Abstract
What is already known about this subject
Systemic esomeprazole exposure is decreased when administered simultaneously with food.
What this study adds
Taking esomeprazole within 15 min of eating a high-fat, high-calorie meal results in reduced systemic drug exposure.
Aim
To investigate the pharmacokinetics of esomeprazole before a high-fat meal vs. fasting.
Methods
This open-label, randomized, crossover study consisted of two 5-day dosing periods of esomeprazole 40 mg per day. On days 1 and 5, subjects received esomeprazole 15 min before a high-fat meal (fed) or 4 h before a non-high-fat meal (fasting).
Results
On days 1 and 5, ratio of fed to fasting area under the plasma concentration–time curve [0.56, 90% confidence interval (CI) 0.50, 0.64, and 0.78, 90% CI 0.74, 0.82, respectively] and peak plasma concentration (0.34, 90% CI 0.28, 0.41, and 0.47, 90% CI 0.41, 0.52, respectively) were outside of the limits of bioequivalence.
Conclusions
Esomeprazole bioavailability was reduced when taken within 15 min before eating a high-fat meal vs. that while fasting.
Keywords: esomeprazole capsules, food–drug interactions, pharmacokinetics
Introduction
Esomeprazole, the S-isomer of the racemate omeprazole, is a proton pump inhibitor (PPI) used to treat acid-related disorders, including gastro-oesophageal reflux disease and erosive oesophagitis and their symptoms [1, 2]. The pharmacodynamic effect of esomeprazole, inhibition of gastric acid secretion and percentage of time with intragastric pH > 4.0, correlates with its area under the plasma concentration–time curve (AUC) [3, 4]. However, esomeprazole AUC is decreased when administered simultaneously with food [4]. The effect of timing of food and administration of esomeprazole on the pharmacokinetic profile of esomeprazole has not been studied previously. Therefore, the objective of this study was to compare the effects of dose administration, 15 min before eating vs. under fasting conditions, on the pharmacokinetics of esomeprazole on the first day of dosing and at steady state (day 5) in healthy volunteers.
Methods
Subjects
Healthy adults (aged 18–50 years, inclusive) with a body weight within 20% of ideal for their height and frame were eligible to participate. Inclusion and exclusion criteria were consistent with those of a previous pharmacokinetic study [5], except that subjects in the present study did not need to accept a nasogastric tube, nor were they screened for Helicobacter pylori infection or cytochrome P450 2C19 polymorphism. Subjects were required to sign informed consent statements before enrolment and to comply with study procedures. The study protocol was approved by the Institutional Review Board at MDS Pharma Services (Lincoln, NE, USA) and study procedures were performed in accordance with the ethical principles of the Declaration of Helsinki [6] and its amendments and in compliance with Good Clinical Practice regulations [7].
Study design
In this single-centre (MDS Pharma Services), open-label, randomized, two-way crossover trial (NEXIUM Study 314), subjects were randomly assigned to one of two dosing sequences, each consisting of two 5-day dosing periods separated by a 7–14-day wash-out period. In one dosing period, on days 1 and 5, subjects received esomeprazole 40-mg capsules 15 min before a standardized, high-fat meal (fed) consisting of eggs, bacon, buttered toast, hash brown potatoes and whole milk. In the other dosing period, on days 1 and 5, subjects received esomeprazole 40 mg 4 h before a standardized, non-high-fat meal (fasting). On days 2–4 of both dosing periods, esomeprazole was administered 30 min before a standardized, medium-fat breakfast. Subjects remained at the study centre for the entire 5 days of each study period.
Pharmacokinetic and statistical analyses
On days 1 and 5 of each dosing period, 5-ml venous blood samples were collected from each subject 5 min before and 0.5, 0.75, 1.0, 1.25, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 7.0, 8.0, 9.0, 10.0 and 12.0 h after dose administration. The samples were collected in heparinized tubes and centrifuged. Plasma was shipped frozen to Quintiles AB (Uppsala, Sweden) for analysis using an achiral column, normal-phase liquid chromatography, and UV detection [8]. The lower limit of quantification (LOQ) was 25 nmol l−1. Interassay repeatability and inaccuracy were calculated from control sample concentrations of 50, 500 and 6000 nmol l−1. Control samples were analysed twice in each analytical run. The interassay repeatability was 4.9%, 3.2% and 2.8% for each concentration, respectively, and the inaccuracy was 0.9%, −1.3% and −1.3%, respectively.
The primary variables were maximum plasma concentration (Cmax) and AUC. AUC was calculated as AUC0–t + Ct/λz, where AUC0–t was AUC from time 0 to the time of the last quantifiable esomeprazole concentration (linear trapezoidal method), Ct was the last quantifiable esomeprazole concentration and λz was the terminal phase elimination rate constant. Secondary variables were AUC0–t, time to maximum plasma concentration (tmax) and elimination half-life (t1/2, defined as 0.693/λz). To ensure sufficient data were available to calculate AUC, the percentage extrapolated of AUC0–t could not exceed 20%. On day 1, AUC data were analysed as a single-dose treatment and extrapolated to infinity. On day 5, AUC was extrapolated from time 0–24 h as AUC0−24 = AUC0–t + [1 − exp(– λz) • (24 − t)]•Ct/λz, where Ct and t were the last quantifiable concentrations and the corresponding time and λz was the terminal phase elimination rate constant and represented one dosing interval under multiple-dosing steady-state conditions. The observed Cmax and tmax were recorded. Concentration data below the LOQ occurring before Cmax were assigned a value of 0, and those occurring after Cmax were excluded from pharmacokinetic analysis.
Subjects were considered evaluable if they finished both treatment periods without major protocol violations and had sufficient data to determine both primary variables (AUC and Cmax). Plasma concentrations were summarized by the following procedure: if ≥50% of concentrations were not quantifiable, mean and standard deviation (SD) were not calculated; if <50% of concentrations were not quantifiable, a value of 12.5 nmol l−1 was assigned for each concentration below LOQ to calculate mean and SD. AUC and Cmax were logarithm-transformed and analysed using an analysis of variance (anova) model fitted for the effects of sequence and subjects within sequence, period and feeding regimen. Contrasts between regimens were calculated and the results presented as geometric least-square mean of the ratio of fed vs. fasting with its 90% confidence interval (CI). Bioequivalence was concluded if the 90% CIs fell within the range of 0.80–1.25. Results for tmax were summarized using median and range; t1/2 was summarized using mean and SD.
Previous pharmacokinetic studies suggested that the variability in AUC and Cmax (fed vs. fasting) would be 10% and 15%, respectively, below the bioequivalence limit of 20%. A sample-size population of 36 evaluable subjects was estimated to provide 95% overall power (97.5% for either Cmax or AUC) to show ‘no food effect’ with a significance level of 0.05.
Results
Subjects
Of the 47 subjects randomized, 44 completed the study (two withdrew consent, one had a positive urine screen for drugs of abuse). Approximately half of the subjects were men (53.2%), most were White (91.5%), mean age was 31.9 years (range 19–50 years) and the mean body mass index was 24.4 kg m−2 (range 18.8–28.4 kg m−2). The number of subjects with sufficient data to calculate both AUC and Cmax on days 1 and 5 were 35 and 43, respectively.
Pharmacokinetics
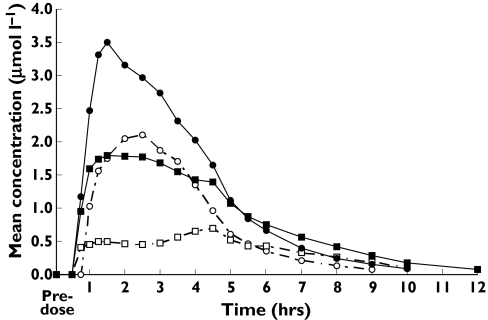
On days 1 and 5, Cmax and AUC were lower when esomeprazole was administered under fed vs. fasting conditions; however, the effect of meal timing was not as great on day 5 as that observed on day 1 (Figure 1; Table 1). The 90% CIs for the ratio of fed vs. fasting AUC and Cmax values were outside the range of 0.80–1.25 on days 1 and 5; therefore, the two regimens were not bioequivalent (Table 1). On days 1 and 5, tmax and t1/2 values were longer under fed conditions (Table 1).
Figure 1.
Mean plasma esomeprazole concentration vs. time on days 1 and 5 for subjects (N = 44) eating 15 min (fed) or 4 h (fasting) after esomeprazole administration. ( , Day 1 Fasting;
, Day 1 Fasting;  , Day 1 Fed;
, Day 1 Fed;  , Day 5 Fasting;
, Day 5 Fasting;  , Day 5 Fed)
, Day 5 Fed)
Table 1.
Pharmacokinetic parameters of esomeprazole 40 mg once daily in fed and fasting healthy adult volunteers
| Day 1 | Day 5 | |||||
|---|---|---|---|---|---|---|
| Parameter | Fed | Fasting | Ratio* | Fed | Fasting | Ratio* |
| AUC | ||||||
| n | 35 | 43 | 35 | 43 | 44 | 43 |
| µmol h−1 l−1 | 4.04 (3.2) | 6.71 (4.3) | 0.56 (0.50, 0.64) | 9.47 (3.3) | 12.31 (4.1) | 0.78 (0.74, 0.82) |
| AUC0–t | ||||||
| n | 44 | 44 | NC | 44 | 44 | NC |
| µmol h−1 l−1 | 2.80 (3.2) | 6.66 (4.1) | 9.22 (3.3) | 12.22 (4.1) | ||
| Cmax | ||||||
| n | 44 | 44 | 35 | 44 | 44 | 43 |
| µmol l−1 | 0.88 (1.1) | 3.48 (1.5) | 0.34 (0.28, 0.41) | 2.45 (1.4) | 5.37 (1.4) | 0.47 (0.41, 0.52) |
| tmax, h† | ||||||
| n | 44 | 44 | 44 | 44 | ||
| Median | 4.02 | 2.00 | — | 2.50 | 1.50 | — |
| Range | 0.77–9.00 | 1.00–4.02 | 0.77–8.00 | 0.77–4.50 | ||
| t1/2, h† | ||||||
| n | 37 | 44 | 43 | 44 | ||
| Mean | 1.53 | 1.07 | — | 1.47 | 1.28 | — |
| Range | 0.59–4.10 | 0.55–2.05 | 0.90–4.30 | 0.85–1.73 | ||
Values shown are geometric means (SD) unless otherwise indicated. AUC, Area under the plasma concentration–time curve; AUC0–t, area under the plasma concentration–time curve from time 0 to the time of last quantifiable concentration; CI, confidence interval; Cmax, maximum plasma esomeprazole concentration; tmax, time to peak esomeprazole concentration; t1/2, elimination half-life.
Ratio (90% CI) of fed to fasting calculated for those subjects with profiles from both periods.
Arithmetic mean (range) or median (range).
Discussion
The pharmacokinetic results of this study in fasting subjects are consistent with those of previous reports of the pharmacokinetics of esomeprazole administered under fasting conditions [9, 10]. In the present study of healthy volunteers, the results show that taking esomeprazole within 15 min of eating a high-fat, high-calorie meal reduced systemic drug exposure, although the reduction seemed less pronounced with repeated dosing.
Delayed gastric emptying can result in decreased absorption of the drug and, hence, lower AUC and Cmax values [11]. Meals with a high fat content slow gastric emptying [12]. A high-fat meal, i.e. a common American breakfast, was chosen for this study to provide the greatest likelihood of detecting a food effect and to mimic a situation in which gastric emptying would be expected to be delayed [12]. The 15-min interval between esomeprazole administration and feeding was chosen to approximate patterns of real-life use by patients.
Inhibition of intragastric acid secretion by esomeprazole increases with higher exposure (AUC) [3]. Because a direct relationship exists between plasma AUC and the antisecretory effects of PPIs, it might be expected that administration of esomeprazole with food would decrease acid suppression [3, 4]. However, Junghard et al.[4] have found that food has no significant effect on the percentage of time that intragastric pH is >4.0, even though AUC and Cmax are decreased. Furthermore, Junghard et al.[4] reported that the relative decrease in Cmax was more pronounced than that of AUC; therefore, the plasma concentration profile was more extended/wider, indicating a longer duration with esomeprazole exposure, which may explain the lack of food effect on the percentage of time with pH > 4.0. Food activates proton pumps, which results in acid secretion, but also buffers gastric acid, which may increase the therapeutic effect of a PPI. The clinical effect of food may be a balance of all of these factors, and it is not possible to know definitively based on the results of this study.
The acid labile nature of esomeprazole may explain the decreased bioavailability. Under fed conditions, food delays gastric emptying (prolonged tmax) and esomeprazole degradation increases with increased time in the stomach. The increased bioavailability on day 5 vs. day 1 may be a result of reduced gastric acidity due to the antisecretory effect of esomeprazole and/or a decreased delay in gastric emptying on day 5 vs. day 1.
In conclusion, administration of food 15 min after dosing with esomeprazole reduces bioavailability on days 1 and 5 of dosing in healthy volunteers.
Competing interests: This study was conducted and funded by AstraZeneca LP, Wilmington, DE, USA; all authors are employees of AstraZeneca LP. The authors thank the principal investigator, James C. Kisicki MD (MDS Pharma Services, Lincoln, NE, USA), the study site staff and the study subjects for their participation; Michael Theisen PhD, Lisa M. Klumpp PhD and Judy Fallon PharmD (Thomson Scientific Connexions, Newtown, PA, USA) for medical writing services (funded by AstraZeneca LP); and Mary Wiggin (AstraZeneca LP) for editorial assistance.
References
- 1.Richter JE, Kahrilas PJ, Johanson J, Maton P, Breiter JR, Hwang C, Marino V, Hamelin B, Levine JG for the Esomeprazole Study Investigators. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–65. doi: 10.1111/j.1572-0241.2001.3600_b.x. [DOI] [PubMed] [Google Scholar]
- 2.Fennerty MB, Johanson JF, Hwang C, Sostek M. Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitis. Aliment Pharmacol Ther. 2005;21:455–63. doi: 10.1111/j.1365-2036.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersson T, Röhss K, Bredberg E, Hassan-Alin M. Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Aliment Pharmacol Ther. 2001;15:1563–9. doi: 10.1046/j.1365-2036.2001.01087.x. [DOI] [PubMed] [Google Scholar]
- 4.Junghard O, Hassan-Alin M, Hasselgren G. The effect of the area under the plasma concentration vs time curve and the maximum plasma concentration of esomeprazole on intragastric pH. Eur J Clin Pharmacol. 2002;58:453–8. doi: 10.1007/s00228-002-0502-1. [DOI] [PubMed] [Google Scholar]
- 5.Sostek MB, Chen Y, Skammer W, Winter H, Zhao J, Andersson T. Esomeprazole administered through a nasogastric tube provides bioavailability similar to oral dosing. Aliment Pharmacol Ther. 2003;18:581–6. doi: 10.1046/j.1365-2036.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association Declaration of Helsinki: Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects. Ferney-Voltaire, France: WMA; 1989. Available at http://www.wma.net. [Google Scholar]
- 7.European Agency for the Evaluation of Medicinal ProductsInternational Conference on Harmonisation–World Health Organization, Guideline for Good Clinical Practice. Geneva: WHO; 2002. Available at http://www.emea.eu.int ICH Topic E6. [Google Scholar]
- 8.Lagerström PO, Persson BA. Determination of omeprazole and metabolites in plasma and urine by liquid chromatography. J Chromatogr. 1984;309:347–56. doi: 10.1016/0378-4347(84)80042-0. [DOI] [PubMed] [Google Scholar]
- 9.Hassan-Alin M, Andersson T, Bredberg E, Röhss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56:665–70. doi: 10.1007/s002280000206. [DOI] [PubMed] [Google Scholar]
- 10.Hassan-Alin M, Andersson T, Niazi M, Röhss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, Y-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779–84. doi: 10.1007/s00228-004-0841-1. [DOI] [PubMed] [Google Scholar]
- 11.Benet LZ, Kroetz DL, Sheiner LB. Pharmacokinetics. the dynamics of drug absorption, distribution, and elimination. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman's: the Pharmacological Basis of Therapeutics. 9. New York: McGraw-Hill; 1996. pp. 3–28. [Google Scholar]
- 12.Mayer EA. The physiology of gastric storage and emptying. In: Johnson LR, Alpers DH, Jacobson ED, Christensen J, Walsh JH, editors. Physiology of the Gastrointestinal Tract. 3. New York: Raven Press; 1994. pp. 929–76. [Google Scholar]