight-normalized AUC0–∞ and Cmax between the renal impairment groups (mild, moderate and ESRD) and the control group using data from multiple trials*
| Pharmacokinetic parameter | Renal function group | n | LS geometric mean | LS geometric mean ratio renal group/normal (90% CI) | P-value |
|---|---|---|---|---|---|
| AUC0–∞ (pg h ml−1)/(µg kg−1) | Normal | 71 | 10 913 | – | – |
| Mild RI | 12 | 12 842 | 1.18 (0.97, 1.42) | 0.156 | |
| Moderate RI | 6 | 17 751 | 1.63 (1.25, 2.12) | 0.003 | |
| ESRD | 8 | 68 054 | 6.24 (4.94, 7.87) | <0.001 | |
| Cmax (pg ml−1)/(µg kg−1) | Normal | 71 | 2 312 | – | – |
| Mild RI | 12 | 2 708 | 1.17 (0.92, 1.49) | 0.284 | |
| Moderate RI | 6 | 3 098 | 1.34 (0.95, 1.89) | 0.160 | |
| ESRD | 8 | 7 595 | 3.28 (2.43, 4.44) | <0.001 | |
| CLp/F (l h−1) | Normal | 71 | 8.14 | – | – |
| Mild RI | 12 | 7.11 | 0.87 (0.69, 1.11) | 0.258 | |
| Moderate RI* | 6 | 5.19 | 0.64 (0.46, 0.89) | 0.008 | |
| ESRD | 8 | 1.3 | 0.16 (0.12, 0.22) | <0.001 |
Clearance estimates for subjects with normal renal function were obtained by combining the control group of the renal study and four historical studies in subjects with Type 2 diabetes. ESRD, End-stage renal disease; RI, renal impairment.
Exenatide exposure (Cmax and AUC) from the combined-analysis dataset was compared between subjects with RI and those with normal renal function (Table 5). In these results, patients with moderate RI had an AUC 1.63 times that of the control group (P= 0.003) and patients with ESRD had an increase in AUC 6.24 times that of the control group (P< 0.001). To illustrate further the significance of these comparisons, simulations of steady-state exenatide plasma profiles using different regimens were conducted and are described in the section below.
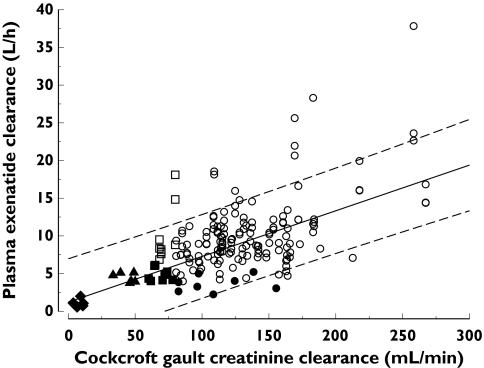
The relationship between exenatide clearance and creatinine clearance was evaluated by mixed-effects regression analysis (Figure 2). The majority of observed data were contained within the 95% confidence bands of the regression line. Based on the linear regression between exenatide clearance and creatinine clearance, predicted mean exenatide clearance for patients with severe RI (CrCL 10–30 ml min−1) ranged from 1.82 to 3.03 l h−1.
Figure 2.
Relationship between exenatide clearance and creatinine clearance (r = 0.63, P < 0.001) showing the regression (solid line) and 95% confidence interval (broken lines). (○, Previous Studies Combined (Healthy); •, Renal Study (Healthy); ▪, Renal Study (Mild RI); □, Previous Studies Combined (Mild RI); ▴, Renal Study (Moderate RI); ♦, Renal Study (ESRD))
Simulations based on the single-dose data from renal study
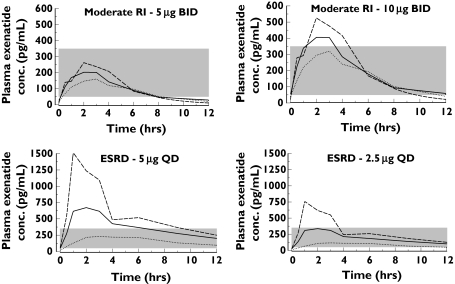
Figure 3 shows the simulated exenatide plasma concentration–time profiles for several treatment regimens in patients with moderate RI or ESRD at steady state. These profiles overlay a reference therapeutic range of 50–350 pg ml−1 (shaded area), which is typical among patients with normal renal function administered 5 and 10 µg [19, 20]. Exenatide concentrations >350 pg ml−1 resulted in a notable increased incidence of nausea [19]. The mean simulated exenatide concentration profile in patients with moderate RI following a 5-µg bid regimen remained below 350 pg ml−1, whereas much of the simulated concentration profile following 10 µg bid exceeded this range for 2–4 h postdose. For patients with ESRD, most of the mean simulated exenatide concentrations following a 5-µg q.d. regimen were >350 pg ml−1 for much of the dosing interval; however, following a 2.5-µg q.d. regimen the mean simulated concentrations remained below 350 pg ml−1.
Figure 3.
Simulated steady-state profiles based on the nonparametric superposition of single-dose data for exenatide at several regimens in patients with moderate renal impairment (RI) or end-stage renal disease (ESRD). Solid and broken lines represent nonparametric simulation of lowest, mean and highest concentration profile of each group. The shaded area (50–350 pg ml−1) is the typical therapeutically efficacious and tolerable range for exenatide in patients with normal renal function
Discussion
This study explored the influence of varying degrees of RI on exenatide safety, tolerability and PK to assess potential dosage adjustment that may be needed for patients at different stages of renal disease. Therapeutic exenatide dosage regimens (5–10 µg bid) were assessed in patients at different stages of renal disease in keeping with recommendations from the European Medicines Evaluation Agency guidance on RI [21]. However, the CLp/F in the healthy group was substantially lower than that observed previously in subjects with a normal renal function [1]. Therefore, previous data from four single-dose studies in 67 patients with Type 2 diabetes were combined with the data of the renal study. The combined analysis showed that, whilst exenatide clearance decreased 13% in the mild-RI group compared with subjects with a normal renal function, tolerability of exenatide (10 µg) was acceptable and this reduction in clearance was not considered clinically relevant; therefore, no dosage adjustment is recommended for patients with mild RI. In patients with moderate RI, exenatide clearance decreased by 36%. Patients with moderate RI tolerated 5 and 10 µg exenatide, although PK simulations for 10 µg bid suggested many patients with moderate RI could briefly experience exenatide concentrations >350 mg ml−1 and therefore might experience periods of reduced tolerance following 10 µg exenatide. Patients with ESRD had significantly reduced clearance (84% decrease) and did not adequately tolerate 5 µg exenatide; therefore, current therapeutic dosages of exenatide are not recommended for this group.
The moderate RI group had a statistically significant increase in exenatide exposure, with a dose–weight normalized AUC of 1.63 times the control group; however, exenatide (5 and 10 µg) was well tolerated in the moderate RI group. PK simulations for this subpopulation suggest steady-state concentrations following a 5-µg dose given bid are likely to be retained in a therapeutically effective and tolerable range (50–350 pg ml−1). However, simulations also show a 10-µg dose given bid to patients with moderate RI will be likely to result in concentrations that exceed this range, although for relatively short duration and by a relatively small magnitude. Dosing with exenatide is recommended to commence at 5 µg bid with escalation to 10 µg bid after at least 1 month based on the individual's tolerability and efficacy profile [1]. Although simulations have shown relatively minor excursions above tolerable concentrations in patients with moderate RI, and the two patients in this study with moderate RI who were administered 10 µg exenatide tolerated it well, therapeutic escalation from 5 to 10 µg bid by patients with moderate RI should be done conservatively.
The ESRD group had a statistically significant increase in exenatide exposure with a dose-normalized AUC of 6.24 times the healthy group. A substantial reduction in exenatide clearance was expected given the results of the preclinical studies that indicated a major contribution of the kidneys to the elimination of exenatide. The simulated steady-state concentration profiles after 5 µg q.d. in patients with ESRD were substantially higher than 350 pg ml−1 threshold for a major portion of the dosing interval, suggesting prolonged periods of poor tolerability with ESRD. Given the poor tolerability observed in this study with 5 µg exenatide, which is currently the lowest therapeutic dosing option, it is recommended that exenatide not be administered to patients with ESRD. The lower 2.5-µg q.d. regimen simulated for ESRD resulted in a mean exenatide concentration profile within the therapeutically efficacious and tolerable range (<350 pg ml−1); however, this has not been clinically tested.
This study did not include a group with severe RI (CrCL ≤ 30 ml min−1 and not on haemodialysis). However, interpolation from a linear fit of exenatide clearance vs. creatinine clearance for patients with severe RI showed a relatively low mean exenatide clearance of 1.82–3.03 l h−1 for this subgroup. Given this low clearance and the poor tolerability of a single 5-µg dose in patients with ESRD, currently available therapeutic regimens of exenatide are also not recommended for patients with severe RI.
Antidiabetic therapy in patients with ESRD and severe RI could be an unmet medical need due to limited therapy choices, and alternate dosing regimens of exenatide could be explored. Given our simulation results showing the predicted mean exenatide plasma concentration profile <350 pg ml−1 with a 2.5-µg q.d. regimen, it would be reasonable to explore further a reduced dosage in patients with ESRD or severe RI. The design of any such future clinical studies to target efficacious and tolerable exposures at lower doses in patients with severe RI or ESRD will be guided by additional PK simulations.
In this renal study, mean apparent clearance in the mild RI group was higher than in the healthy control group. This was an artefact of the relatively low mean clearance estimate obtained in the control group due to four patients with substantially higher exposures than previously observed in patients with normal renal function. While plausible explanations such as bioanalytical or dosing error were investigated, a definitive reason for the observation was not established. Therefore, PK results from this study were combined with those from four previous single-dose PK studies to provide an evaluation of the relationship of individual clearance estimates over a wide continuum of creatinine clearance. The historical database was composed of patients with Type 2 diabetes compared with the renal study, where all except one patient did not have diabetes. As previous analyses have established that exenatide clearance is similar in patients with Type 2 diabetes and healthy volunteers [9] and the bioanalytical methodology was the same in every study, combining these datasets was justified. Notably, the historical database contributed not only data from subjects with normal renal function, but also 11 observations with mild renal impairment. As a result, numerical differences exist in exposure estimates shown in Tables 4 and 5 for subjects with normal renal function and mild renal impairment and could be explained by the addition of these data. In order to avoid bias, statistical analyses from both the renal study alone and the combined analysis are presented, and are considered complementary.
In this study, a single 5- or 10-µg subcutaneous dose of exenatide appeared generally well tolerated in all evaluated stages of renal function except the ESRD group. Overall, the incidences of nausea and vomiting in each group were generally related to exenatide plasma concentrations, which can be mitigated by dosage management. In long-term exenatide clinical trials, the incidence of nausea with 5 µg bid was less than with 10 µg bid [2–4]. The same studies also found that the incidence of nausea decreased over time with either dosage due to increased tolerance.
Transient increases from baseline in blood pressure and heart rate were observed with exenatide treatment in this study; however, the lack of placebo control and the potential confounding effects of renal disease and concomitant medications limit interpretation of these results. Although acute increases were demonstrated here after a single dose, no differences between placebo and exenatide treatments were observed for blood pressure or heart rate (measured at clinic visits), or cardiovascular adverse events in the 30-week exenatide clinical trials [2–4].
In conclusion, no dosage adjustment of exenatide is required for patients with mild to moderate RI because the recommended starting dosage of 5 µg was well tolerated and any subsequent increase in dosage would be based on the patient's individual tolerance and glycaemic response. However, as mean exenatide clearance was significantly reduced and a single 5-µg dose of exenatide was not well tolerated in subjects with ESRD, this dosage may not be suitable for use in patients with ESRD or severe RI (creatinine clearance <30 ml min−1).
Competing interests
This work was sponsored by Eli Lilly and Co. and Amylin Pharmaceuticals, Inc. and is related to study protocols H8O-EW-GWAB, 2993-102, 2993-103, 2993-110 and 2993-118.
The authors thank Dr Jacques Bagon, Dr Annemie Knops and Dr Patricia van der Niepen for conducting the clinical evaluations, Michael Kallin Carter for technical assistance in statistical evaluation of data, David Webb PhD and Dr Jie Mao for assistance in preparing the manuscript, and Eric Vandeloise for monitoring the study sites.
References
- 1.BYETTA®. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2006. [Google Scholar]
- 2.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 4.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 5.Thorens B, Porret A, Bûhler L, Deng S-P, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–82. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 6.Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Pharmacol Toxicol. 2004;95:252–62. doi: 10.1111/j.1742-7843.2004.t01-1-pto950502.x. [DOI] [PubMed] [Google Scholar]
- 7.Gromada J, Rorsman P. New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res. 2004;36:822–9. doi: 10.1055/s-2004-826169. [DOI] [PubMed] [Google Scholar]
- 8.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–8. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 9.Reddy S, Park S, Fineman M, Jay L, Carter M, Reynolds L, Sanburn N, Kothare PA. Clinical pharmacokinetics of exenatide in patients with type 2 diabetes. The AAPS J. 2005;7(S2):M1285. [Google Scholar]
- 10.Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetized pigs. Diabetologia. 2006;49:706–12. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
- 11.Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, Chen K, Young A. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–7. [Google Scholar]
- 12.Copley K, McCowen K, Hiles R, Nielsen LL, Young A, Parkes DG. Investigation of exenatide elimination and its in vivo and in vitro degradation. Curr Drug Metab. 2006;7:367–74. doi: 10.2174/138920006776873490. [DOI] [PubMed] [Google Scholar]
- 13.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes. The United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 14.USRDS. Bethesda, MD: National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases; 2004. US Renal Data System Annual Data ReportAtlas of End-Stage Renal Disease in the United States; pp. 218–20. [Google Scholar]
- 15.Molyneaux LM, Constantino MI, McGill M, Zilkens R, Yue DK. Better glycaemic control and risk reduction of diabetic complications in Type 2 diabetes: comparison with the DCCT. Diabetes Res Clin Pract. 1998;42:77–83. doi: 10.1016/s0168-8227(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 16.Nosadini R, Tonolo G. Relationship between blood glucose control, pathogenesis and progression of diabetic nephropathy. J Am Soc Nephrol. 2004;15:S1–S5. doi: 10.1097/01.asn.0000093372.84929.ba. [DOI] [PubMed] [Google Scholar]
- 17.Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–7. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 18.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–9. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 19.Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health-Syst Pharm. 2005;62:173–81. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 20.Calara F, Taylor K, Han J, Zabala E, Carr E, Wintle M, Fineman M. A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin-4) Clin Therapeutics. 2005;27:210–5. doi: 10.1016/j.clinthera.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency. Note for Guidance on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Impaired Renal Function. London: EMEA Committee for Medical Prod Hum Use; 2004. Publication CHMP/EWP/225/02. [Google Scholar]