Abstract
We have examined the effects of administration of testosterone for 7 days on monocarboxylate transporter (MCT) 1 and MCT4 mRNAs and proteins in seven metabolically heterogeneous rat hindlimb muscles and in the heart. In addition, we also examined the effects of testosterone treatment on plasmalemmal MCT1 and MCT4, and lactate transport into giant sarcolemmal vesicles prepared from red and white hindlimb muscles and the heart. Testosterone did not alter MCT1 or MCT4 mRNA, except in the plantaris muscle. Testosterone increased MCT1 (20%–77%, P < 0.05) and MCT4 protein (29%–110%, P < 0.05) in five out of seven muscles examined. In contrast, in the heart MCT1 protein was not increased (P > 0.05), and MCT 4 mRNA and protein were not detected. There was no correlation between the testosterone-induced increments in MCT1 and MCT4 proteins. Muscle fibre composition was not associated with testosterone-induced increments in MCT1 protein. In contrast, there was a strong positive relationship between the testosterone-induced increments in MCT4 protein and the fast-twitch fibre composition of rat muscles. Lactate transport into giant sarcolemmal vesicles was increased in red (23%, P < 0.05) and white muscles (21%, P < 0.05), and in the heart (58%, P < 0.05) of testosterone-treated animals (P < 0.05). However, plasmalemmal MCT1 protein (red, +40%, P < 0.05; white, +39%, P < 0.05) and plasmalemmal MCT4 protein (red, +25%, P < 0.05; white, +48%, P < 0.05) were increased only in skeletal muscle. In the heart, plasmalemmal MCT1 protein was reduced (−20%, P < 0.05). In conclusion, these studies have shown that testosterone induces an increase in both MCT1 and MCT4 proteins and their plasmalemmal content in skeletal muscle. However, the testosterone-induced effect was tissue-specific, as MCT1 protein expression was not altered in the heart. In the heart, the testosterone-induced increase in lactate transport cannot be explained by changes in plasmalemmal MCT1 content, but in skeletal muscle the increase in the rate of lactate transport was associated with increases in plasmalemmal MCT1 and MCT4.
Lactate is produced by many tissues, with skeletal muscle being the most prominent of these. In addition, muscle can oxidize lactate (Gladden, 1996). Thus, lactate fluxes into and out of the muscle cells. This movement of lactate across the plasma membrane is associated with a pH-dependent monocarboxylate transporter (MCT) system (Juel, 1997; Juel & Halestrap, 1999). In the past few years, a family of MCT proteins (MCT1–14) has been identified (Halestrap & Meredith, 2004). Among these MCT isoforms, MCT1 and MCT4 are thought to be the key transporters involved in regulating the lactate flux across the plasma membrane. MCT1 is ubiquitously expressed in many tissues, whereas MCT4 is present primarily in skeletal muscle (Bonen, 2001). In human and rat skeletal muscle, MCT1 expression is highly correlated with indices of the oxidative capacities of muscle (Pilegaard et al. 1999b; Bonen, 2001). In contrast, MCT4 is expressed mainly in fast-twitch fibres and its expression has been associated with indices of glycolytic capacities (Bonen et al. 2000b).
It has been shown that lactate transport and MCT protein expression can be altered in relation to the metabolic demands placed on muscle by contractile activity. For example, skeletal muscle lactate transport can be increased when muscle activity is chronically increased, either by training (McDermott & Bonen, 1993; Pilegaard et al. 1993, 1999a; Bonen et al. 1998b; Dubouchaud et al. 2000) or by chronic electrical stimulation of rat hindlimb muscles (McCullagh et al. 1996a, 1997; Bonen et al. 2000c) In contrast, when muscle activity is decreased by denervation (McCullagh & Bonen, 1995) or hindlimb suspension (Dubouchaud et al. 1996), lactate transport is decreased. Alterations in MCT1 protein expression appear to be tightly associated with these increased (Bonen et al. 1998b; Pilegaard et al. 1999a) or decreased (Wilson et al. 1998) energy demands placed on muscle. MCT4 protein expression is less easily perturbed by muscle activity, apparently requiring more intense exercise before it is up-regulated (Pilegaard et al. 1999a; Bonen et al. 2000c; Yoshida et al. 2004). Thus, there appears to be a good concordance between alterations in muscle activity patterns, the expression of MCT1 and MCT4, and the rates of lactate transport.
Because lactate is an important substrate for hepatic gluconeogenesis (McDermott & Bonen, 1992) and muscle glyconeogenesis (Bonen et al. 1990; Bonen & Homonko, 1994), and for oxidation, particularly in the heart (Chatham et al. 1999), it has been of interest to examine whether there are changes in MCTs and/or lactate transport when the substrate endocrine milieu is altered. In insulin-resistant muscles of obese Zucker rats, there was a decrease in MCT4 expression and lactate influx into small sarcolemmal vesicles (Py et al. 2001b). With streptozotocin-induced diabetes, there was a significant decrease in sarcolemmal lactate transport (Py et al. 2001a), which may be related to the reductions in MCT1 and MCT4 protein expression in this model of diabetes (Enoki et al. 2003). Triiodothyronine (T3) administration (for 7 days) increased lactate transport, an effect that was associated with increases in MCT4 protein and mRNA, as MCT1 protein was not increased, despite a marked increase in MCT1 mRNA (Wang et al. 2003). Thus, the substrate hormonal milieu contributes to the regulation of MCT protein expression and consequently to the rates of lactate transport.
It is known that prolonged testosterone administration increases muscle mass (Brodsky et al. 1996; Bhasin et al. 2001; Sinha-Hikim et al. 2002), presumably via mechanisms involving alterations in the expression of multiple muscle growth factors (Bhasin et al. 2001) and changes in protein turnover (Ferrando et al. 1998, 2002). In contrast, the effects of testosterone on substrate transport and metabolism have been equivocal. In an early study, testosterone administration (for 35 days) did not appear to alter rates of substrate metabolism, as neither the activities of selected enzymes nor the rates of pyruvate oxidation were altered (Kuhn & Max, 1985). However in more recent studies, testosterone has been shown to stimulate glycogenesis and inhibit glycolysis in muscle (Ramamani et al. 1999; Van Breda et al. 2003). In addition, long-term administration (8–12 weeks) of this hormone has been shown to reduce skeletal glucose uptake by ∼50% (Holmang et al. 1990; Rincon et al. 1996). This reduction appeared to be associated with an impaired insulin-induced translocation of glucose transporter 4 (GLUT-4), as GLUT-4 expression was not altered (Rincon et al. 1996). Taken altogether, it appears that testosterone administration can alter carbohydrate metabolism in skeletal muscle.
Whether testosterone also reduces lactate transport, and MCT1 and MCT4 expression, as well as glucose transport (Holmang et al. 1990; Rincon et al. 1996), in muscle is not known. Recently, it was shown that testosterone reduced MCT2 mRNA in a dose-dependent manner in seminiferous tubules (Carson et al. 2002; Boussouar et al. 2003). However, skeletal muscle MCT1 and MCT4 may not respond in a similar manner, as tissue-specific regulation (heart versus muscle) (Hatta et al. 2001) and isoform-specific regulation of MCTs have been shown (Bonen et al. 2000c; Wang et al. 2003). In muscle, there are conflicting reports as to the androgen receptor number in fast-twitch and slow-twitch skeletal muscle (Deschenes et al. 1994; Bricout et al. 1994, 1999; Carson et al. 2002), but the affinity of androgen receptors appears to be greater in fast-twitch muscle than in slow-twitch muscle (Bricout et al. 1999). In addition, the androgen receptor increase in response to the anabolic steroid treatment is greater in fast twitch muscle than in slow twitch muscle (Carson et al. 2002). Therefore, muscles with a higher proportion of fast-twitch glycolytic (FG) fibres may be more predisposed to respond to testosterone treatment. Moreover, as MCT4 is also highly expressed in FG fibres (Bonen et al. 2000b), it may well be that testosterone treatment would be particularly effective in altering MCT4 in muscles that are rich in FG fibres. Therefore, in the present study, we have examined the effects of 7 days of testosterone administration on MCT1 and MCT4 mRNA and protein levels in seven, metabolically heterogeneous rat hindlimb muscles, as well as in the heart. In addition, we also examined the effects of testosterone treatment on the plasmalemmal MCT1 and MCT4 content and on the rate of lactate transport into giant sarcolemmal vesicles prepared from red and white hindlimb muscles and the heart.
Methods
Animals
Male Wistar rats (6 weeks old) were purchased (Nihon Seibutsu Zairyou Center, Tokyo) and housed in an air-conditioned room on a 12 h light–12 h dark cycle. All rats were fed a diet of Purina rat chow and water ad libitum. Their body weights were checked daily. At 7 weeks of age, the animals were assigned randomly to the control and testosterone-injected groups. Rats were anaesthetized with isoflurane (2.5%) in an anaesthesia induction chamber. Thereafter, testosterone, dissolved in sesame oil, was injected at the back of the neck (10 mg (100 μl)−1 (100 g body weight)−1). Control animals were treated similarly with vehicle (sesame oil) alone. Injections were given daily for 7 days. Ethical approval for these studies was obtained from the Committees on Animal Care at the University of Tokyo and the University of Guelph.
Detection of MCT1 and MCT4 mRNA and protein
Animals were anaesthetized with sodium pentobarbital (60 mg (100 g body weight)−1, i.p.) 24 h after the last testosterone injection. Blood was taken from the heart and tissues were excised rapidly (i.e. heart and hindlimb muscles: soleus (SOL), plantaris (PL), red and white gastrocnemius (RG and WG, respectively), red and white tibialis anterior (RTA and WTA, respectively) and extensor digitorum longus (EDL)). Tissues were frozen in liquid nitrogen, and stored at −80°C until analysed for MCT1 and MCT4 mRNA and protein levels.
MCT1 and MCT4 mRNA
RNA was isolated from muscle using Trizol reagent (Invitrogen, Burlington, Ontario, Canada). Approximately 50 mg frozen muscle was added to 1 ml ice-cold Trizol and homogenized for two 10 s bursts at 15 000 r.m.p. using a Polytron 3100 homogenizer (Kinematica, Littau, Switzerland). Homogenates were centrifuged at 12 000 g for 10 min at 4°C to pellet cellular debris. Chloroform (200 μl) was added to the supernatant fraction and shaken vigorously for 15 s. The organic and aqueous phases were separated by centrifugation at 12 000 g for 15 min. The aqueous phase was removed and 600 μl isopropanol was added. After mixing thoroughly, the solution was added to an RNAeasy Mini-spin column (Qiagen, Mississauga, Ontario, Canada) and RNA was isolated according to the manufacturer's instructions. DNase (Qiagen) treatment was performed on the column. RNA concentration and purity was estimated by OD 260/280 in TE buffer.
cDNA synthesis was performed using First Strand cDNA Synthesis Kit for RT-PCR (AMV) (Roche Diagnostics, Laval, Quebec, Canada). Reverse transcriptase reactions were performed according to the manufacturer's instructions with 1 μg RNA in a total reaction volume of 26 μl using random hexamer oligonucleotides and including gelatin. cDNA reactions were diluted 5-fold before addition to the PCR cocktail.
Quantitative real-time PCR was performed using a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA). The PCR reaction was carried out using qPCR SuperMix UDG (Invitrogen, Burlington, Ontario, Canada) as recommended by the manufacturer, using ROX as a reference dye. The concentration of each primer was 10 μm. Five microlitres of dilute cDNA was added to each reaction for a total volume of 25 μl. The thermocycling conditions were: 2 min 50°C, 5 min 95°C, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. A melt curve analysis was performed to ensure a single product was generated. The primer sequences used are listed in Table 1. For MCTs, one primer from each set was designed across an intron/exon junction to prevent genomic DNA amplification. The 18S RNA primer set sequence is available in the public RTPrimerDB database (http:\\medgen.UGent.be\rtprimerdb\), RTPrimerDB ID: 1490.
Table 1.
Primer sequences used for real-time PCR
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | GenBank accession number | Size (bp) |
|---|---|---|---|---|
| MCT1 | GGTGTCATTGGAGGTCTTGGG | GGCCAATGGTCGCTTCTTG | NM_012716 | 90 |
| MCT4 | GGGTCATCACTGGCTTGGGT | GGAACACGGGACTGCCTGC | NM_030834 | 123 |
| 18S RNA | GTTGGTTTTCGGAACTGAGGC | GTCGGCATCGTTTATGGTCG | See Pattyn et al. (2003) | 204 |
Data quantification was carried out using 7500 System SDS Software using the 2−ΔΔCT method (Livak & Schmittgen, 2001). Primer amplification efficiencies were determined to be approximately equal as outlined by Pattyn et al. (2003) using 18S RNA as an internal standard.
MCT1 and MCT4 protein
Proteins from muscles and heart were isolated and separated using SDS-PAGE and MCT1 and MCT4 detected as previously described (Bonen et al. 2000c; Hatta et al. 2001; Enoki et al. 2003; Coles et al. 2004; Yoshida et al. 2004). For each set of Western blots, equal quantities of protein were loaded (30 μg), and data from muscles and heart from each of the two groups as well as a muscle standard were included. This permitted normalization of the data to the standard across the different blots. Densities of the MCT1 and MCT4 protein bands were quantified by scanning the resultant films on a densitometer connected to a computer with appropriate software.
Preparation of giant sarcolemmal vesicles and plasmalemmal MCT detection
In a subset of rats, muscles were also taken after 7 days of testosterone treatment to examine rates of lactate transport into giant vesicles, as well as determining MCT1 and MCT4 at the plasma membrane of these vesicles. Sarcolemmal vesicles were purified as we have previously described (Bonen et al. 1998a, 2000a; Tonouchi et al. 2002). Briefly, red (RG + RTA) and white (WG + WTA) muscles were combined and scissored lengthwise into thin slices. These slices were incubated in a solution containing 140 mm KCl, 5 mm MOPS (pH 7.4), 150 U ml−1 collagenase (Sigma type VII), and 1.0 mg ml−1 of the protease inhibitor aprotinin (Sigma-Aldrich, St Louis, MO, USA) for 1 h at 34°C. A three-layer step-density gradient was used to isolate the vesicles. The upper layer was composed of KCl-MOPS (3 ml), the middle layer was composed of 4% Nicodenz in KCl-MOPS (3 ml), and the bottom layer contained the vesicle suspension (8 ml). The vesicles were removed from the interface of the two upper layers after centrifugation (60 g for 45 min) at room temperature (20°C). Thereafter, the vesicles were diluted with KCl-MOPS and recovered with a final centrifugation step (12 000 g for 4 min). A portion of the vesicles was saved to ascertain the plasma membrane content of MCT1 and MCT4. Plasma membrane proteins were separated using SDS-PAGE followed by Western blotting, as we have recently reported in detail (Bonen et al. 2000c; Hatta et al. 2001; Enoki et al. 2003; Yoshida et al. 2004).
Lactate uptake by giant sarcolemmal vesicles
Lactate uptake measurements were performed under zero-trans conditions in giant vesicles. For these purposes, lactate (1 mm lactate, 0.1 μCi l-[U-14C]lactate per tube) was added to the vesicle suspension and vortexed briefly. As preliminary studies demonstrated that lactate uptake increased linearly up to 30 s, lactate uptake was terminated after 10 s by the addition of an ice-cold stop solution (3 mm HgCl in 0.1% bovine serum albumin, KCl-MOPS). The vesicles were then centrifuged (12 000 g for 2 min), and the supernatant fraction was discarded. To determine non-specific 14C-lactate associated with vesicles, the stop solution was added to the vesicles before the lactate solution was loaded. 14C activity was determined using standard liquid scintillation procedures.
Other measurements
After 1 week of testosterone treatment, serum testosterone concentrations were measured with a commercially available radioimmunoassay kit (Diagnostic Product Corporation, Los Angeles, CA, USA). In addition, blood glucose and lactate concentrations were measured using blood lactate and glucose analysers (Arkray, Kyoto, Japan). The wet muscle weights of SOL, PL, gastrocnemius (RG + WG), tibialis anterior (RTA + WTA) and EDL were measured, when they were excised.
Muscle fibre composition
We have previously characterized the muscle fibre composition of the hindlimb muscles in rats (Megeney et al. 1993). These results compared well with those of Armstrong & Phelps (1984) and with recent studies in our laboratory (Benton & Bonen, 2005). Therefore, the muscle fibre composition data from our laboratory (Megeney et al. 1993) were used to compare the testosterone-induced changes in MCT1 and MCT4 proteins in relation to the fibre composition of the seven rat hindlimb muscles.
Data analysis
A two-way (control versus testosterone) analysis of variance (ANOVA) was used to analyse the data. All data are reported as means ± s.e.m.
Results
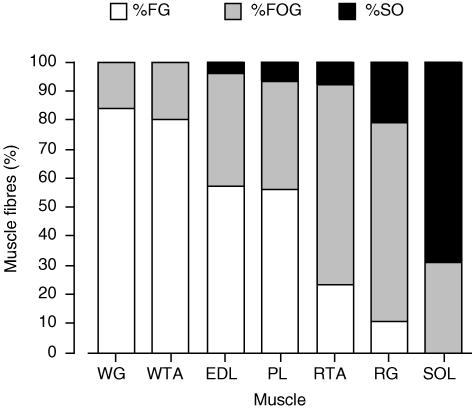
Initial body weights of each group did not differ (Table 2). After 1 week there were no significant differences in the body weights of the control and testosterone-treated animals (Table 2). Circulating testosterone concentrations were significantly increased in the testosterone-treated group (P < 0.05; Table 2). There were no differences between the control and testosterone-treated groups in circulating blood glucose and lactate concentrations (Table 2). With the testosterone treatment, the muscle wet weight was significantly increased in PL (+11%, P < 0.05), gastrocnemius (+10%, P < 0.01) and tibialis anterior (+6%, P < 0.01), but not in SOL (P > 0.05) and EDL (P > 0.05, Table 2). The seven rat hindlimb muscles used in the present study encompass a wide range of muscle fibre composition (Fig. 1). Examination of one representative hindlimb muscle (PL) revealed that the muscle fibre composition was not altered with 1 week of testosterone treatment (P > 0.05, data not shown).
Table 2.
Body and muscle weights, and circulating glucose, lactate and testosterone in control and 7 day testosterone-treated rats (mean ±s.e.m.)
| Parameter | Control (n = 7) | Testosterone-treated (n = 6) |
|---|---|---|
| Body weight (g) | ||
| Pre-treatment | 225.5 ± 7.3 | 227.4 ± 5.7 |
| Post-treatment | 282.9 ± 4.3** | 276.0 ± 3.7** |
| Muscle weight (mg) | ||
| Soleus | 166 ± 3 | 167 ± 7 |
| Plantaris | 497 ± 12 | 540 ± 11* |
| Gastrocnemius | 2399 ± 44 | 2643 ± 58* |
| Tibialis anterior | 941 ± 14 | 998 ± 10* |
| Extensor digitorum longus | 240 ± 7 | 251 ± 5 |
| Testosterone (nm) | 0.36 ± 0.04 | 15.8 ± 5.4 |
| Glucose (mm) | 8.5 ± 0.4 | 8.0 ± 0.4 |
| Lactate (mm) | 2.0 ± 0.4 | 1.9 ± 0.2 |
P < 0.05, change in body weight within each group
P < 0.05, testosterone versus control.
Figure 1. Muscle fibre composition of seven rat hindlimb muscles.
FG, fast-twitch glycolytic; FOG, fast-twitch oxidative glycolytic; SO, slow twitch oxidative. The data have been redrawn from a previous study completed in our laboratory (Megeney et al. 1993).
Effects of testosterone treatment on MCT mRNA abundance
In control hindlimb muscles, MCT4 mRNA abundance was greatest in the most glycolytic muscles (WTA and WG) and was low in the highly oxidative SOL muscle. The converse was observed for MCT1 mRNA, its abundance was greatest in the more oxidative muscles (SOL and RG) and the heart. These results confirm previously reported observations (Bonen et al. 2000b). There was, however, no linear relationship between the fibre composition of the hindlimb muscles and their MCT1 or MCT4 mRNA abundances (data not shown).
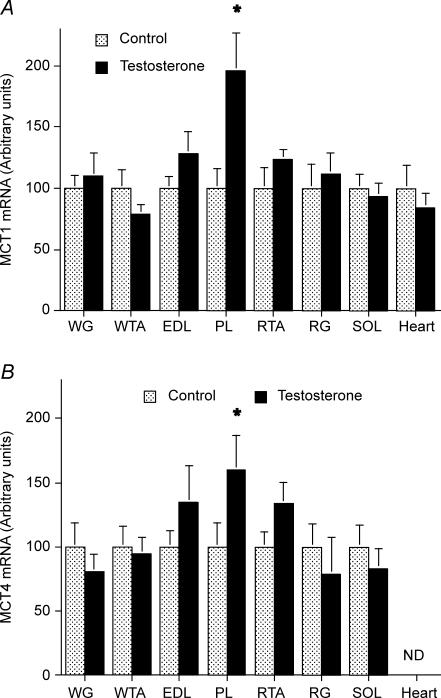
To facilitate the presentation of the relative effects of the testosterone treatment on the MCT1 and MCT4 mRNAs in the seven rat hindlimb muscles, we set the MCT1 and MCT4 mRNA abundance in each muscle in the control group to 100. Except for PL, testosterone administration did not alter either the MCT1 or MCT4 mRNA abundances in muscle or in the heart (Fig. 2).
Figure 2. MCT1 (A) and MCT4 (B) mRNA in seven rat hindlimb muscles and the heart, in control and testosterone-treated animals (mean ± s.e.m.).
Data were normalized to 18S RNA and the control muscle data were set to 100 for each muscle. Control, n = 7; testosterone-treated animals, n = 7. ND, not detected. pco.05 testosteroneus control.
Effects of testosterone treatment on MCT protein expression
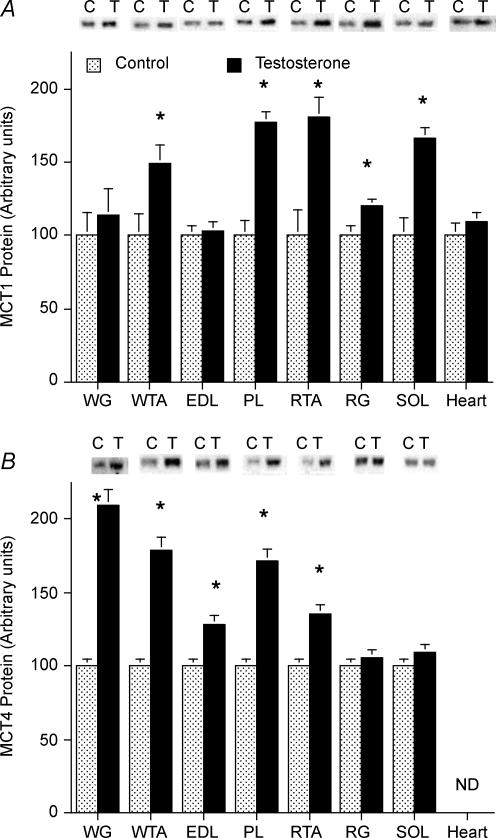
Previously, we have reported that in rat hindlimb muscles, MCT1 expression is highly correlated with the oxidative muscle fibre composition (McCullagh et al. 1996b; Bonen et al. 2000b), and conversely, that MCT4 expression is highly correlated with the glycolytic muscle fibre composition (Bonen et al. 2000b). Although these relationships were also observed in the present study (data not shown), we have chosen to set the MCT1 and MCT4 content of each muscle in the control group to 100, to facilitate the presentation of the relative effects of the testosterone treatment on the expression of these proteins in the seven rat hindlimb muscles that were examined.
MCT1 protein
After 1 week of testosterone treatment, there were marked changes in MCT1 protein expression. In the testosterone-treated group, MCT1 was increased in PL (+77%), SOL (+67%), RTA (+51%), RG (+20%) and WTA (+50%) muscles (P < 0.05, Fig. 2A). In WG (+14%) and EDL (+3%) muscles, the changes were not significant (P > 0.05). In contrast, testosterone failed to alter MCT1 protein expression in the heart (Fig. 3A).
Figure 3. MCT1 (A) and MCT4 (B) proteins in seven rat hindlimb muscles and the heart, in control (C) and testosterone (T)-treated animals (mean ± s.e.m.).
Data were normalized to a common standard in each gel and the control muscle data were set to a mean of 100 for each muscle. Control, n = 7; testosterone-treated animals, n = 6. ND, not detected. Equal quantities of protein (30 μg) were loaded for each muscle. pco.05 testosteroneus control
MCT4 protein
There were also marked changes in MCT4 protein expression after 1 week of testosterone treatment. The largest increases were observed in muscles rich in FG fibres, namely, WG (+110%) and WTA (+80%, P < 0.05, Fig. 3B). There were also increases in PL (+71%, P < 0.05, Fig. 2B) and EDL (+29%, P < 0.05, Fig. 3B), muscles rich in both FG fibres and fast-twitch oxidative (FOG) fibres (Fig. 1). In muscles rich in FOG fibres there was an increase in MCT4 in RTA (+35%, P < 0.05, Fig. 3B) but not in RG (+6%, P > 0.05, Fig. 3B). No increase in MCT4 was observed in SOL muscle (Fig. 2B), which is comprised primarily of slow-twitch oxidative fibres (Fig. 1) and in which MCT4 expression is minimal (Bonen et al. 2000b). The adult heart does not appear to express MCT4 protein (Bonen et al. 2000b).
MCT1, MCT4 and muscle fibre composition
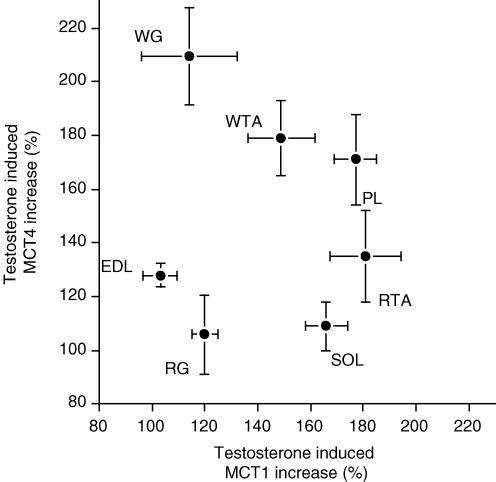
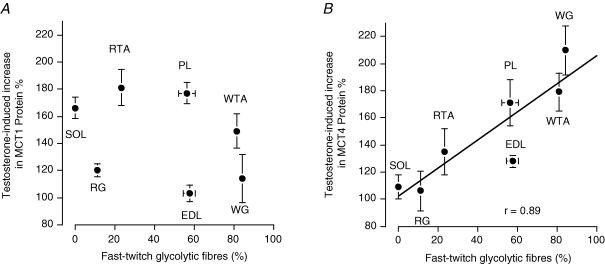
Within the same muscles, the testosterone-induced increases in MCT1 and MCT4 were not correlated (Fig. 4). Similarly, there was no relationship between the muscle fibre composition and the testosterone-induced increase in MCT1 protein (Fig. 5A). In contrast, there was a positive relationship between the proportion (%) of FG fibres in the seven hindlimb muscles and the testosterone-induced increase in MCT4 protein (Fig. 5B).
Figure 4. Relationship between testosterone-induced increases (%) in MCT1 and MCT4 proteins in rat hindlimb muscles.
For each muscle, the mean ± s.e.m. of MCT1 and MCT4 are plotted against each other. The data are from Fig. 3A and B.
Figure 5. Relationship between the fast-twitch glycolytic (FG) muscle fibre composition of rat hindlimb muscles and the testosterone-induced increases (%) in MCT1 (A) and MCT4 (B) proteins.
The MCT1 and MCT4 data are from Fig. 3 and the muscle fibre data are from Fig. 1. Data are mean ± s.e.m. (in some instances the error bars are smaller than the plotting symbol).
Plasma membrane MCTs and lactate uptake by giant sarcolemmal vesicles
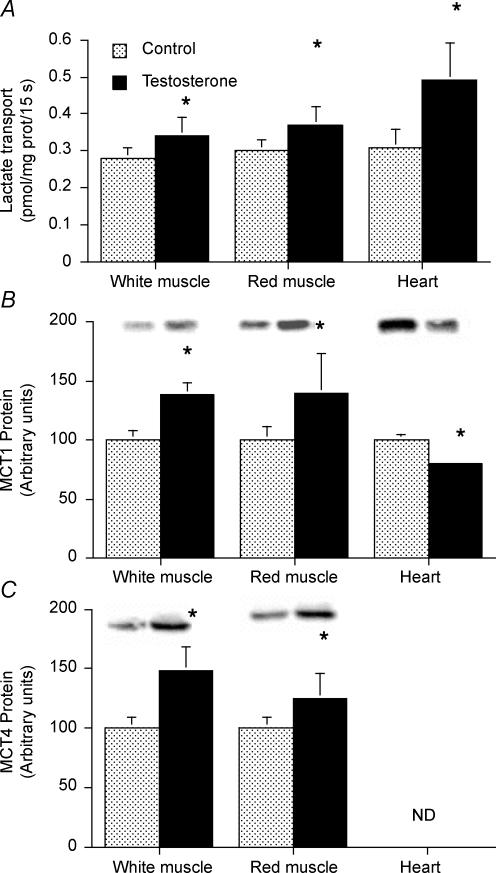
Plasma membrane MCTs and lactate transport were examined in giant sarcolemmal vesicles in control and testosterone-treated animals. To obtain sufficient quantities of these vesicles for transport studies, we combined WG and WTA muscles, and RG and RTA muscles to provide white and red giant sarcolemmal vesicles, respectively. With testosterone treatment, the rates of lactate transport were increased in both red (+21%, P < 0.05) and in white (+23%, P < 0.05) giant sarcolemmal vesicles (Fig. 6A). Testosterone administration increased plasma membrane MCT1 in both white (+39%) and red muscles (+40%, P < 0.05, Fig. 6B). Similarly, plasma membrane MCT4 protein was also increased in white (+48%) and red muscle (+25%, P < 0.05, Fig. 6C).
Figure 6. Rates of lactate transport into giant sarcolemmal vesicles (A) and plasmalemmal MCT1 (B) and plasmalemmal MCT4 (C) proteins obtained from red and white skeletal muscle and the heart.
For each preparation of giant vesicles, the heart, white muscle (WTA and WG) and red muscle (RTA and RG) from two rats were pooled. Lactate transport rates and plasmalemmal MCT1 and MCT4 determinations were made in five independent experiments. ND, not detected. For Western blotting, equal quantities of protein (10 μg) were loaded for each muscle. pco.05 testosteroneus control
MCT4 protein was not detected in giant vesicle plasma membranes prepared from the heart, in either the control or testosterone-treated animals. Plasma membrane MCT1 protein in the heart was reduced by 20% in the testosterone-treated group (P < 0.05, Fig. 6B). Concomitantly, lactate transport was increased 58% in the testosterone-treated group (P < 0.05, Fig. 6A).
Discussion
This is the first study to demonstrate that testosterone treatment can induce MCT protein expression in skeletal muscle but not in the heart. The novel observations of this study are that following 7 days of testosterone treatment: (a) skeletal muscle MCT1 and MCT4 protein expression were increased, while MCT1 and MCT4 mRNA abundances were generally not altered; (b) sarcolemmal MCT1 and MCT4 were increased; (c) rates of lactate transport were increased; (d) within the same muscle, the changes in MCT1 and MCT4 expression were not correlated; (e) among the metabolically heterogeneous rat muscles there was no relationship between muscle fibre composition and the increase in MCT1; (f) there was a positive association between FG muscle fibre composition and the changes in MCT4; (g) in the heart, neither MCT1 expression nor its plasmalemmal content were increased; and (h) rates of lactate transport were increased in the testosterone treated animals. Thus, these studies have shown that in skeletal muscle, but not in the heart, testosterone regulates the expression of MCT1. Moreover, muscles rich in FG fibres are particularly susceptible to testosterone-induced up-regulation of MCT4.
Seven days of testosterone administration significantly increased the circulating blood testosterone concentration, but not circulating glucose and lactate levels, nor body weight. Increases in muscle weight (6–10%) were observed in muscles with a large proportion of FG fibres. No change in muscle mass was observed in SOL, which is composed primarily of slow twitch fibres. Others have shown that prolonged testosterone treatment (3–20 weeks) did not alter muscle fibre composition in slow- and fast-twich muscles in rats (Bricout et al. 1999) or humans (Sinha-Hikim et al. 2002). Similarly, we observed no changes in the fibre composition of the PL muscle after 1 week of testosterone treatment (data not shown). The different testosterone-susceptibility in muscles with different fibre composition, in the present study, may be related to the greater affinity (Bricout et al. 1999) and/or number (Monks et al. 2006) of androgen receptors in fast-twitch muscle fibres, which may affect responsiveness to androgens. Others have observed that muscles rich in fast-twitch fibres increase their androgen receptors relatively more when exposed to an anabolic steroid (Carson et al. 2002). Our study is in line with these results, because, except for the EDL muscle, muscles rich in fast-twitch fibres exhibit a greater gain in muscle mass in response to testosterone treatment.
Recently Boussouar et al. (2003) reported that testosterone inhibited MCT2 mRNA abundance in a dose-dependent manner. However, protein expression was not examined. This may be critical as there are indications that MCT protein expression is regulated via post-transcriptional mechanisms, at least in skeletal muscle (Bonen et al. 2000b,c). Indeed, the present results also indicate that MCT protein expression in muscle is regulated via post-transcriptional mechanisms, presumably a testosterone-induced net increase in protein synthesis, due to a reutilization of intracellular amino acids (Ferrando et al. 1998, 2002). Presently, there are no other studies available that have examined the effect of testosterone administration on MCT protein expression. In comparing the results of the present study in skeletal muscle and the heart, and the study of Boussouar et al. (2003) in seminiferous tubules, it appears that the effects of testosterone on MCT1 and MCT4 mRNAs are different from the effects on MCT2 mRNA. Moreover, tissue-specific responses to testosterone can also occur (i.e. muscle versus heart: present study). There has also been previous evidence for tissue-specific and isoform-specific regulation of MCTs (Bonen et al. 2000c; Hatta et al. 2001; Wang et al. 2003).
In general, the MCT1 protein isoform is abundantly expressed in oxidative muscle fibres, whereas the MCT4 protein isoform predominates in muscles rich in glycolytic fibre (Bonen et al. 2000b). In the present study we have shown that testosterone treatment increased both MCT1 and MCT4 expression in most muscles examined. However, there was no correlation between the testosterone-induced MCT1 protein increase and the proportion of FG fibres in muscle. In contrast, a strong positive correlation was found between the FG fibre content of skeletal muscles and the testosterone-induced MCT4 protein increment. This may be related to the higher affinity of androgen receptors in these FG fibres (Bricout et al. 1999). As the increased testosterone susceptibility applies to MCT4, but not MCT1, in muscles rich in FG fibres, it appears that the mechanisms regulating the expression of MCT1 and MCT4 in skeletal muscle may differ. We have previously observed that protein expression of MCT1 and MCT4 occurs via post-transcriptional regulation or a combination of post-transcriptional regulation and pre-translational mechanisms (Bonen et al. 2000b,c; Wang et al. 2003). This would also seem to be the case in the present study, as except for one muscle, no changes in MCT1 or MCT4 mRNA abundance was observed (we have no simple explanation for this discrepancy in PL). Thus, changes in mRNA abundance by themselves appear not to provide a particularly good explanation for altered MCT1 and MCT4 protein expression. Models describing the mechanisms that regulate gene expression have undergone a number of revisions in the past 50 years. A recent series of reviews have addressed the current understandings of the complexities involved in regulating gene expression (Baker & Parker, 2004; Chambeyron & Bickmore, 2004; Dahlberg & Lund, 2004; Grewal & Rice, 2004; Huang & Richter, 2004; Murchison & Hannon, 2004; Proudfoot, 2004; Van De Bor & Davis, 2004). These reviews clearly show that there is not a simple concordance between the rate of transcription, mRNA abundance and the changes in protein levels. It is, however, the change at the level of the protein that is physiologically most relevant, and testosterone clearly induces the up-regulation of MCT1 and MCT4 proteins in skeletal muscle.
We also examined the effect of testosterone on lactate transport into giant sarcolemmal vesicles. For this purpose, we used the giant sarcolemmal vesicle preparation, which has been used by us (McCullagh et al. 1996a; Tonouchi et al. 2002; Wang et al. 2003), and others (Juel, 1991; Juel et al. 1991, 1994) to examine the rate of lactate uptake across the plasma membrane. Because rodent skeletal muscles express both MCT1 and MCT4, it is difficult to examine lactate uptake in relation to either MCT1 or MCT4 alone. Therefore, we examined lactate transport at a low lactate concentration (1 mm), below the Km of each MCT (Km for MCT1, ∼5 mm; Km for MCT4, ∼20 mm) (Broer et al. 1998; Dimmer et al. 2000; Manning Fox et al. 2000). The testosterone-induced increase in the rate of lactate transport was associated with concomitantly increased plasmalemmal MCT1 and MCT4, presumably as a result of their increased expression. However, the relative increase in lactate transport into red (+21%) and white vesicles (+23%) was less than the increase in plasmalemmal MCT1 (red vesicles, +41%; white vesicles, +39%) and plasmalemmal MCT4 (red vesicles, +25%; white vesicles, +48%). Whether other MCTs in muscle were down-regulated with testosterone treatment is not known.
The increments in the protein expression of MCT1 and MCT4 were qualitatively similar to the increments in the plasmalemmal MCT1 and MCT4, whereas the quantitative increments in protein expression and plasmalemmal content differed somewhat. There would seem to be a relatively simple explanation for this latter difference. We have previously shown that both MCT1 and MCT4 are present at the plasma membrane, the mitochondria and the T-tubules (Bonen et al. 2000b; Benton et al. 2004), and additionally, MCT4 is present in an intracellular depot (Bonen et al. 2000b). By measuring the total MCT1 or MCT4 proteins in muscle homogenates, all the various compartments are included, but in the giant vesicles only the MCTs in a single compartment (i.e. the plasma membrane) are being measured. Given that MCTs are probably not equally distributed between each of the various compartments (i.e. plasma membrane, T-tubules, mitochondria and intracellular depot), it is not too surprising that the relative changes in muscle homogenate and plasma membrane are quantitatively, but not qualitatively, somewhat dissimilar.
Testosterone treatment resulted in a markedly different effect on MCT1 in the heart than in skeletal muscle. The heart expresses MCT1, not MCT4, in mature rats (Bonen et al. 2000b; Hatta et al. 2001). Whereas testosterone treatment did not alter MCT1 protein expression, there was, however, a reduction (−20%) in plasmalemmal MCT1. It is perhaps possible that testosterone treatment increased the mitochondrial pool of MCT1 (Brooks et al. 1999; Benton et al. 2004) in the heart at the expense of the plasmalemmal MCT1 pool. This needs to be confirmed. Clearly, the increased rate of lactate transport in the heart in the testosterone-treated animals cannot be attributed to the reduced plasmalemmal MCT1. The heart probably expresses other MCTs (cf. Poole & Halestrap, 1993; Price et al. 1998). One of these could have been up-regulated by testosterone, and thereby account for the increase in lactate transport into the heart.
In summary, we have shown that testosterone treatment increased the protein expression and plasmalemmal content of MCT1 and MCT4 in skeletal muscles, whereas MCT1 expression in the heart was not altered. The magnitude of the testosterone-induced increases in MCT4, but not MCT1, was highly correlated with the presence of FG muscle fibres. Testosterone treatment also increased the rate of lactate transport into giant sarcolemmal vesicles prepared from red and white muscles, and from the heart. This was associated with an increase in plasma membrane MCT1 and MCT4 in skeletal muscle. In contrast, in the heart, the testosterone-induced increase in the rate of lactate transport could not be attributed to concomitant changes in plasmalemmal MCT1, as this was reduced. We surmise that testosterone treatment may have altered the subcellular distribution of MCT1 and MCT4, and that therefore the changes in plasmalemmal MCT1 and MCT4 and their expression within skeletal muscle were qualitatively but not quantitatively similar. In the heart, however, the changes in plasmalemmal MCT1 and MCT4 protein expression were very different.
Acknowledgments
These studies were supported by the Ministry of Education, Culture, Sports, Science & Technology (MEXT) of Japan and the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chair program. A.B. is the Canada Research Chair in Metabolism and Health.
References
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16:293–299. doi: 10.1016/j.ceb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Benton CR, Bonen A. Experimental Biology. FASEB, San Diego: 2005. PGC-1α expression correlates with skeletal muscle fibre type and fuel handling proteins (Abstract) p. A1121. [Google Scholar]
- Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Commun. 2004;323:249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Storer TW. Proof of the effect of testosterone on skeletal muscle. J Endocrinol. 2001;170:27–38. doi: 10.1677/joe.0.1700027. [DOI] [PubMed] [Google Scholar]
- Bonen A. Expression of lactate transporters (MCT1, MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86:6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- Bonen A, Homonko D. Effects of exercise and glycogen depletion on glyconeogenesis in muscle. J Appl Physiol. 1994;76:1753–1758. doi: 10.1152/jappl.1994.76.4.1753. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Arumugam Y, Glatz JFC, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000a;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Lui S, Dyck DJ, Kiens B, Kristiansen S, Turcotte L, Van Der Vusse GJ, Glatz JFC. Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol Endocrinol Metab. 1998a;275:E471–E478. doi: 10.1152/ajpendo.1998.275.3.E471. [DOI] [PubMed] [Google Scholar]
- Bonen A, McCullagh KJA, Putman CT, Hultman E, Jones NL, Heigenhauser GJF. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am J Physiol Endocrinol Metab. 1998b;274:E102–E107. doi: 10.1152/ajpendo.1998.274.1.E102. [DOI] [PubMed] [Google Scholar]
- Bonen A, McDermott JC, Tan MH. Glycogenesis and glyconeogenesis in skeletal muscle: effects of pH and hormones. Am J Physiol Endocrinol Metab. 1990;258:E693–E700. doi: 10.1152/ajpendo.1990.258.4.E693. [DOI] [PubMed] [Google Scholar]
- Bonen A, Miskovic D, Tonouchi M, Lemieux K, Wilson MC, Marette A, Halestrap AP. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. Am J Physiol Endocrinol Metab. 2000b;278:E1067–E1077. doi: 10.1152/ajpendo.2000.278.6.E1067. [DOI] [PubMed] [Google Scholar]
- Bonen A, Tonuchi M, Miskovic D, Heddle C, Heikkila JJ, Halestrap AP. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am J Physiol Endocrinol Metab. 2000c;279:E1131–E1138. doi: 10.1152/ajpendo.2000.279.5.E1131. [DOI] [PubMed] [Google Scholar]
- Boussouar F, Mauduit C, Tabone E, Pellerin L, Magistretti PJ, Benahmed M. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol Reprod. 2003;69:1069–1078. doi: 10.1095/biolreprod.102.010074. [DOI] [PubMed] [Google Scholar]
- Bricout VA, Germain PS, Serrurier BD, Guezennec CY. Changes in testosterone muscle receptors: effects of an androgen treatment on physically trained rats. Cell Mol Biol (Noisy-le-Grand) 1994;40:291–294. [PubMed] [Google Scholar]
- Bricout VA, Serrurier BD, Bigard AX, Guezennec CY. Effects of hindlimb suspension and androgen treatment on testosterone receptors in rat skeletal muscles. Eur J Appl Physiol. 1999;79:443–448. doi: 10.1007/s004210050535. [DOI] [PubMed] [Google Scholar]
- Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men – a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- Broer S, Schneider HP, Broer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–174. doi: 10.1042/bj3330167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol. 1999;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- Carson JA, Lee WJ, McClung J, Hand GA. Steroid receptor concentration in aged rat hindlimb muscle: effect of anabolic steroid administration. J Appl Physiol. 2002;93:242–250. doi: 10.1152/japplphysiol.01212.2001. [DOI] [PubMed] [Google Scholar]
- Chambeyron S, Bickmore WA. Does looping and clustering in the nucleus regulate gene expression? Curr Opin Cell Biol. 2004;16:256–262. doi: 10.1016/j.ceb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Chatham JC, Gao Z-P, Bonen A, Forder JR. Preferential inhibition of lactate oxidation relative to glucose oxidation in the rat heart following diabetes. Cardiovasc Res. 1999;43:96–106. doi: 10.1016/s0008-6363(99)00056-5. [DOI] [PubMed] [Google Scholar]
- Coles L, Litt J, Hatta H, Bonen A. Exercise rapidly increases expression of the monocarboxylate transporters MCT1 and MCT4 in rat muscle. J Physiol. 2004;561:253–261. doi: 10.1113/jphysiol.2004.073478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg JE, Lund E. Does protein synthesis occur in the nucleus? Curr Opin Cell Biol. 2004;16:308–313. doi: 10.1016/j.ceb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Maresh CM, Armstrong LE, Covault J, Kraemer WJ, Crivello JF. Endurance and resistance exercise induce fiber type specific responses in androgen binding capacity. J Steroid Biochem Mol Biol. 1994;50:175–179. doi: 10.1016/0960-0760(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Dimmer K-S, Friedrich B, Lang F, Deitmer JW, Broer S. The low affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Granier P, Mercier J, Le Peuch C, Prefaut C. Lactate uptake by skeletal muscle sarcolemmal vesicles decreases after 4 wk of hindlimb unweighting in rats. J Appl Physiol. 1996;80:416–421. doi: 10.1152/jappl.1996.80.2.416. [DOI] [PubMed] [Google Scholar]
- Enoki T, Yoshida Y, Hatta H, Bonen A. Exercise training alleviates MCT1 and 4 reductions in heart and skeletal muscles of STZ-induced diabetic rats. J Appl Physiol. 2003;94:2433–2438. doi: 10.1152/japplphysiol.01155.2002. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate Transport and Exchange During Exercise. Vol. 12. New York: Oxord University Press; 1996. [Google Scholar]
- Grewal SIS, Rice JC. Regulation of heterochromatin by histone methylation and small RNAs. Curr Opin Cell Biol. 2004;16:230–238. doi: 10.1016/j.ceb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family – from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Hatta H, Tonouchi M, Bonen A. Tissue-specific and isoform-specific changes in MCT1 and 4 in heart and soleus muscle during a 1-yr period. Am J Physiol Endocrinol Metab. 2001;281:E749–E756. doi: 10.1152/ajpendo.2001.281.4.E749. [DOI] [PubMed] [Google Scholar]
- Holmang A, Svedberg J, Jennische E, Bjorntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol Endocrinol Metab. 1990;259:E555–E560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Richter JD. Regulation of local mRNA translation. Curr Opin Cell Biol. 2004;16:308–313. doi: 10.1016/j.ceb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Juel C. Muscle lactate transport studied in sarcolemmal giant vesicles. Biochim Biophys Acta. 1991;1065:15–20. doi: 10.1016/0005-2736(91)90004-r. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate – proton cotransport in skeletal muscle. Physiol Rev. 1997;77:1–37. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. J Physiol. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Honig A, Pilegaard H. Muscle lactate transport studied in sarcolemmal giant vesicles: dependence on fiber type and age. Acta Physiol Scand. 1991;143:361–365. doi: 10.1111/j.1748-1716.1991.tb09246.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Kristiansen S, Pilegaard H, Wojtaszewski J, Richter EA. Kinetics of lactate transport in sarcolemmal giant vesicles obtained from human skeletal muscle. J Appl Physiol. 1994;76:1031–1036. doi: 10.1152/jappl.1994.76.3.1031. [DOI] [PubMed] [Google Scholar]
- Kuhn FE, Max SR. Testosterone and muscle hypertrophy in female rats. J Appl Physiol. 1985;59:24–27. doi: 10.1152/jappl.1985.59.1.24. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Bonen A. Reduced lactate transport in denervated rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1995;268:R884–R888. doi: 10.1152/ajpregu.1995.268.4.R884. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Juel C, O'Brien M, Bonen A. Chronic muscle stimulation increases lactate transport in rat skeletal muscle. Mol Cell Biochem. 1996a;156:51–57. doi: 10.1007/BF00239319. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol Endocrinol Metab. 1996b;271:E143–E150. doi: 10.1152/ajpendo.1996.271.1.E143. [DOI] [PubMed] [Google Scholar]
- McCullagh KJA, Poole RC, Halestrap AP, O'Brien M, Bonen A. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am J Physiol Endocrinol Metab. 1997;273:E239–E246. doi: 10.1152/ajpendo.1997.273.2.E239. [DOI] [PubMed] [Google Scholar]
- McDermott JC, Bonen A. Glyconeogenic and oxidative lactate utilization in skeletal muscle. Can J Physiol Pharmacol. 1992;70:142–149. doi: 10.1139/y92-021. [DOI] [PubMed] [Google Scholar]
- McDermott JC, Bonen A. Endurance training increases skeletal muscle lactate transport. Acta Physiol Scand. 1993;147:323–327. doi: 10.1111/j.1748-1716.1993.tb09505.x. [DOI] [PubMed] [Google Scholar]
- Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol. 2000;529:285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney LA, Neufer PD, Dohm GL, Tan MH, Blewett CA, Elder GCB, Bonen A. Effects of muscle activity and fiber composition on glucose transport and GLUT4. Am J Physiol Endocrinol Metab. 1993;264:E583–E593. doi: 10.1152/ajpendo.1993.264.4.E583. [DOI] [PubMed] [Google Scholar]
- Monks DA, Kopachik W, Breedlove SM, Jordan CL. Anabolic responsiveness of skeletal muscles correlates with androgen receptor protein but not mRNA. Can J Physiol Pharmacol. 2006;84:273–277. doi: 10.1139/y05-157. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16:223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Pattyn F, Speleman F, De Paepe A, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database. Nucleic Acids Res. 2003;31:122–123. doi: 10.1093/nar/gkg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol Endocrinol Metab. 1999a;276:E255–E261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Juel C, Wibrand F. Lactate transport studied in sarcolemmal giant vesicles from rats: effects of training. Am J Physiol Endocrinol Metab. 1993;264:E156–E160. doi: 10.1152/ajpendo.1993.264.2.E156. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 1999b;276:E843–E848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol Cell Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. New perspectives on connecting messenger RNA-3′ end formation to transcription. Curr Opin Cell Biol. 2004;16:272–278. doi: 10.1016/j.ceb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Py G, Eydoux N, Perez-Martin A, Raynaud E, Brun J-F, Prefaut C, Mercier J. Streptozotocin-induced diabetes decreases rat sarcolemmal lactate transport. Metabolism. 2001a;50:418–424. doi: 10.1053/meta.2001.21692. [DOI] [PubMed] [Google Scholar]
- Py G, Lambert K, Perez-Martin A, Raynaud E, Prefaut C, Mercier J. Impaired sarcolemmal vesicle lactate uptake and skeletal muscle MCT1 and MCT4 expression in obese Zucker rats. Am J Physiol Endocrinol Metab. 2001b;281:E1308–E1315. doi: 10.1152/ajpendo.2001.281.6.E1308. [DOI] [PubMed] [Google Scholar]
- Ramamani A, Aruldhas MM, Govindarajulu P. Differential response of rat skeletal muscle glycogen metabolism to testosterone and estradiol. Can J Physiol Pharmacol. 1999;77:300–304. [PubMed] [Google Scholar]
- Rincon J, Holmang A, Wahlstrom EO, Lonnroth P, Bjorntorp P, Zierath JR, Wallberg-Henriksson H. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes. 1996;45:615–621. doi: 10.2337/diab.45.5.615. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- Tonouchi M, Hatta H, Bonen A. Muscle contraction increases lactate transport while reducing sarcolemmal MCT4, but not MCT1. Am J Physiol Endocrinol Metab. 2002;282:E1062–E1069. doi: 10.1152/ajpendo.00358.2001. [DOI] [PubMed] [Google Scholar]
- Van Breda E, Keizer H, Kuipers H, Kranenburg G. Effect of testosterone and endurance training on glycogen metabolism in skeletal muscle of chronic hyperglycaemic female rats. Br J Sports Med. 2003;37:345–350. doi: 10.1136/bjsm.37.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Bor M, Davis I. mRNA localisation gets more complex. Curr Opin Cell Biol. 2004;16:300–307. doi: 10.1016/j.ceb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tonouchi M, Miskovic D, Hatta H, Bonen A. T3 increases lactate transport and the expression of MCT4, but not MCT1, in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2003;285:E622–E628. doi: 10.1152/ajpendo.00069.2003. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Jackson VN, Hedle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter MCT3. J Biol Chem. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Hatta H, Kato M, Enoki T, Kato H, Bonen A. Relationship between skeletal muscle MCT1 and accumulated exercise during voluntary wheel running. J Appl Physiol. 2004;97:527–534. doi: 10.1152/japplphysiol.01347.2003. [DOI] [PubMed] [Google Scholar]