Abstract
What is already known about this subject
CHF 4227 is a selective oestrogen receptor modulator (SERM) that compares favourably in efficacy and potency with raloxifene in preventing bone loss and lowering serum cholesterol concentrations in OVX rats.
The compound prevented the development of DMBA-induced mammary carcinoma in rats.
In preclinical studies, CHF 4227 differs from oestrogens and from others SERMs, such as levormeloxifene, in its lack of oestrogenic effects on uterine tissue.
What this study adds
CHF 4227 is a well-tolerated SERM for postmenopausal women dosed once daily for 28 days. It appears to effect beneficially bone tissue and serum lipids without inducing oestrogenic actions on the endometrium, negative effects on fibrinolytic system, and without worsening menopausal symptoms, in particular hot flushes.
CHF 4227 is a promising agent for the treatment of several conditions in postmenopausal women.
Aims
We evaluated the tolerability, adverse events profile, pharmacokinetics, and pharmacodynamics of CHF 4227, a new selective oestrogen receptor modulator (SERM), in healthy postmenopausal women.
Methods
Two phase I studies were conducted according to a double-bind, placebo-controlled design. Subjects were randomized to receive six single (5–400 mg) or five multiple oral doses of CHF 4227 for 28 days (5–100 mg).
Results
No vaginal bleeding and no changes in either endometrial thickness or the placenta protein 14 marker were found after 4 weeks of treatment. The compound did not induce negative effects on the fibrinolytic system. After 28 days of treatment, CHF 4227 decreased both total and LDL cholesterol concentrations (maximum decreases from baseline of 17.4% (95% CI 7.0, 27.7) and 27.6% (95% CI 9.0, 46.3), respectively). Decreases in both serum and urinary type-I C-terminal collagen telopeptide were also observed producing maximum changes of 40.6% (95% CI 29.5, 51.7), and 41.7% (95% CI 20.3, 56.8), respectively. CHF4227 (5 and 10 mg) induced near maximal oestrogen-like effects on bone markers and serum lipids without causing hot flushes. The pharmacokinetics of CHF 4227 were characterized by a slow absorption, a long elimination half-life (31–42 h after single administration) and dose linearity with respect to Cmax and AUC up to 100 mg.
Conclusions
CHF 4227 is a well-tolerated SERM when administered once daily for 28 days. It is potentially active on bone resorption and serum lipids, without affecting the endometrium and without worsening hot flushes. CHF 4227 is a promising agent for the treatment of several conditions in postmenopausal women.
Keywords: SERM, CHF 4227, single and multiple dose trial, LDL cholesterol, markers of bone metabolism
Introduction
Bone loss is accelerated in women after the menopause [1–3], often leading to osteoporosis and a subsequent increase in fracture risk [4]. Until recently, hormone replacement treatment (HRT) has been widely used for the prevention of menopausal symptoms in addition to preventing and treating osteoporosis [5]. Additionally, the epidemiological relationship between oestrogen therapy and a decrease coronary heart disease and Alzheimer's disease has encouraged a greater use of oestrogens in postmenopausal women [6–8]. Unfortunately, the benefits of oestrogens based on prospective clinical trials have not been demonstrated in the Women's Health Initiative trial [9]. In fact, although oestrogen/progesterone replacement therapy significantly decreases the incidence of vertebral and hip fractures [10], it also induces harmful effects on the cardiovascular system, the central nervous system and reproductive tissues (increase in breast cancer incidence of 26% after about 5 years treatment) [9, 11]. Moreover, the risk of ovarian cancer is increased in postmenopausal women who use unopposed oestrogens [12]. These findings on the negative effects of long-term oestrogen therapy have brought to an end the Women's Health Initiative study (originally scheduled to last 8.5 years), thus discouraging the use of HRT for long-term prevention of postmenopausal conditions.
These considerations strengthen the rationale for developing alternative approaches to osteoporosis prevention and treatment. Selective oestrogen receptor modulators (SERMs) are one therapeutic alternative to HRT for postmenopausal women, because of their potential to mimic most of the beneficial effects of oestrogens, without inducing most of their adverse effects [13]. Currently, one SERM, raloxifene, is approved for use in the prevention and treatment of osteoporosis in postmenopausal women [14, 15].
CHF 4227 (3-(4-methoxy) phenyl-4-[[4-[2-(1-piperidinyl) ethoxy] phenyl] methyl]-2H-1-benzopyran-7-ol hydrochloride) is a novel benzopyran derivative, which was identified during a search for tissue-selective oestrogens characterized by an improved agonist/antagonist activity compared with raloxifene in bone, in serum lipids, and in uterine tissue [16, 17]. The compound, administered orally before the oestrogen stimulus, is a full oestrogen antagonist on the uterus in immature rats (ED50 = 0.016 mg kg−1 day−1, which is about 25 times more potent than raloxifene) [16]. In ovariectomized rats (9–10 months old) treated orally for 4 weeks, 0.1 mg kg−1 day−1 CHF 4227 was sufficient to maintain bone mineral density at the level of sham-operated animals and to produce a marked hypocholesterolaemic effect (with mean serum cholesterol conentrations 74% lower than control rats) [16]. CHF 4227 showed a protective effect on bone and serum lipids similar to that achieved with 17α-ethynyl estradiol (EE2), whereas raloxifene was significantly less effective and about 100 times less potent [16]. Volumetric bone mineral density measured by peripheral quantitative computed tomography (pQCT), confirmed protection from bone loss in the spine and proximal femur among rats treated with CHF 4227 for 4 months [17]. This effect was associated with strong inhibition of bone resorption both histologically and biochemically. Furthermore, CHF 4227 preserved trabecular microarchitecture, analyzed by microcomputed tomography (µCT), and maintained biomechanical indices of bone strength in the spine and proximal femur [17]. CHF 4227 has a substantial advantage over EE2 and other SERMs characterized by a benzopyran structure, such as levormeloxifene, in its lack of oestrogenic effects on uterine tissue [16, 18]. The preclinical pharmacokinetics of CHF 4227 in rats and monkeys, are suggestive of once a day dosing in humans (unpublished results).
The present article describes the first two clinical studies of CHF 4227 carried out in healthy postmenopausal female subjects. The aim of these studies was to evaluate the tolerability, adverse effect profile and pharmacokinetics of CHF 4227 and to confirm its promising preclinical profile in humans.
Methods
The studies were conducted in the Clinical Pharmacology Unit of Biotrial, Rennes, France, in accordance with the amended Declaration of Helsinki in 2000 (updated in a clarification note in 2002), the Good Clinical Practice (GCP) as recommended by the International Conference on Harmonization (ICH) in 1996, and by the French Huriet-Sérusclat law on biomedical research.
Both studies were approved by the local independent Ethics Committee (Comite Consultative de Protection des Personnes dans la Recherce Biomedical), Brest Cedex, France. Before starting the studies, each participant gave her written consent.
Both trials followed a randomized, double-blind, placebo controlled design. CHF 4227 was administered to healthy postmenopausal women, as confirmed by estradiol (E2) plasma concentrations below 20 pg ml−1, follicle stimulating hormone (FSH) plasma concentrations above 40 IU l−1 and with at least a 1 year history of menstrual cycle suppression. CHF was formulated as a gelatine white capsule, characterized by 99–100% release of the drug after 120 min.
None of the subjects had concomitant disease as confirmed by a prestudy physical, gynaecological examination, blood chemistry, haematology, urinalysis and a 12-lead ECG. All subjects had a normal mammogram or ultrasound breast examination and a normal cervical smear within 1 year of the first dose of CHF 4227. Reasonable efforts were made to document all relevant treatments received by the subjects within the month preceding administration of CHF 4227. If possible, concomitant treatment was not allowed during the study. If the use of a concomitant treatment became necessary, it was recorded in the case report form. Ingestion of alcohol was not allowed 48 h before the first drug administration, throughout the inpatient period and up to the end of the study visit. Only subjects who were not taking hormone replacement therapy during the 2 months before the study were recruited.
Single ascending dose safety study
A two-rotating panel (A and B), four-period, double-blind, randomized, placebo controlled study was performed in 24 subjects with single oral escalating doses and interspersed placebo. Within each panel, a washout period of at least 2 weeks between the two doses for each subject, and an interval of at least 1 week between two incremental doses were selected. In each panel, 12 subjects were randomized to receive either CHF 4227 or placebo. Panel A subjects were administered single oral doses of 5 mg, 50 mg, 200 mg or a placebo; panel B subjects were administered single oral doses of 25 mg, 100 mg, 400 mg or a placebo. Each subject received three incremental doses of CHF 4227 and one placebo. In each period nine subjects received active treatment and three subjects received a placebo. Treatment was administered in the morning to subjects under fasting conditions.
Multiple ascending dose safety trial
A five-parallel group, dose ranging, double-blind, randomized, placebo controlled repeat dose study was performed in 56 subjects with five escalating doses administered orally once daily for 4 weeks. In each group, except at the 100 mg dose, 12 subjects were randomized to receive CHF 4227 (n = 9) or placebo (n = 3). In the 100 mg dose group, eight subjects were randomized to receive the drug (n = 6) or placebo (n = 2). The progression of administration of the, 5 mg, 10 mg, 25 mg, 50 mg and 100 mg doses was based on the safety profile of the first single ascending dose study and on pharmacokinetic considerations. Escalation to the next period was decided on a case-by-case basis, dependent upon the absence of clinical adverse effects, ECG and/or laboratory abnormalities considered to be medically significant at the previous dose.
At the Clinical Pharmacology Unit, the drug was administered once daily in the morning under fasting conditions. The subjects were asked to comply with this dosing regimen when at home.
Clinical and pharmacodynamic assessments
Vital signs (blood pressure, heart rate, body weight and temperature), ECG, haematology, blood chemistry, urinalysis and adverse events were assessed at regular intervals during both trials. All the participants had experienced episodes of hot flushes in the previous 3 months, and were able to recognize the symptoms. Their intensity, onset during the treatment period, duration and number of episodes were documented by the patient before, on day 14 and after treatment.
The intensity of the adverse events was graded using the following definitions: mild (minor discomfort that did not lead to either modification of the dosage or did not require corrective treatment), moderate (affected normal daily activity, or led to a lowering of the dosage, or a temporary interruption in its administration, or required corrective treatment), and severe (prevented normal daily activity or led to cessation of treatment).
Patients suffering an adverse event at the 28 day visit were followed up until it dissipated. The patient without adverse events was requested to report any new ones during the month after the offset of the treatment period. The concentrations of E2, the biomarkers for the hypothalamo-pituitary axis FSH and luteinizing hormone (LH), blood lipids (triglycerides, HDL, LDL and total cholesterol) and coagulation parameters such as fibrinogen, prothrombin time (PT), activated partial thromboplastin time (aPTT), and plasminogen activator inhibitor (PAI-1) were assessed in both trials. In addition to these parameters, prolactin was assessed in the single ascending dose safety study and the markers of bone resorption, serum and urinary (corrected for creatinine excretion) type-I C-terminal collagen telopeptide (CTX-S and CTX-U), were evaluated in the multiple ascending dose safety study. In addition to the physical and gynaecological examinations carried out at the beginning and at the end of both trials, endometrial safety was monitored in the multiple ascending dose safety study by measuring endometrial thickness using transvaginal ultrasonography performed with endovaginal probes operating at a frequency of 5–7 MHz (thickness sensitivity 1 mm), and a placental protein 14 assay (PP14, a well established surrogate marker of SERM activity on the postmenopausal endometrium) [19]. PP14 was determined by radioimmunoassay; the detection limit was 0.3 µg l−1 and intra-assay and interassay variations were 4 and 10%, respectively. A thickening of more than 4 mm, confirmed by hysterosonography, led to withdrawal of the subject from the study.
Blood and urine sampling
In the single ascending dose study, blood samples were collected before and at 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, 60, 72, 96, 120 h after dosing. Urine samples were taken at 24 h intervals up to 120 h.
In the multiple dose trial, blood after the first dose on day 1 was drawn predose and at the same times as the single dose study up to 24 h. Pre-dose blood samples were also collected on days 2, 3, 6, 14 and 21. The final dose was administered on day 28 and blood samples were drawn at the same times as day 1 up to 24 h, with additional sampling at 48, 72, 96, 120 and 168 h post dose. Urine samples were taken at 12 h intervals up to 24 h on day 1 and on day 28.
Drug and metabolite analysis
Free CHF 4227 in plasma and free and total (free + conjugated) drug in urine was extracted using with Oasis HLB solid phase cartridges (30 mg ml−1). The extraction was carried out with an automated sample processor (Model ASPEC XL, Gilson). The cartridges were conditioned with 1 ml methanol, 1 ml milli-Q deionized water, and 0.5 ml 0.5 m sodium phosphate buffer pH 11.7. Plasma and urine samples (600 µl) to which the internal standard (the deuterated drug) and 1 ml 0.5 m sodium phosphate buffer pH 11.7 had been added, were loaded on the cartridges. Potentially co-eluting compounds were washed out with 3 ml of 0.25 m sodium phosphate buffer pH 11.7/methanol (70 : 30 v : v) and 0.5 ml of water. Elution of the analyte was performed with 0.3 ml of methanol acidified with formic acid (final pH = 2.2). The eluted samples were then evaporated under nitrogen flow to dryness and reconstituted with 0.3 ml of methanol acidified with formic acid (final pH = 2.2), and 10 µl was injected onto the HPLC/MS/MS system.
For the determination of total (free + conjugated) CHF 4227 in urine, 600 µl of urine was added to 100 µl of 10 mg ml−1β-glucuronidase enzyme solution and 50 µl of 1 m sodium phosphate pH 5.9 buffer. Samples were incubated at 37°C for 16 h. After the incubation, samples were vortex mixed, loaded onto the automated sample processor, and processed as described previously for free CHF 4227.
For the determination of total CHF 4227 in plasma, samples (250 µl) were added to 25 µl of 1 m sodium phosphate pH 5.9 buffer and 50 µl of 10 mg ml−1β-glucuronidase enzyme solution, and incubated at 37°C for 16 h. Twenty-five µl of deuterated internal standard and of 375 µl of CH3CN acidified with formic acid (final pH = 2.2) were added to the incubates, followed by vortex mixing and centrifugation at 17 800 g. An aliquot (5 µl) of the supernatant was injected into the HPLC/MS/MS.
The lower limits of determination were 50 and 500 pg ml−1 for free and total CHF 4227 in plasma, respectively, and 50 pg ml−1 for both free and total drug in urine. Inter- and intra-assay coefficients of variation were less than 15% for both free and total CHF 4227 analysis.
Pharmacokinetic analysis
Pharmacokinetic analysis was performed using noncompartmental methods and with WinNonlin Professional 4.0 software (Pharsight Corporation, Cary, North Carolina, USA). Cmax, and tmax were obtained directly from the plasma concentration data. The apparent elimination rate constant, λz, was estimated by log-linear regression analysis from the terminal phase of the plasma concentration vs. time curve, taking at least the final three sampling points with concentrations equal to or greater than the limit of determination. The terminal half life, t1/2, was calculated from the expression ln2/λz. In the single dose study, the area under the plasma vs. time curve was calculated using the linear trapezoidal rule from time 0 to the last measurable concentration (AUC(0,last)). In the multiple ascending dose trial, after the first dose AUC(τ) was calculated, with τ equal to 24 h (one dosing interval) and at steady state AUCss was determined, calculated from time 0 up to the last measurable data point in the dosing interval using the trapezoidal method. The accumulation ratio (Rac) was calculated as the repeat dose AUCss to single dose AUC(0,24 h), ratio on day 28. The peak trough fluctuation (PTF%), indicating the degree of change of plasma concentrations at steady state, was calculated from the expression 100 × (Css,max – Css,min)/Css,average.
Statistical analysis
Safety and pharmacodynamic data were analyzed by the Data Management and Biostatistics Unit of BIOTRIAL using SAS® software, version 8.2 (SAS institute Inc. Cary NC USA). In the single ascending dose safety study, descriptive statistics were determined with respect to panel, treatment and time. In the multiple ascending dose study, the comparison of treatments at baseline were tested using one-way anova. Probability values of <0.05 were considered to be statistically significant. Post-dose values were compared between treatments by covariance analysis with baseline as the covariate. When treatment effect was significant, comparisons of the mean values at the different doses of CHF 4227 from those obtained during placebo treatment were performed using Dunnett's test. For CTX-S, CTX-U and blood lipids, the overall percent decrease during drug treatment compared with baseline was calculated with 95% confidence intervals.
Pharmacokinetic data were analyzed using WinNonlin 4.0 software (Pharsight Corporation, Cary, North Carolina, USA). Dose proportionality for CHF 4227 AUC(0,last) and Cmax was assessed after natural logarithmic transformation of parameters, and using Prism 4 for Windows software (version 4.03).
Results
Tolerability and adverse event profile
Single ascending dose study
No serious or severe adverse events were recorded. CHF 4227 did not produce any clinically significant change in blood pressure, ECG intervals or laboratory test parameters compared with placebo. A total of 57 treatment emergent adverse events (TEAEs) were observed in 15 subjects; all were mild in intensity (except for one case of vomiting and four of postural hypotension, which were classified as moderate) and all the subjects recovered spontaneously. No maximum tolerated dose (dose at which in more than 30% of haematology and blood chemistry profiles were significantly modified) was identified, and no differences between the adverse event profiles, with respect to either frequency or type of event, after CHF 4227 or placebo were observed. The TEAEs did not appear to be dose-related. The most frequent in subjects taking CHF 4227 were hot flushes (16.7%), and leucorrhoea (8.3%). In subjects randomized to placebo the frequency of hot flushes and leucorrhoea was the same (16.7% and 8.3%, respectively) as that in patients taking CHF 4227.
Multiple ascending dose study
Six of the 56 subjects did not complete the study. One taking placebo withdrew her consent 3 days after inclusion for personal reasons, and five withdrew because of the following events, all diagnosed on day 14 during the planned transvaginal ultrasound scan: adrenocortical carcinoma of the left kidney (one subject), uterine polyps (two subjects), both classified as being unrelated to CHF 4227, a localized thickening of the endometrium (one subject) not confirmed after hysteroscopy, fibro-glandular cystic formation in the endometrium (one subject), whose relationship to treatment was unlikely considering the size, the very low cell proliferation index (1% as measured by immunohistochemical analysis), the time elapsed since the beginning of treatment (14 days), and the otherwise healthy status of the endometrium (the thickness and the PP14 marker value were within normal values). A total of 141 TEAEs were reported (90 in 39 subjects administered CHF4227 and 51 in 11 subjects given placebo). All TEAEs were of mild to moderate intensity. The most frequent (Table 1) were musculoskeletal and connective tissue disorders (mostly muscle cramps and myalgia), reproductive system and breast disorders (mostly leucorrhoea), nervous system disorders (headaches), gastrointestinal disorders (abdominal pain), and vascular disorders (hot flushes). No clear treatment or dose-dependent relationship could be established, as the TEAEs occurred to the same extent in patients given CHF 4227 and placebo. Subjects treated with 5 and 10 mg CHF 4227 did not experience hot flushes (Table 1).
Table 1.
Adverse events reported after repeated dose administration of CHF 4227, expressed as number of treatment emergent adverse events (AE), number (n) and percentage (%) of subjects with at least one adverse event
| CHF 4227 dose | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg (n = 9) | 10 mg (n = 9) | 25 mg (n = 9) | 50 mg (n = 9) | 100 mg (n = 6) | Placebo (n = 14) | |||||||
| AE | n | AE | n | AE | n | AE | n | AE | n | AE | n | |
| Overall | 10 | 8 | 14 | 8 | 24 | 8 | 37 | 9 | 10 | 6 | 51 | 11 |
| (88.9%) | (88.9%) | (88.9%) | (100.0%) | (100.0%) | (78.8%) | |||||||
| Abdominal pain | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 6 | 2 |
| (11.1%) | (11.1%) | (11.1%) | (0.0%) | (0.0%) | (14.3%) | |||||||
| Muscle cramp | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 10 | 3 |
| (11.1%) | (11.1%) | (0.0%) | (11.1%) | (0.0%) | (21.4%) | |||||||
| Myalgia | 0 | 0 | 1 | 1 | 5 | 4 | 1 | 1 | 0 | 0 | 5 | 3 |
| (0.0%) | (11.1%) | (44.4%) | (11.1%) | (0.0%) | (21.4%) | |||||||
| Headache | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 2 | 2 | 5 | 3 |
| (11.1%) | (11.1%) | (33.3%) | (33.3%) | (33.3%) | (21.4%) | |||||||
| Leucorrhoea | 2 | 2 | 0 | 0 | 6 | 4 | 2 | 2 | 3 | 1 | 5 | 4 |
| (22.2%) | (0.0%) | (44.4%) | (22.2%) | (16.7%) | (28.6%) | |||||||
| Hot flushes | 0 | 0 | 0 | 0 | 2 | 2 | 6 | 4 | 1 | 1 | 4 | 2 |
| (0.0%) | (0.0%) | (22.2%) | (44.4%) | (16.7%) | (14.3%) | |||||||
CHF 4227 did not produce clinically significant changes in vital signs (except for one case of symptomatic postural hypotension), ECG intervals or laboratory test values compared with placebo. There were no statistically significant changes in endometrial thickness and PP14 when comparing placebo and treatment groups at any dose on day 14 and 29 (data not shown). No vaginal bleeding was observed in women taking CHF 4227.
Statistically significant decreases in fibrinogen concentration after 5 and 10 mg CHF 4227 treatment were observed at the final visit (Table 2). No clinically or statistically significant change in PT or aPTT was found (data not shown). Moreover there was no clinically or statistically significant change in PAI 1 concentration, a finding that excluded any profibrinolytic activity of CHF 4227 (data not shown).
Table 2.
Effect of CHF 4227 on fibrinogen concentrations after 4 weeks of treatment. Baseline, day 29 values, absolute and % changes from baseline (mean ± SEM), least square means (LSM) of the difference from placebo and its 95% confidence intervals (95% CI) are reported
| CHF 4227 dose | ||||||
|---|---|---|---|---|---|---|
| Fibrinogen (g l−1) | 5 mg | 10 mg | 25 mg | 50 mg | 100 mg | Placebo |
| Baseline | 3.30 ± 0.24 | 3.31 ± 0.15 | 3.38 ± 0.19 | 3.51 ± 0.15 | 3.66 ± 0.24 | 3.45 ± 0.16 |
| Day 29 | 2.96 ± 0.18** | 3.00 ± 0.13* | 3.51 ± 0.23 | 3.28 ± 0.14 | 3.62 ± 0.26 | 3.69 ± 0.11 |
| Absolute change | −0.34 ± 0.13 | −0.31 ± 0.13 | 0.10 ± 0.15 | −0.24 ± 0.12 | 0.05 ± 0.42 | 0.17 ± 0.14 |
| % change | −9.14 ± 3.61 | −8.84 ± 4.19 | 3.31 ± 4.06 | −6.31 ± 3.70 | 3.11 ± 11.03 | 6.67 ± 4.59 |
| LSM difference | −0.73** | −0.69* | −0.18 | −0.42 | −0.07 | |
| 95% CI | −1.32, −0.14 | −1.31, −0.08 | −0.75, 0.39 | −1.00, 0.17 | −0.76, 0.62 | |
Statistically significant difference between active and placebo, P < 0.05 and P < 0.01, respectively, Dunnett's test.
Pharmacodynamics
The single dose administration of CHF 4227 had no significant effect on LH, E2 or prolactin. Whereas FSH concentrations tended to remain stable or to increase during placebo treatment, the opposite was observed for CHF 4227, when decreases from baseline were observed 24 and 48 h after administration. Despite these variations, all FSH values remained within the normal range (data not shown). In the multiple dose study there was no significant change in E2, LH and FSH concentrations (not shown).
Total cholesterol concentrations decreased between baseline and day 29 following CHF 4227 with a mean %change ranging from 13.1% (95% CI 6.1, 20.2) at 50 mg to 17.4% (95% CI 7.0, 27.7) at 5 mg (Table 3). Statistical significance was found for the 5 mg and 100 mg doses when compared with placebo. LDL cholesterol concentrations decreased between baseline and day 29 after CHF 4227 for all doses with a mean % change ranging from 17.1% (95% CI 13.2, 21.0) at 25 mg and 27.6% (95% CI 9.0, 46.3) at 100 mg. The treatment effect was significant with respect to placebo at all doses except 25 mg (Table 3). HDL cholesterol and triglyceride concentrations remained unchanged throughout the study (Table 3).
Table 3.
Effect of CHF 4227 on total cholesterol, HDL cholesterol, LDL cholesterol and triglyceride concentrations after 4 weeks of treatment. Baseline, day 29 values, absolute and % changes from baseline (mean ± SEM), least square means (LSM) of the difference from placebo ant its 95% confidence intervals (95% C.I) are reported
| CHF 4227 dose | |||||||
|---|---|---|---|---|---|---|---|
| 5 mg | 10 mg | 25 mg | 50 mg | 100 mg | Placebo | ||
| Total cholesterol (mmol l−1) | Baseline | 6.93 ± 0.34 | 6.79 ± 0.34 | 6.51 ± 0.39 | 6.00 ± 0.20 | 5.62 ± 0.50 | 6.26 ± 0.25 |
| Day 29 | 5.64 ± 0.20* | 5.70 ± 0.37 | 5.51 ± 0.39 | 5.21 ± 0.26 | 4.70 ± 0.20* | 5.92 ± 0.18 | |
| Absolute change | −1.29 ± 0.32 | −1.09 ± 0.13 | −1.00 ± 0.09 | −0.79 ± 0.20 | −0.88 ± 0.39 | −0.35 ± 0.14 | |
| % change | −17.4 ± 4.11 | −15.7 ± 2.12 | −15.7 ± 1.61 | −13.1 ± 2.99 | −14.1 ± 4.93 | −4.79 ± 2.18 | |
| LSM difference | −0.72* | −0.56 | −0.57 | −0.53 | −0.75* | ||
| 95% CI | −1.34; −0.09 | −1.20; 0.07 | −1.15; 0.01 | −1.13; 0.07 | −1.47; −0.02 | ||
| HDL cholesterol (mmol l−1) | Baseline | 2.15 ± 0.17 | 1.86 ± 0.20 | 1.83 ± 0.20 | 1.58 ± 0.11 | 1.62 ± 0.13 | 1.91 ± 0.14 |
| Day 29 | 1.71 ± 0.14 | 1.70 ± 0.17 | 1.58 ± 0.16 | 1.66 ± 0.18 | 1.68 ± 0.22 | 1.65 ± 0.12 | |
| Absolute change | −0.41 ± 0.12 | −0.16 ± 0.11 | −0.26 ± 0.10 | 0.09 ± 0.19 | 0.06 ± 0.12 | −0.25 ± 0.05 | |
| % change | −17.4 ± 6.62 | −6.5 ± 5.48 | −13.% ± 3.97 | 8.7 ± 14.36 | 2.7 ± 8.27 | −12.67 ± 2.81 | |
| LSM difference | −0.09 | 0.08 | −0.02 | 0.25 | 0.24 | ||
| 95% CI | −0.46; 0.28 | −0.30; 0.46 | −0.37; 0.33 | −0.12; 0.62 | −0.20; 0.67 | ||
| LDL cholesterol (mmol l−1) | Baseline | 4.23 ± 0.25 | 4.36 ± 0.30 | 4.14 ± 0.38 | 4.01 ± 0.26 | 3.54 ± 0.57 | 3.91 ± 0.24 |
| Day 29 | 3.20 ± 0.17** | 3.50 ± 0.28* | 3.48 ± 0.36 | 3.08 ± 0.17** | 2.52 ± 0.36** | 3.81 ± 0.21 | |
| Absolute change | −1.03 ± 0.25 | −0.86 ± 0.17 | −0.67 ± 0.07 | −0.94 ± 0.25 | −1.02 ± 0.38 | −0.10 ± 0.12 | |
| % change | −22.7 ± 5.23 | −19.4% ± 3.63 | −17.1 ± 1.66 | −24.0 ± 4.59 | −27.6 ± 6.72 | −1.08 ± 3.69 | |
| LSM difference | −0.82** | −0.60* | −0.48 | −0.80** | −1.05** | ||
| 95% CI | −1.37; −0.25 | −1.19; −0.01 | −1.02; 0.05 | −1.35; −0.24 | −1.70; −0.38 | ||
| Triglycerides (mmol l−1) | Baseline | 1.22 ± 0.11 | 1.34 ± 0.27 | 1.14 ± 0.13 | 0.92 ± 0.08 | 1.11 ± 0.12 | 0.99 ± 0.08 |
| Day 29 | 1.25 ± 0.03 | 1.11 ± 0.08 | 0.99 ± 0.11 | 1.05 ± 0.14 | 0.92 ± 0.10 | 1.01 ± 0.07 | |
| Absolute change | 0.04 ± 0.11 | −0.23 ± 0.20 | −0.14 ± 0.11 | 0.13 ± 0.08 | −0.08 ± 0.07 | −0.09 ± 0.15 | |
| % change | 13.1 ± 11.0 | −6.00 ± 11.43 | −9.94 ± 8.27 | 12.04 ± 7.30 | −8.19 ± 7.23 | −0.05 ± 11.93 | |
| LSM difference | 0.38 | −0.05 | −0.08 | 0.08 | 0.17 | ||
| 95% CI | −0.10; 0.85 | −0.55; 0.44 | −0.54; 0.37 | −0.38; 0.55 | −0.38; 0.71 | ||
Statistically significant difference between active and placebo, P < 0.05 and P < 0.01, respectively, Dunnett's test.
Decreases in both CTX-S and CTX-U concentrations were observed between baseline and day 29, with a mean % change of 40.6% (95% CI 29.5, 51.7) at 10 mg and 41.7% (95% CI 20.3, 56.8) at 50 mg, compared with 13.1% (95% CI 0.2. 26.5) and 13.8% (95% CI 3.8, 23.7) after placebo (Figure 1). A statistical significant difference in this change was found between 50 mg CHF 4227 and placebo.
Figure 1.
Metabolic bone marker (CTX-S and CTX-U) variations during 4 weeks of CHF 4227 administration, expressed as a % of baseline values (mean ± SEM). *Statistically significant difference between CHF 4227 and placebo after 28 days of treatment, P < 0.05, Dunnett's test. 5 mg (▪), 10 mg (□), 25 mg (▴), 50 mg (▵), 100 mg (•), Placebo (○)
Pharmacokinetics
Single ascending dose study
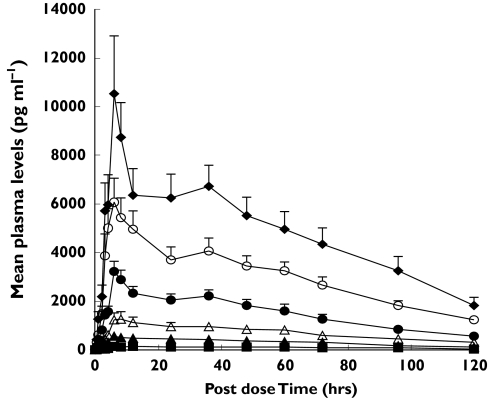
The pharmacokinetic parameters for CHF 4227 and the plasma concentration-time data are reported in Table 4 and in Figure 2. At all doses CHF 4227 was absorbed slowly with a median tmax ranging from 6 to 18 h. The peak plasma concentration decreased slowly and the mean elimination half-life ranged from 31 h to 42 h. Plasma CHF 4227 concentrations were found to be proportional to the dose (Cmax, r2 = 0.9906 and P < 0.0001; AUC(0,24 h), r2 = 0.9823 and P < 0.0001). Less than 0.02% of CHF 4227 was eliminated as unchanged drug in urine over 120 h after administration. Renal clearance was less than 0.2 l h−1.
Table 4.
Pharmacokinetic parameters for CHF 4227 after administration of single oral doses. Data are shown as mean ± SEM
| CHF 4227 dose | ||||||
|---|---|---|---|---|---|---|
| 5 mg | 25 mg | 50 mg | 100 mg | 200 mg | 400 mg | |
| Cmax (ng ml−1) | 0.22 ± 0.09 | 0.62 ± 0.17 | 1.46 ± 0.61 | 3.40 ± 1.59 | 6.78 ± 1.87 | 11.53 ± 2.66 |
| tmax (h)# | 18.0 (1.0–96.0) | 7.0 (3.0–60.0) | 8.0 (6.0–60.0) | 6.0 (6.0–36.0) | 6.0 (3.0–72.0) | 6.0 (1.0–60.0) |
| t1/2 (h) | 31.0 ± 27.7 | 33.5 ± 21.8 | 42.2 ± 32.8 | 34.5 ± 23.9 | 37.3 ± 33.2 | 33.6 ± 13.8 |
| (n = 2) | (n = 7) | (n = 9) | (n = 9) | (n = 7) | (n = 9) | |
| AUC(0,last) (ng ml−1 h) | 11.5 ± 3.1 | 36.9 ± 6.7 | 85.3 ± 41.2 | 181.3 ± 75.2 | 362.7 ± 134.4 | 575.2 ± 158.1 |
| AUC(0,∞) (ng ml−1 h) | * | 51.5 ± 31.4 | 103.5 ± 48.6 | 202.8 ± 92.5 | 475.9 ± 103.9 | 651.1 ± 286.5 |
| (n = 7) | (n = 9) | (n = 9) | (n = 7) | (n = 9) | ||
Median and range
not calculated.
Figure 2.
Mean plasma profiles for CHF 4227 in 12 postmenopausal women after single escalating doses of the drug (mean ± SEM). 5 mg (▪), 25 mg (▴), 50 mg (▵), 100 mg (•), 200 mg (○), 400 mg (♦)
Multiple ascending dose study
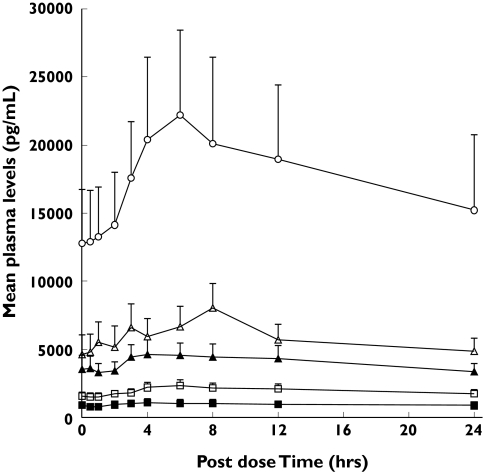
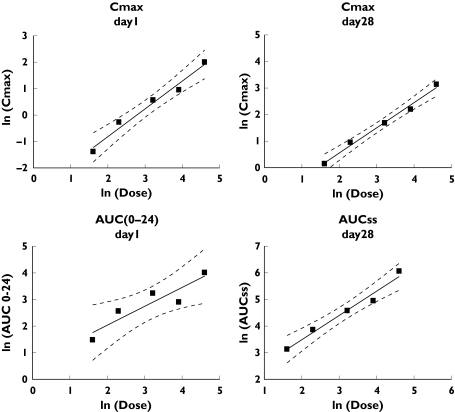
Steady state concentrations of CHF 4227 were reached 6–14 days after the start of dosing. Absorption was slow with tmax ranging from 4 to 12 h (Table 5 and Figure 3). As observed in the single dose study, the elimination half-life was not dose-dependent, mean values ranging between 35 and 53 h. The accumulation index was significantly greater than 1 (ranging between 3.3 and 9.8). The fluctuations of the steady-state concentrations, represented by the PTF% index, ranged from 52 to 99%, indicating a large variability between patients and doses. The rate and extent of exposure of women to CHF 4227 generally increased proportionately with increasing dose (Figure 4) (Cmax slope = 1.05, 95% CI 0.75, 1.35, r2 = 0.98; AUC(0,24 h) slope = 0.71, 95% CI 0.15, 1.27, r2 = 0.84 on day 1 and Cmax slope = 0.95, 95% CI 0.77, 1.13, r2 = 0.99; AUC(0,24 h) slope = 0.91, 95% CI 0.63, 1.19, r2 = 0.97 on day 28). Less than 0.005% of CHF 4227 was eliminated as unchanged drug in urine on day 1 and less than 0.009% on day 28. Renal clearance was less than 1 ml min−1. Conjugated CHF 4227 plasma concentrations were about 10 times greater than those of parent compound, and urinary excretion of the former was about 0.3% of the dose.
Table 5.
Pharmacokinetic parameters for CHF 4227 after administration of repeated oral doses. Data are shown as mean ± SEM
| CHF 4227 dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 mg | 10 mg | 25 mg | 50 mg | 100 mg | ||||||
| Day 1 | Day 28 | Day 1 | Day 28 | Day 1 | Day 28 | Day 1 | Day 28 | Day 1 | Day 28 | |
| Cmax (ng ml−1) | 0.25 ± 0.031 | 1.16 ± 0.28 | 0.76 ± 0.12 | 2.59 ± 0.42 | 1.76 ± 0.35 | 5.37 ± 3.23 | 2.58 ± 0.39 | 9.02 ± 1.75 | 7.37 ± 1.79 | 22.97 ± 5.93 |
| tmax (h)# | 6.0 | 4.0 | 12.0 | 6.0 | 8.0 | 4.0 | 6.0 | 6.0 | 6.0 | 6.0 |
| (3.0–24.0) | (0–6.0) | (3.0–24.0) | (2.0–8.0) | (3.0–12.0) | (0–6.0) | (2.0–12.0) | (1.0–8.0) | (4.0–6.0) | (3.0–6.0) | |
| t1/2 (h) | – | 52.3 | – | 51.8 | – | 53.2 | – | 35.4 | – | 47.0 |
| AUC(0,24 h) (ng ml−1 h) | 4.4 ± 0.6 | 22.9 ± 6.2 | 13.0 ± 2.1 | 47.6 ± 7.5 | 25.4 ± 4.1 | 97.3 ± 19.9 | 18.3 ± 3.1 | 140.6 ± 28.6 | 55.1 ± 11.7 | 429.6 ± 130.0 |
| Rac | – | 5.04 | – | 3.26 | – | 3.9 | – | 9.83 | – | 7.28 |
| PTF (%) | 52.1 | 61.8 | 65.8 | 98.6 | 65.8 | |||||
Median and range on day 28; AUC(0,24 h) stands for AUCss.
Figure 3.
Mean plasma concentration-time profiles for CHF 4227 in nine postmenopausal women on day 28 after repeated doses of the drug (mean ± SEM). 5 mg (▪), 10 mg (□), 25 mg (▴), 50 mg (▵), 100 mg (○)
Figure 4.
Assessment of dose proportionality for CHF 4227 Cmax, on day 1 and on day 28 and for AUC(0,24 h), on day 1 and AUCss on day 28
Discussion
SERMs are one alternative to oestrogen-replacement therapy for postmenopausal women. Raloxifene, the only SERM approved for the treatment and prevention of osteoporosis, increases bone mineral density with a consequent significant decrease in vertebral fractures [14, 20]. It is also effective in lowering the incidence of breast cancer [21], and in normalizing the lipid profile in a manner that may be favourable to cardiovascular disease prevention, without stimulating the endometrium in postmenopausal women [14, 22, 23].
However, raloxifene is less effective than other inhibitors of bone resorption, in that it increases bone mineral density of the spine by only 1.5–2% [14] compared with 4–5% for oestrogen replacement therapy [10], and 7–9% for bisphosphonates [24]. In addition, raloxifene has been shown to induce menopausal symptoms in many patients, especially hot flushes [14, 20].
CHF 4227 is a new orally active nonsteroidal oestrogen agonist/antagonist currently under development for the prevention and treatment of postmenopausal disorders. Preclinical studies have shown that CHF 4227 compares favourably in efficacy and potency to raloxifene in preventing bone loss, lowering serumcholesterol concentrations, and antagonizing oestrogen stimulation of the uterus [16, 17]. Moreover, results obtained using the DMBA-induced mammary tumour model indicate that CHF 4227 has significant chemopreventive effects [16].
Single and multiple dose (28 days of regular treatment) clinical studies were conducted to evaluate the tolerability, adverse event profile and pharmacokinetics of CHF 4227, and to confirm its preclinical pharmacodynamic characteristics. In the single dose study there were no differences between the adverse event profiles after CHF 4227 and placebo even at the highest dose of 400 mg. In particular, CHF 4227 did not increase leucorrhoea and hot flushes when compared with placebo.
In the 28-day treatment study, CHF 4227 at daily doses from 5 to 100 mg was found to be well tolerated in postmenopausal women, at least up to 1 month after the last dose. All adverse events were mild and reversible, and none was dose related. There was no clinically significant abnormality in any of the haematology, blood chemistry or urinalysis parameters investigated. Furthermore, there were no clinically significant changes in vital signs or the ECG in subjects receiving CHF 4227.
CHF 4227 showed no significant effect on FSH, LH or estradiol plasma concentrations. The absence of an effect on plasma oestrogen concentrations with CHF 4227 seems to be unique to this compound, as an increase in estradiol concentrations has been reported during tamoxifene, raloxifene and ospemifene administration [23, 25, 26].
Preclinical investigations in rodents showed that CHF4227 had no stimulatory effects on the endometrium [16]. In the present study, transvaginal ultrasonography, hysterosonography and serum placenta protein 14 (a well established biomarker of the effect SERM on the postmenopausal endometrium) [19] were used to evaluate the potential oestrogenic effect of CHF 4227 on uterine tissue. No statistically significant changes in both endometrial thickness and the PP 14 marker were found between placebo and any dose of CHF 4227 on day 14 and 29. Furthermore, no vaginal bleeding was observed in women taking CHF 4227. These results suggest that CHF 4227 is not uterothrophic after daily dosing of 5–100 mg for 4 weeks, confirming the preclinical data.
In the current study, CHF 4227 administered for 28 days decreased both total and LDL cholesterol concentrations. The effect on total cholesterol was significant with respect to placebo at 5 and 100 mg, whereas the decrease in LDL-cholesterol was statistically significant at 5, 10, 50 and 100 mg. These changes were greater than those reported during raloxifene therapy (6–11% reduction from baseline) [27]. Decreases in CTX-S and CTX-U concentrations following CHF 4227 administration were also observed
The lowering effect of raloxifene on serum lipids and biochemical markers of bone metabolism is apparent only after 3–6 months of regular dosing [14]. In addition ERA-923, a SERM under clinical investigation for use in tamoxifen refractory metastatic breast cancer, does not affect serum lipid and markers of bone resorption after 28 days of treatment [28]. Previous studies have also shown decreased LDL and/or total cholesterol after tamoxifen treatment for 2 months or longer [29–32]. Thus, CHF 4227 appears to be the first SERM capable of significantly decreasing plasma lipids and markers of bone turnover after 1 month of treatment. Although this potentially beneficial effect on bone tissue and on cardiovascular risk will require confirmation by appropriately designed clinical trials, the bone resorption and lipid lowering activity indicates that CHF 4227 has therapeutic potential in postmenopausal women.
CHF 4227 administration did not modify PTT and PAI 1, confirming the absence of profibrinolytic effects. However, a decrease in fibrinogen concentrations was observed. Therefore, these results suggest that CHF 4227 is free from the detrimental effects on the fibrinolytic system, which have been reported with oestrogens and raloxifene [9, 33].
After 28 days of dosing with 5 and 10 mg of CHF 4227 the mean steady state concentrations of the drug were 1.16 and 2.59 ng ml−1, respectively. Using oestrogen depleted OVX rats, a dose of 0.1 mg kg−1 day−1 of CHF 4227 p.o. for 4 weeks, corresponding to a mean steady state plasma concentration of 500–600 pg ml−1, was sufficient to maintain bone mineral density at the sham level and to lower plasma cholesterol concentrations significantly [16]. Assuming that the effects on bone tissue and plasma lipids are correlated with steady-state concentrations and are species independent, an effective human daily dose should be about 2–4 mg of CHF 4227. In line with this hypothesis, a significant effect on total and LDL-cholesterol, and a trend towards a decrease in bone turnover were observed with CHF 4227 at 5 mg daily.
Hot flushes are thought to be the result of disturbances of thermoregulation within the hypothalamus. Oestrogens are used to treat hot flushes, indicating that in the CNS an oestrogen agonist activity is necessary to decrease vasomotor disturbances. In contrast, raloxifene given at 60 mg day−1 (the approved human daily dose) significantly increased hot flushes, particularly in early postmenopausal women [14, 20, 27]. Thus, raloxifene at therapeutic doses is unable to discriminate between the oestrogen agonist effect on bone and serum lipids and the oestrogen antagonist effect on vasomotor symptoms. In the present study, subjects treated with 5 and 10 mg CHF 4227 did not experience hot flushes. Although we are aware that phase I studies are not designed to and do not have enough power to compare the prevalence and frequency of hot flushes between groups (because of a low sample size) and that hot flushes are highly variable in their frequency during the day, the effects observed on vasomotor symptoms suggest that CHF 4227, at a putative therapeutic dose, induces evident oestrogen-like effects on bone markers and serum lipids without producing an increased frequency of hot flushes. Whether this selectivity might be due to a unique structural alteration induced by CHF 4227 within the oestrogen receptor in the hypothalamus or by other mechanisms (e.g. differences in tissue distribution) is not clear, and will be the subject of future investigations, involving, long-term clinical trials.
The pharmacokinetics of CHF 4227 showed proportionality with dose over the range studied after single and repeated administration of the drug. The long elimination half-life of CHF 4227 explains why its accumulation index was significantly greater than 1. Conjugated plasma CHF 4227 concentrations after enzymatic hydrolysis were about 10 times higher than those of parent drug. After single and repeated administration of CHF 4227, the urinary excretion of both unchanged and conjugated drug was negligible Steady-state plasma concentrations were reached 6–14 days after treatment started.
In conclusion, CHF 4227 is a well-tolerated SERM for postmenopausal women dosed once daily for 28 days. It appears to be active on bone tissue and serum lipid concentrations, without inducing stimulatory oestrogenic effects on the endometrium, and without worsening menopausal symptoms, in particular hot flushes. These data suggest that CHF 4227 is a promising agent for the treatment of several conditions in postmenopausal women.
The study was funded by Chiesi Farmaceutici S.p.A.
References
- 1.Gallagher JC, Goldgar D, Moy A. Total bone calcium in normal women: effect of age and menopause status. J Bone Miner Res. 1987;2:491–6. doi: 10.1002/jbmr.5650020605. [DOI] [PubMed] [Google Scholar]
- 2.Gotfredsen A, Hadberg A, Nilas L, Christiansen C. Total body bone mineral in healthy adults. J Lab Clin Med. 1987;110:362–8. [PubMed] [Google Scholar]
- 3.Nordin BEC, Need AG, Chatterton BE, Horowitz M, Morris HA. The relative contribution of age and years since menopause to postmenopausal bone loss. J Clin Endocrinol Metab. 1990;70:83–8. doi: 10.1210/jcem-70-1-83. [DOI] [PubMed] [Google Scholar]
- 4.Riggs BL, Melton LJ., III Involutional osteoporosis. N Engl J Med. 1986;314:1676–86. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 5.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and use of oestrogens in postmenopausal women. The Framingham Study. N Engl J Med. 1987;317:1169–74. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- 6.Barret-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Paganini-Hill A, Henderson V. Oestrogen replacement therapy and risk of Alzheimer's disease. Arch Intern Med. 1996;156:2213–7. [PubMed] [Google Scholar]
- 8.Tang MX, Jacobs D, Stem Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 9.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of oestrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomised controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Cauley JA, Robbins J, Chen Z, Cummings SR, Jacjson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB. Effects of oestrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomised trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, III, Assaf AR, Jackson RD, Morley KJ, Wassertheil-Smoller S, Wactawski-Wende J. Oestrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative memory study: a randomised controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 12.Lacey JV, jr Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzin A, Schairer C. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–41. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 13.Jordan CV. Antioestrogen and selective oestrogen receptor modulators as multifunctional medicines. 2. Clinical consideration and new agents. J Med Chem. 2003;46:1081–111. doi: 10.1021/jm020450x. [DOI] [PubMed] [Google Scholar]
- 14.Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper MW, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–7. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 15.Bryant HG, Glasebrook AL, Yang NN, Sato M. A pharmacological review of raloxifene. J Bone Miner Metab. 1996;14:1–9. [Google Scholar]
- 16.Galbiati E, Caruso PL, Amari G, Armani E, Ghirardi S, Delcanale M, Civelli M. Pharmacological actions of a novel, potent, tissue-selective benzopyran oestrogen. J Pharmacol Exp Ther. 2002;303:196–203. doi: 10.1124/jpet.102.038034. [DOI] [PubMed] [Google Scholar]
- 17.Reina Armamento-Villareal Shein S, Nawaz A, Napoli N, Mueller C, Halstead LR, Brodt MD, Silva MJ, Galbiati E, Caruso PL, Civelli M, Civitelli R. A new selective oestrogen receptor modulator, CHF 4227.01, preserves bone mass and microarchitecture in ovariectomized rats. J Bone Mineral Res. 2005;20:2178–88. doi: 10.1359/JBMR.050801. [DOI] [PubMed] [Google Scholar]
- 18.Galbiati E, Caruso PL, Amari G, Armani E, Ghirardi S, Delcanale M, Civelli M. Effect of 3-phenil-4-[[4-[2-(1-piperidinil) ethoxy]phenyl]methyl]-2H-1-benzopyran-7-ol (CHF 4056), a novel nonsteroidal oestrogen agonist/antagonist, on reproductive and nonreproductive tissue. J Pharmacol Exp Ther. 2002;300:802–9. doi: 10.1124/jpet.300.3.802. [DOI] [PubMed] [Google Scholar]
- 19.Tankó LB, Warming L, Bagger YZ, Byrjalsen I, Jensen S, Christiansen C. Serum placental protein 14: a novel marker of selective oestrogen receptor modulator action on the postmenopausal endometrium. Biomarkers. 2002;7:257–66. doi: 10.1080/13547500210125031. [DOI] [PubMed] [Google Scholar]
- 20.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in pstmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomised clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Eckert S, Krueger KA, Grady S, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomised trial. Multiple Outcomes Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 22.Boss SM, Huster WJ, Neild JA, Glant MD, Eisenhut CC, Draper MW. Effects of raloxifene hydrochloride on the endometrium of postmenopausal women. Am J Obstet Gynaecol. 1997;177:1458–64. doi: 10.1016/s0002-9378(97)70091-7. [DOI] [PubMed] [Google Scholar]
- 23.Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med. 1999;130:431–9. doi: 10.7326/0003-4819-130-5-199903020-00015. [DOI] [PubMed] [Google Scholar]
- 24.Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Jr, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment Study Group. N Engl J Med. 1995;333:1437–43. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 25.Voipio SK, Komi J, Kangas L, Halonen K, De Gregorio MW, Erkkola RU. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas. 2002;43:207–14. doi: 10.1016/s0378-5122(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 26.Jordan VC, Fritz NF, Langan-Fahey S, Thompson M, Tormey DC. Alteration of endocrine parameters in premenopausal women with breast cancer during long-term adjuvant therapy with tamoxifen as the single agent. J Natl Cancer Inst. 1991. p. 83. 1488-91. [DOI] [PubMed]
- 27.Clemett D, Spencer CM. Raloxifene: a review of its use in postmenopausal osteoporosis. Drugs. 2000;60:379–411. doi: 10.2165/00003495-200060020-00013. [DOI] [PubMed] [Google Scholar]
- 28.Cotreau MM, Stonis L, Dykstra KH, Gandhi T, Gutierrez M, Xu J, Park Y, Burghart PH Schwertschlag US. Multiple-dose, safety, pharmacokinetics, and pharmacodynamics of a new selective oestrogen receptor modulator, ERA-923, in healthy postmenopausal women. J Clin Pharmacol. 2002;42:157–65. doi: 10.1177/00912700222011193. [DOI] [PubMed] [Google Scholar]
- 29.Guetta V, Lush RM, Figg WD, Waclawiw MA, Cannon RO. Effects of the antioestrogen tamoxifen on low-density lipoprotein concentration and oxidation in postmenopausal women. Am J Cardiol. 1995;76:1072–3. doi: 10.1016/s0002-9149(99)80302-6. [DOI] [PubMed] [Google Scholar]
- 30.Vrbanec D, Reiner Z, Belev B, Plestina S. Changes in serum lipid and lipoprotein levels in postmenopausal patients with node-positive breast cancer treated with tamoxifen. Tumori. 1998;84:687–90. doi: 10.1177/030089169808400615. [DOI] [PubMed] [Google Scholar]
- 31.Wasan KM, Ramaswamy M, Haley J, Dunn BP. Administration of long-term tamoxifen therapy modifies the plasma lipoprotein-lipid concentration and lipid transfer protein I activity in postmenopausal women with breast cancer. J Pharm Sci. 1997;86:876–9. doi: 10.1021/js970097w. [DOI] [PubMed] [Google Scholar]
- 32.Marttunen MB, Hietanen P, Titinen A, Roth HJ, Viinikka L, Ylikorkala O. Effect of tamoxifen and toremifene on urinary excretion of pyridinoline and deoxypyridinoline and bone density in postmenopausal patients with breast cancer. Calcif. Tissue Int. 1999;65:365–8. doi: 10.1007/s002239900714. [DOI] [PubMed] [Google Scholar]
- 33.Barret-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Komitzer M, McNabb MA, Wenger NK raloxifene use for the heart (RUTH) trial investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]